Development and Assessment of Regeneration Methods for Peptide-Based QCM Biosensors in VOCs Analysis Applications
Abstract
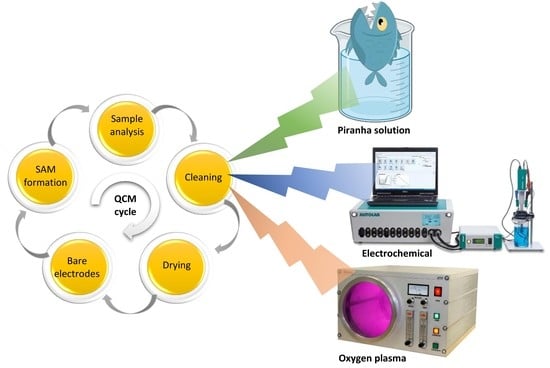
:1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis and Deposition on QCM Transducers
2.2. Piranha Cleaning
2.3. Oxygen Plasma Cleaning
2.4. Electrochemical Cleaning
2.5. Surface Characterization
2.6. Measurement Setup
3. Results
3.1. Bare Gold Electrodes Cleaning
3.2. Cleaning after Deposition
3.3. AFM Analysis
3.4. Biosensors Responses to Gaseous Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Full, J.; Baumgarten, Y.; Delbrück, L.; Sauer, A.; Miehe, R. Market Perspectives and Future Fields of Application of Odor Detection Biosensors within the Biological Transformation—A Systematic Analysis. Biosensors 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Guo, H.; Sun, X. Recent progress on cell-based biosensors for analysis of food safety and quality control. Biosens. Bioelectron. 2019, 126, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, C.; Rajkumar, R.; Bhargava, K. Introduction to Biosensors. In Biosensors and Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2015; T. 9; pp. 1–68. ISBN 9780128031018. [Google Scholar]
- Migoń, D.; Wasilewski, T.; Suchy, D. Application of QCM in Peptide and Protein-Based Drug Product Development. Molecules 2020, 25, 3950. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Davis, J.J. Characterising the biosensing interface. Anal. Chim. Acta, 2022; in press. 339759. [Google Scholar] [CrossRef]
- Pérez, R.L.; Ayala, C.E.; Park, J.Y.; Choi, J.W.; Warner, I.M. Coating-based quartz crystal microbalance detection methods of environmentally relevant volatile organic compounds. Chemosensors 2021, 9, 153. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Dickert, F.L. An Overview of High Frequency Acoustic Sensors—QCMs, SAWs and FBARs—Chemical and Biochemical Applications. Sensors 2019, 19, 4395. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, T.; Szulczyński, B.; Kamysz, W.; Gębicki, J.; Namieśnik, J. Evaluation of three peptide immobilization techniques on a qcm surface related to acetaldehyde responses in the gas phase. Sensors 2018, 18, 3942. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Advances in olfaction-inspired biomaterials applied to bioelectronic noses. Sens. Actuators B Chem. 2018, 257, 511–537. [Google Scholar] [CrossRef]
- Saitoh, A.; Nomura, T.; Munoz, S.; Moriizumi, T. Quartz crystal microbalance odor sensor coated with mixed-thiol-compound sensing film. In Proceedings of the Annual IEEE International Frequency Control Symposium, Pasadena, CA, USA, 29 May 1998; pp. 685–690. [Google Scholar]
- Wang, J.; Tanaka, M.; Okochi, M. Quartz Crystal Microbalance Sensor Based on Peptide Anchored Single-Walled Carbon Nanotubes for Highly Selective TNT Explosive Detection. Proc. IEEE Sensors 2020, 2020. [Google Scholar] [CrossRef]
- Wasilewski, T.; Neubauer, D.; Kamysz, W.; Gębicki, J. Recent progress in the development of peptide-based gas biosensors for environmental monitoring. Case Stud. Chem. Environ. Eng. 2022, 5, 100197. [Google Scholar] [CrossRef]
- Gaggiotti, S.; Palmieri, S.; Della Pelle, F.; Sergi, M.; Cichelli, A.; Mascini, M.; Compagnone, D. Piezoelectric peptide-hpDNA based electronic nose for the detection of terpenes; Evaluation of the aroma profile in different Cannabis sativa L. (hemp) samples. Sens. Actuators B Chem. 2020, 308, 127697. [Google Scholar] [CrossRef]
- Masoumi, S.; Hajghassem, H. Design of the trinitrotoluene biosensor using polydiacetylene conjugated with peptide receptors coated on GR-FETs with colorimetric response. Sens. Rev. 2019, 39, 819–827. [Google Scholar] [CrossRef]
- Tertis, M.; Hosu, O.; Feier, B.; Cernat, A.; Florea, A.; Cristea, C. Electrochemical Peptide-Based Sensors for Foodborne Pathogens Detection. Molecules 2021, 26, 3200. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.M.; Barros, H.L.S.; Flauzino, J.M.R.; Guedes, P.H.G.; Pereira, J.M.; Fujiwara, R.T.; Mineo, T.W.P.; Mineo, J.R.; de Oliveira, R.J.; Madurro, J.M.; et al. A novel peptide-based sensor platform for detection of anti-Toxoplasma gondii immunoglobulins. J. Pharm. Biomed. Anal. 2019, 175. [Google Scholar] [CrossRef] [PubMed]
