Electroanalytical Enzyme Biosensor Based on Cordia superba Enzyme Extract for the Detection of Phytomarkers in Kombucha
Abstract
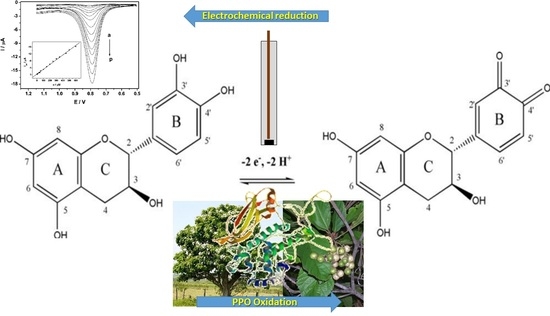
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Preparation of the Raw Vegetable Extract
2.3. Preparation of Kombucha (Fermented Tea)
2.4. Biosensor Preparation, Electrochemical Cell and Voltammetric Parameters
2.5. Antioxidant Activity Determinations
2.6. Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiorcea-Paquim, A.M.; Enache, T.E.; Gil, E.S.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Pelle, F.D.; Compagnone, D. Nanomaterial-based sensing and biosensing of phenolic compounds and related antioxidant capacity in food. Sensors 2018, 18, 2. [Google Scholar]
- Batista, É.A.; Silva, G.N.; Sgobbi, L.F.; Machado, F.B.; Macedo, I.Y.; Moreno, E.K.; Neto, J.R.; Scalize, P.S.; Gil, E.S. Enzymatic electroanalytical biosensor based on maramiellus colocasiae fungus for detection of phytomarkers in infusions and green tea kombucha. Biosensors 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Valente, I.M.; Cabrita, A.R.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.; Maia, M.R. Unravelling the phytonutrients and antioxidant properties of European Vicia faba L. seeds. Int. Food Res. J. 2019, 116, 888–896. [Google Scholar] [CrossRef]
- Gul, I.; Ahmad, M.S.; Naqvi, S.S.; Hussain, A.; Wali, R.; Farooqi, A.A.; Ahmed, I. Polyphenol oxidase (PPO) based biosensors for detection of phenolic compounds: A Review. J. Appl. Biol. 2017, 5, 072–085. [Google Scholar]
- Raymundo-Pereira, P.A.; Silva, T.A.; Caetano, F.R.; Ribovski, L.; Zapp, E.; Brondani, D.; Bergamini, M.F.; Marcolino, L.H., Jr.; Banks, C.E.; Oliveira, O.N., Jr.; et al. Polyphenol oxidase-based electrochemical biosensors: A review. Anal. Chim. Acta. 2020, 1139, 198–221. [Google Scholar] [CrossRef]
- Antunes, R.S.; Ferraz, D.; Garcia, L.G.; Thomaz, D.V.; Luque, R.; Lobón, G.S.; Gil, E.S.; Lopes, F.L. Development of a polyphenol oxidase biosensor from Jenipapo fruit extract (Genipa americana L.) and determination of phenolic compounds in textile industrial effluents. Biosensors 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.F.O.; David, J.P.; David, J.M.; Giulietti, A.M.; Queiroz, L.P.; Santos, R.R.; Soares, M.B.P. Immunomodulatory activity of extracts from Cordia superba Cham. and Cordia rufescens A. DC. (Boraginaceae), plant species native from Brazilian Semi-arid. Braz. J. Pharmacog. 2008, 18, 11–15. [Google Scholar] [CrossRef]
- Lopes, T.N.; Verçoza, F.C.; Missagia, C.C.C. Fenologia reprodutiva e visitantes florais de Cordia superba Cham. (Boraginaceae) na vegetação da restinga de Grumari, Rio de Janeiro. Ver. Biol. Neotrop. 2015, 12, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Matias, E.F.F.; Alves, E.F.; Silva, M.K.D.N.; Carvalho, V.R.D.A.; Coutinho, H.D.M.; Costa, J.G.M.D. The genus Cordia: Botanists, ethno, chemical and pharmacological aspects. Rev. Bras. Farmacogn. 2015, 25, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Souza, G.M.; Balmant, B.D.; Vítolo, H.F.; Gomes, K.B.P.; Florentino, T.M.; Catuchi, T.A.; Vieira, W.L. Estratégias de utilização de luz e estabilidade do desenvolvimento de plântulas de Cordia superba Cham. (Boraginaceae) crescidas em diferentes ambientes luminosos. Acta Bot. Bras. 2009, 23, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.F.; Hikal, M.S.; Abou-Taleb, K.A. Biological, chemical and antioxidant activities of different types Kombucha. Ann. Agric. Sci. 2020, 65, 35–41. [Google Scholar] [CrossRef]
- Mizuta, A.G.; de Menezes, J.L.; Dutra, T.V.; Ferreira, T.V.; Castro, J.C.; da Silva, C.A.J.; Pilau, E.J.; Junior, M.M.; de Abreu Filho, B.A. Evaluation of antimicrobial activity of green tea kombucha at two fermentation time points against Alicyclobacillus spp. LWT 2020, 130, 109641. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.F.; Benjamin, S.R.; Antunes, R.S.; Lopes, F.M.; Somerset, V.S.; Gil, E.D.S. Solanum melongena polyphenol oxidase biosensor for the electrochemical analysis of paracetamol. Prep. Biochem. Biotechnol. 2016, 46, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C.L.W.T. Kinetics and Mechanisms of Antioxidant Activity using the DPPH Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of the Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Pérez, J.H.; López, M.S.P.; López-Cabarcos, E.; López-Ruiz, B. Amperometric tyrosinase biosensor based on polyacrylamide microgels. Biosens. Bioelectron. 2006, 22, 429–439. [Google Scholar] [CrossRef]
