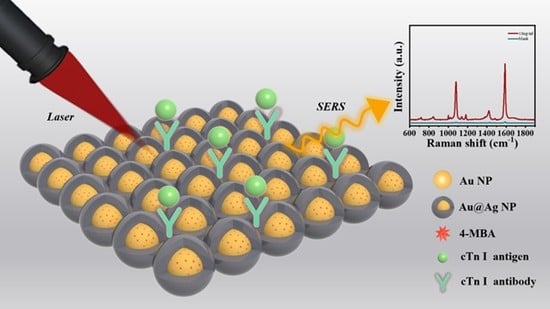
Reporter Molecules Embedded Au@Ag Core-Shell Nanospheres as SERS Nanotags for Cardiac Troponin I Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Au@Ag Nanospheres
2.3. Synthesis of Au@4-MBA@Ag SERS Nanotags
2.4. Materials Characterization and SERS Test
3. Results and Discussion
3.1. Morphological and Raman Characterization of Au and Au@Ag
3.2. Characterization of Core-Shell Structure
3.3. SERS Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Zhao, A.; Zuo, F.; Khan, R.; Hussain, H.M.J.; Chang, J. Au@Ag core-shell nanoparticles for microRNA-21 determination based on duplex-specific nuclease signal amplification and surface-enhanced Raman scattering. Microchim. Acta 2020, 187, 384. [Google Scholar] [CrossRef]
- Daubert, M.A.; Jeremias, A. The utility of troponin measurement to detect myocardial infarction: Review of the current findings. Vasc. Health Risk Manag. 2010, 6, 691–699. [Google Scholar]
- Apple, F.S. Cardiac troponin assays: Analytical issues and clinical reference range cutpoints. Cardiovasc. Toxicol. 2001, 1, 93–98. [Google Scholar] [CrossRef]
- Rezaee, M.A.; Rasaee, M.J.; Mohammadnejad, J. Selection of specific inhibitor peptides in enzyme-linked immunosorbent assay (ELISA) of cardiac troponin I using immuno-dominant epitopes as competitor. J. Immunoass. Immunochem. 2017, 38, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Y.; Li, M.; Ye, X.; Wu, T.; Li, C. Enhanced photoelectrochemical immunosensing of cardiac troponin I based on energy transfer between N-acetyl-L-cysteine capped CdAgTe quantum dots and dodecahedral Au nanoparticles. Biosens. Bioelectron. 2017, 91, 741–746. [Google Scholar] [CrossRef]
- White, H.D. Pathobiology of Troponin Elevations Do Elevations Occur with Myocardial Ischemia as Well as Necrosis? J. Am. Coll. Cardiol. 2011, 57, 2406–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Dou, W.; Liu, X.; Ping, J.; Li, D.; Ying, Y.; Xie, L. Nano-labeled materials as detection tags for signal amplification in immunochromatographic assay. Trac-Trends Anal. Chem. 2022, 154, 116673. [Google Scholar] [CrossRef]
- Shen, W.; Tian, D.; Cui, H.; Yang, D.; Bian, Z. Nanoparticle-based electrochemiluminescence immunosensor with enhanced sensitivity for cardiac troponin I using N-(aminobutyl)-N-(ethylisoluminol)-functionalized gold nanoparticles as labels. Biosens. Bioelectron. 2011, 27, 18–24. [Google Scholar] [CrossRef]
- Lee, T.-H.; Chen, L.-C.; Wang, E.; Wang, C.-C.; Lin, Y.-R.; Chen, W.-L. Development of an Electrochemical Immunosensor for Detection of Cardiac Troponin I at the Point-of-Care. Biosensors 2021, 11, 210. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, R.; Li, M.; Min, R.; Wu, Q.; Kaushik, B.K.; Jha, R.; Zhang, B.; Kumar, S. Cardiac Troponin I Detection using Gold/Cerium-Oxide Nanoparticles assisted Hetro-Core Fiber Structure. IEEE Trans. Nanobiosci. 2022, 3192491. [Google Scholar] [CrossRef]