- Cernat, A.; Canciu, A.; Tertis, M.; Graur, F.; Cristea, C. Synergic action of thermosensitive hydrogel and Au/Ag nanoalloy for sensitive and selective detection of pyocyanin. Anal. Bioanal. Chem. 2019, 411, 3829–3838. [Google Scholar] [CrossRef]
- Cova, C.M.; Rincón, E.; Espinosa, E.; Serrano, L.; Zuliani, A. Paving the Way for a Green Transition in the Design of Sensors and Biosensors for the Detection of Volatile Organic Compounds (VOCs). Biosensors 2022, 12, 51. [Google Scholar] [CrossRef]
- Schmidt, H.G. Safe Piranhas: A Review of Methods and Protocols. ACS Chem. Health Saf. 2022, 29, 54–61. [Google Scholar] [CrossRef]
- Wasilewski, T.; Szulczyński, B.; Wojciechowski, M.; Kamysz, W.; Gębicki, J. A Highly Selective Biosensor Based on Peptide Directly Derived from the HarmOBP7 Aldehyde Binding Site. Sensors 2019, 19, 4284. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, D.; Jaśkiewicz, M.; Bauer, M.; Gołacki, K.; Kamysz, W. Ultrashort cationic lipopeptides–effect of N-terminal amino acid and fatty acid type on antimicrobial activity and hemolysis. Molecules 2020, 25, 257. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, T.; Szulczyński, B.; Wojciechowski, M.; Kamysz, W.; Gębicki, J. Determination of long-chain aldehydes using a novel quartz crystal microbalance sensor based on a biomimetic peptide. Microchem. J. 2020, 154, 104509. [Google Scholar] [CrossRef]
- Zhang, H. Surface characterization techniques for polyurethane biomaterials. In Advances in Polyurethane Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 23–73. ISBN 9780081006221. [Google Scholar]
- Latif, U.; Can, S.; Hayden, O.; Grillberger, P.; Dickert, F.L. Sauerbrey and anti-Sauerbrey behavioral studies in QCM sensors—Detection of bioanalytes. Sens. Actuators B Chem. 2013, 176, 825–830. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Kreuh, D.; Vesel, A. Removal of surface impurities from qcm substrates with the low-pressure oxygen-plasma treatment. Mater. Tehnol. 2013, 47, 795–797. [Google Scholar]
- Berman, D.; Krim, J. Impact of oxygen and argon plasma exposure on the roughness of gold film surfaces. Thin Solid Films 2012, 520, 6201–6206. [Google Scholar] [CrossRef]
- Khusnah, N.F.; Sakti, S.P.; Santjojo, D.J.D.H. Oxygen Plasma Effect on QCM Sensor Coated Polystyrene Film. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367. [Google Scholar] [CrossRef]
- Sugawara, E.; Nikaido, H. Properties of AdeABC and AdeIJK efflux systems of Acinetobacter baumannii compared with those of the AcrAB-TolC system of Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 7250–7257. [Google Scholar] [CrossRef] [Green Version]
- Pinto, S.M.; Pinzon, E.F.; Corzo, S.P.; Miranda, D.A. Effect of the electrode surface on the tetrapolar impedance measurements of Hela Cells in suspension. J. Phys. Conf. Ser. 2019, 1272, 12015. [Google Scholar] [CrossRef] [Green Version]
- Canaria, C.A.; So, J.; Maloney, J.R.; Yu, C.J.; Smith, J.O.; Roukes, M.L.; Fraser, S.E.; Lansford, R. Formation and removal of alkylthiolate self-assembled monolayers on gold in aqueous solutions. Lab Chip 2006, 6, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, V.; Carrara, S.; Stagni, C.; Samorì, B. Chip cleaning and regeneration for electrochemical sensor arrays. Thin Solid Films 2010, 518, 3360–3366. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography: Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhong, Z.W.; Diallo, E.M.; Wang, Z.H.; Yue, W.S. Silicon wafer wettability and aging behaviors: Impact on gold thin-film morphology. Mater. Sci. Semicond. Process. 2014, 26, 25–32. [Google Scholar] [CrossRef]






| No. | Method | Cycle Range (vs. Reference Electrode) | Number of CV Cycles | Scan Rate | Solution | Total Time |