- Sandeep, S.; Santhosh, A.S.; Swamy, N.K.; Suresh, G.S.; Melo, J.S. Detection of catechol using a biosensor based on biosynthesized silver nanoparticles and polyphenol oxidase enzymes. Port. Electrochim. Acta. 2019, 37, 257–270. [Google Scholar] [CrossRef]
- Sethuraman, V.; Muthuraja, P.; Manisankar, P. Fabrication of an efficient polyaniline-polyphenol oxidase based biosensor for catechol. Anal. Methods. 2013, 5, 6523–6530. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Wang, S.; Tan, Y.; Kan, J. A new catechol biosensor immobilized polyphenol oxidase by combining electropolymerization and cross-linking process. Int. J. Polym. Mater. 2013, 62, 620–626. [Google Scholar] [CrossRef]
- Nurten, A.; Deniz, F.S.S.; Orhan, I.E. Kombucha-an ancient fermented beverage with desired bioactivities: A narrowed review. Food Chem. X 2020, 14, 72–85. [Google Scholar]
- Anacleto, D.A.; Souza, B.A.; Marchini, L.C.; Moreti, A.C.C.C. Composição de amostras de mel de abelha Jataí (Tetragonisca angustula latreille, 1811). Ciencia Tecnol. Alime. 2019, 29, 535–541. [Google Scholar] [CrossRef]
- Gois, G.C.; Gois, G.C.; Rodrigues, A.E.; de LIma, C.A.B.; Silva, L.T. Vista do Composição do mel de Apis mellifera: Requisitos de qualidade. Acta Vet. Bras. 2013, 7, 137–147. [Google Scholar]
- Lazzarotto, S.; Scherruth, M.S.F.; Calixto, P.S.; Carraro, M.M.; Silveira, A.C.; Lazzarotto, M. Método de Folin Ciocalteou adaptado para quantificar polifenóis em extratos de erva-mate. Revista Movimenta. 2020, 13, 410–426. [Google Scholar]




| Enzyme Source | Method | Linear Range (µM) | Limit of Detection (µM) | Reference |
|---|---|---|---|---|
| C. superba | DPV | 30–300 | 0.13 | This work |
| M. colocosiae | DPV | 50–300 | 0.17 | [3] |
| Jenipapo fruit (Genipa americana L.) | DPV | 10–310 | 7 | [18] |
| Mushrooms | Amperometric | 0.5-24.0 | 0.3 | [19] |
| Manilkara Z. (sapota) Fruit | Chrono amperometric | 1.0–15.0 | 0.47 | [20] |
| Agaricus bisporus | Amperometric | 0.5–101 | 0.15 | [21] |
| Purchased from Sigma | Cyclic voltammetry | 1.0–100 | 0.01 | [22] |
| Samples | Epc (V) (n = 3) | Ipc (µA) ± Standard Deviation (n = 3) | DPPH Catechin Equivalent (µM) ± Standard Deviation (n = 3) | Folin-Ciocalteu Catechin Equivalent (µM) ± Standard Deviation (n = 3) |
|---|---|---|---|---|
| Germany | 0.35 | −9.8 ± 0.38 | −9.8 ± 0.0095 | −2.7 ± 0.02 |
| American | 0.35 | −2.34 ± 0.42 | −0.12 ± 0.055 | −2.4 ± 0.01 |
| Canadian | 0.34 | −0.7 ± 0.44 | −5.08 ± 0.0085 | −2.1 ± 0.11 |
| Jun (honey) | 0.34 | −0.16 ± 0.38 | −0.14 ± 0.006 | −2.06 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, E.A.; Pereira, M.O.A.; Macêdo, I.Y.L.; Machado, F.B.; Moreno, E.K.G.; Diniz, E.P.; Frazzão, I.G.V.; Bernardes, L.S.C.; Oliveira, S.C.B.; Gil, E.S. Electroanalytical Enzyme Biosensor Based on Cordia superba Enzyme Extract for the Detection of Phytomarkers in Kombucha. Biosensors 2022, 12, 1112. https://doi.org/10.3390/bios12121112
Batista EA, Pereira MOA, Macêdo IYL, Machado FB, Moreno EKG, Diniz EP, Frazzão IGV, Bernardes LSC, Oliveira SCB, Gil ES. Electroanalytical Enzyme Biosensor Based on Cordia superba Enzyme Extract for the Detection of Phytomarkers in Kombucha. Biosensors. 2022; 12(12):1112. https://doi.org/10.3390/bios12121112
Chicago/Turabian StyleBatista, Erica A., Marx O. A. Pereira, Isaac Y. L. Macêdo, Fabio B. Machado, Emily K. G. Moreno, Elgia P. Diniz, Italo G. V. Frazzão, Lorrayne S. C. Bernardes, Severino C. B. Oliveira, and Eric S. Gil. 2022. "Electroanalytical Enzyme Biosensor Based on Cordia superba Enzyme Extract for the Detection of Phytomarkers in Kombucha" Biosensors 12, no. 12: 1112. https://doi.org/10.3390/bios12121112