- Mihailescu, C.-M.; Stan, D.; Iosub, R.; Moldovan, C.; Savin, M. A Sensitive capacitive immunosensor for direct detection of human heart fatty acid-binding protein (h-FABP). Talanta 2015, 132, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Cialla-May, D.; Zheng, X.S.; Weber, K.; Popp, J. Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: From cells to clinics. Chem. Soc. Rev. 2017, 46, 3945–3961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, L.; Liu, B.; Ni, H.; Sun, L.; Su, E.; Chen, H.; Gu, Z.; Zhao, X. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens. Bioelectron. 2018, 106, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, S.; Cai, X.; Huang, S.; Cheng, Z.; Shi, Y.; Pang, M.; Ma, P.a.; Lin, J. Yolk-Shell Structured Au Nanostar@Metal-Organic Framework for Synergistic Chemo-photothermal Therapy in the Second Near-Infrared Window. Nano Lett. 2019, 19, 6772–6780. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, Y.; Liu, X.; Xu, Y.; Wang, S.; Xu, Z. The fabrication of polymer-nanocone-based 3D Au nanoparticle array and its SERS performance. Appl. Phys. A-Mater. Sci. Process. 2017, 123, 45. [Google Scholar] [CrossRef]
- Lee, H.-E.; Lee, H.K.; Chang, H.; Ahn, H.-Y.; Erdene, N.; Lee, H.-Y.; Lee, Y.-S.; Jeong, D.H.; Chung, J.; Nam, K.T. Virus Templated Gold Nanocube Chain for SERS Nanoprobe. Small 2014, 10, 3007–3011. [Google Scholar] [CrossRef]
- Jia, H.; Li, F.; Chow, T.H.; Liu, X.; Zhang, H.; Lu, Y.; Wang, J.; Zhang, C.-y. Construction of Spatially Separated Gold Nanocrystal/Cuprous Oxide Architecture for Plasmon-Driven CO2 Reduction. Nano Lett. 2022, 22, 7268–7274. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; He, J.; Thackray, B.D.; Ye, J. Ultrabright gap-enhanced Raman tags for high-speed bioimaging. Nat. Commun. 2019, 10, 3905. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.-I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Driskell, J.D.; Kwarta, K.M.; Lipert, R.J.; Porter, M.D.; Neill, J.D.; Ridpath, J.F. Low-level detection of viral pathogens by a surface-enhanced Raman scattering based immunoassay. Anal. Chem. 2005, 77, 6147–6154. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; You, H. Preparation of Au @ 4-nitrothiophenol @ Ag @ bovine serum albumin internal surface-enhanced Raman scattering tags and its application in cell Raman imaging. Chin. J. Chromatogr. 2018, 36, 317–324. [Google Scholar]
- Ma, Y.; Pronithaveepong, K.; Li, N. Gold Superparticles Functionalized with Azobenzene Derivatives: SERS Nanotags with Strong Signals. Acs Appl. Mater. Interfaces 2017, 9, 10530–10536. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, H.; Zhang, X.; Liu, D.; Chen, D.; Zhang, W.; Zhang, L.; Santos, H.A.; Hai, M. Biodegradable Photothermal and pH Responsive Calcium Carbonate@Phospholipid@Acetalated Dextran Hybrid Platform for Advancing Biomedical Applications. Adv. Funct. Mater. 2016, 26, 6158–6169. [Google Scholar] [CrossRef]
- Liu, R.; Liu, X.; Tang, Y.; Wu, L.; Hou, X.; Lv, Y. Highly Sensitive Immunoassay Based on Immunogold-Silver Amplification and Inductively Coupled Plasma Mass Spectrometric Detection. Anal. Chem. 2011, 83, 2330–2336. [Google Scholar] [CrossRef]
- Hu, C.; Ma, L.; Mi, F.; Guan, M.; Guo, C.; Peng, F.; Sun, S.; Wang, X.; Liu, T.; Li, J. SERS-based immunoassay using core-shell nanotags and magnetic separation for rapid and sensitive detection of cTn I. New J. Chem. 2021, 45, 3088–3094. [Google Scholar] [CrossRef]