|---|---|---|---|---|---|---|
| 1. | Hydrochloric acid potential cycling | −500 to 1500 mV | 10 | 100 mV/s | 50 mM HCl | 400 s |
| 2. | Sulphuric acid potential cycling | −400 to 1400 mV | 12 | 100 mV/s | 50 mM Sulphuric acid | 240 s |
| 3. | Potassium hydroxide potential sweep | −100 to −1200 mV | 10 | 50 mV/s | 50 mM KOH | 440 s |
| Sensor No. | Frequency Change after Immobilization [Hz], Mass Change [µg/cm2] | Frequency Change after Cleaning [Hz], Mass Change [µg/cm2] |
|---|---|---|
| Cycle 1, 2, 3 | Cycle 1, 2, 3 | |
| Electrochemical | ||
| D1 | −276 (+1214), −255 (+1122), −260 (+1144) | 329 (−1448), 333 (−1465), 290 (−1276) |
| D2 | −291 (+1280), −285 (+1254), −281 (+1236) | 297 (−1307), 305 (−1342), 279 (−1228) |
| D3 | −333 (+1465),−311 (+1368), −309 (+1360) | 401 (−1764), 354 (−1558), 331 (−1456) |
| Plasma | ||
| B1 | −325 (+1430), −301 (+1324), −291 (+1280) | 345 (−1518), 322 (−1417), 229 (−1008) |
| B2 | −377 (+1659), −351 (+1544), −300 (+1320) | 429 (−1888), 453 (−1993), 329 (−1448) |
| B3 | −298 (+1311), −251 (+1104), −239 (+1052) | 301 (−1456), 359 (−1580), 363 (−1597) |
| Piranha | ||
| C1 | −401 (+1764), −389 (+1712), −314 (+1382) | 529 (−2328), 444 (−1954), 407 (−1791) |
| C2 | −388 (+1707), −401 (+1764), −312 (+1373) | 505 (−2222), 403 (−1773), 358 (−1575) |
| C3 | −371 (+1632), −388 (+1707), −301 (+1324) | 398 (−1751), 429 (−1888), 444 (−1954) |
| Sensor No. | Cycle | ΔEp,immo | ΔEp,clean | %Δ | %Δmean |
|---|---|---|---|---|---|
| D1 (HCl cleaning) | 1 | 0.14155 | 0.10786 | −23.8 | −22.0 ± 3.5 |
| 2 | 0.13223 | 0.09891 | −25.2 | ||
| 3 | 0.13755 | 0.11403 | −17.1 | ||
| D2 (H2SO4 cleaning) | 1 | 0.16389 | 0.13183 | −19.6 | −16.9 ± 2.7 |
| 2 | 0.14405 | 0.11841 | −17.8 | ||
| 3 | 0.13577 | 0.11785 | −13.2 | ||
| D3 (KOH cleaning) | 1 | 0.16630 | 0.10228 | −38.5 | −31.3 ± 5.2 |
| 2 | 0.16079 | 0.11432 | −28.9 | ||
| 3 | 0.16032 | 0.11800 | −26.4 |
| Cleaning Technique | Advantages | Disadvantages |
|---|---|---|
| Piranha solution | Easy to handle, possibility to clean multiple sensors in single cycle, expensive instruments or reagents are not required | Very toxic, requires safety procedures, leads to surface erosion, significantly reduces sensors’ lifetime and sensitivity after multiple cleaning cycles, changes sensors’ surface wettability |
| Plasma cleaning | Possibility to clean multiple sensors in single cycle, use of expensive or toxic chemicals is eliminated, high control and repeatability | Slightly reduces sensors’ lifetime and sensitivity after multiple cleaning cycles, minor problems with correct plasma generation, expensive instrument, changes sensors’ surface wettability |
| Electrochemical cleaning | Insignificantly reduces sensors’ lifetime and sensitivity after multiple cleaning cycles, non-invasive for sensors’ surfaces, lower consumption of toxic reagents, safe and environmentally friendly, high control and repeatability | Single sensor can be cleaned in one cycle, time-consuming, complicated instrumentation that requires trained personnel, expensive instrument |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewski, T.; Szulczyński, B.; Dobrzyniewski, D.; Jakubaszek, W.; Gębicki, J.; Kamysz, W. Development and Assessment of Regeneration Methods for Peptide-Based QCM Biosensors in VOCs Analysis Applications. Biosensors 2022, 12, 309. https://doi.org/10.3390/bios12050309
Wasilewski T, Szulczyński B, Dobrzyniewski D, Jakubaszek W, Gębicki J, Kamysz W. Development and Assessment of Regeneration Methods for Peptide-Based QCM Biosensors in VOCs Analysis Applications. Biosensors. 2022; 12(5):309. https://doi.org/10.3390/bios12050309
Chicago/Turabian StyleWasilewski, Tomasz, Bartosz Szulczyński, Dominik Dobrzyniewski, Weronika Jakubaszek, Jacek Gębicki, and Wojciech Kamysz. 2022. "Development and Assessment of Regeneration Methods for Peptide-Based QCM Biosensors in VOCs Analysis Applications" Biosensors 12, no. 5: 309. https://doi.org/10.3390/bios12050309