- Wang, D.; Bao, L.; Li, H.; Guo, X.; Liu, W.; Wang, X.; Hou, X.; He, B. Polydopamine stabilizes silver nanoparticles as a SERS substrate for efficient detection of myocardial infarction. Nanoscale 2022, 14, 6212–6219. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.-W.; Pu, H.; Wei, Q. Two-dimensional Au@Ag nanodot array for sensing dual-fungicides in fruit juices with surface-enhanced Raman spectroscopy technique. Food Chem. 2020, 310, 125923. [Google Scholar] [CrossRef]
- Guo, P.; Sikdar, D.; Huang, X.; Si, K.J.; Xiong, W.; Gong, S.; Yap, L.W.; Premaratne, M.; Cheng, W. Plasmonic core-shell nanoparticles for SERS detection of the pesticide thiram: Size- and shape-dependent Raman enhancement. Nanoscale 2015, 7, 2862–2868. [Google Scholar] [CrossRef]
- Hu, C.; Ma, L.; Guan, M.; Mi, F.; Peng, F.; Guo, C.; Sun, S.; Wang, X.; Liu, T.; Li, J. SERS-based magnetic immunoassay for simultaneous detection of cTn I and H-FABP using core-shell nanotags. Anal. Methods 2020, 12, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Shoushtari, A.M.; Rabiee, M.; Uzun, L.; Mak, W.C.; Turner, A.P.F. An electrochemical immunosensor for cardiac Troponin I using electrospun carboxylated multi-walled carbon nanotube-whiskered nanofibres. Talanta 2018, 182, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-H.; Paek, E.-H.; Kim, Y.-K.; Kim, J.-H.; Paek, S.-H. Chemiluminometric enzyme-linked immunosorbent assays (ELISA)-on-a-chip biosensor based on cross-flow chromatography. Anal. Chim. Acta 2009, 632, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Toma, K.; Oishi, K.; Iitani, K.; Arakawa, T.; Mitsubayashi, K. Surface plasmon-enhanced fluorescence immunosensor for monitoring cardiac troponin I. Sens. Actuators B-Chem. 2022, 368, 132132. [Google Scholar] [CrossRef]
- Negahdary, M.; Behjati-Ardakani, M.; Sattarahmady, N.; Yadegari, H.; Heli, H. Electrochemical aptasensing of human cardiac troponin I based on an array of gold nanodumbbells-Applied to early detection of myocardial infarction. Sens. Actuators B-Chem. 2017, 252, 62–71. [Google Scholar] [CrossRef]
- Zuo, J.; Zhao, X.; Ju, X.; Qiu, S.; Hu, W.; Fan, T.; Zhang, J. A New Molecularly Imprinted Polymer (MIP)-based Electrochemical Sensor for Monitoring Cardiac Troponin I (cTnI) in the Serum. Electroanalysis 2016, 28, 2044–2049. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Wang, Y.; Zheng, Y.; Sun, S. An improved portable biosensing system based on enzymatic chemiluminescence and magnetic immunoassay for biological compound detection. Measurement 2014, 47, 200–206. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhao, Y.; Zhang, S.; Bao, L.; Li, H.; Xu, J.; He, B.; Hou, X. Reporter Molecules Embedded Au@Ag Core-Shell Nanospheres as SERS Nanotags for Cardiac Troponin I Detection. Biosensors 2022, 12, 1108. https://doi.org/10.3390/bios12121108
Wang D, Zhao Y, Zhang S, Bao L, Li H, Xu J, He B, Hou X. Reporter Molecules Embedded Au@Ag Core-Shell Nanospheres as SERS Nanotags for Cardiac Troponin I Detection. Biosensors. 2022; 12(12):1108. https://doi.org/10.3390/bios12121108
Chicago/Turabian StyleWang, Ding, Yiru Zhao, Shen Zhang, Liping Bao, Huijun Li, Jingcheng Xu, Bin He, and Xumin Hou. 2022. "Reporter Molecules Embedded Au@Ag Core-Shell Nanospheres as SERS Nanotags for Cardiac Troponin I Detection" Biosensors 12, no. 12: 1108. https://doi.org/10.3390/bios12121108