Characterization and Behaviour of Silica Engineered Nanocontainers in Low and High Ionic Strength Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Engineered Nanomaterials
2.3. Test Solutions and Dispersions
2.4. Engineered Nanomaterial Characterization
2.5. Statistical Analysis
3. Results and Discussion
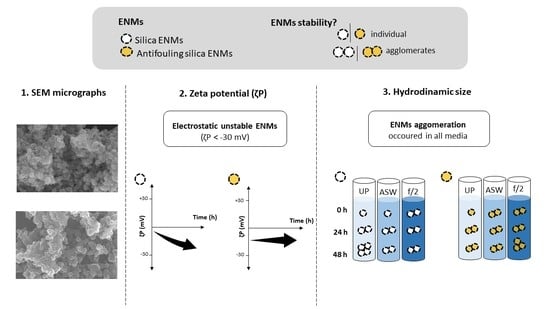
3.1. Engineered Nanomaterial Morphology
3.2. The Zeta Potential of ENMs
3.3. The Influence of Ionic Strength on the ENM Hydrodynamic Diameter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECHA Information on Chemicals (Silicon Dioxide). Available online: https://echa.europa.eu/pt/brief-profile/-/briefprofile/100.028.678 (accessed on 20 January 2022).
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global Life Cycle Releases of Engineered Nanomaterials. J. Nanoparticle Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic Amorphous Silica Nanoparticles: Toxicity, Biomedical and Environmental Implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Duan, L.; Wang, C.; Zhang, W.; Ma, B.; Deng, Y.; Li, W.; Zhao, D. Interfacial Assembly and Applications of Functional Mesoporous Materials. Chem. Rev. 2021, 121, 14349–14429. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering Mesoporous Silica Nanoparticles for Drug Delivery: Where Are We after Two Decades? Chem. Soc. Rev. 2022, 51, 5241–5732. [Google Scholar] [CrossRef] [PubMed]
- Shchukina, E.; Shchukin, D.G. Nanocontainer-Based Active Systems: From Self-Healing Coatings to Thermal Energy Storage. Langmuir 2019, 35, 8603–8611. [Google Scholar] [CrossRef]
- Michailidis, M.; Gutner-Hoch, E.; Wengier, R.; Onderwater, R.; D’Sa, R.A.; Benayahu, Y.; Semenov, A.; Vinokurov, V.; Shchukin, D.G. Highly Effective Functionalized Coatings with Antibacterial and Antifouling Properties. ACS Sustain. Chem. Eng. 2020, 8, 8928–8937. [Google Scholar] [CrossRef]
- Maia, F.; Silva, A.P.; Fernandes, S.; Cunha, A.; Almeida, A.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Incorporation of Biocides in Nanocapsules for Protective Coatings Used in Maritime Applications. Chem. Eng. J. 2015, 270, 150–157. [Google Scholar] [CrossRef]
- Ruggiero, L.; Di Bartolomeo, E.; Gasperi, T.; Luisetto, I.; Talone, A.; Zurlo, F.; Peddis, D.; Ricci, M.A.; Sodo, A. Silica Nanosystems for Active Antifouling Protection: Nanocapsules and Mesoporous Nanoparticles in Controlled Release Applications. J. Alloys Compd. 2019, 798, 144–148. [Google Scholar] [CrossRef]
- Borisova, D.; Mohwald, H.; Shchukin, D.G. Mesoporous Silica Nanoparticles for Active Corrosion Protection. ACS Nano 2011, 5, 1939–1946. [Google Scholar] [CrossRef]
- Maia, F.; Tedim, J.; Lisenkov, A.D.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G.S. Silica Nanocontainers for Active Corrosion Protection. Nanoscale 2012, 4, 1287–1298. [Google Scholar] [CrossRef]
- Martins, R.; Figueiredo, J.; Sushkova, A.; Wilhelm, M.; Tedim, J.; Loureiro, S. “Smart” Nanosensors for Early Detection of Corrosion: Environmental Behavior and Effects on Marine Organisms. Environ. Pollut. 2022, 302, 118973. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.; Loureiro, S.; Martins, R. Hazard of Novel Anti-Fouling Nanomaterials and the Biocides DCOIT and Silver to Marine Organisms. Env. Sci. Nano 2020, 6, 1670–1680. [Google Scholar] [CrossRef]
- Ferreira, V.; Pavlaki, M.D.; Martins, R.; Monteiro, M.S.; Maia, F.; Tedim, J.; Soares, A.M.V.M.; Calado, R.; Loureiro, S. Effects of Nanostructure Antifouling Biocides towards a Coral Species in the Context of Global Changes. Sci. Total. Environ. 2021, 799, 149324. [Google Scholar] [CrossRef] [PubMed]
- Privitera, A.; Ruggiero, L.; Venditti, I.; Pasqual Laverdura, U.; Tuti, S.; De Felicis, D.; Lo Mastro, S.; Duranti, L.; Di Bartolomeo, E.; Gasperi, T.; et al. One Step Nanoencapsulation of Corrosion Inhibitors for Gradual Release Application. Mater. Today Chem. 2022, 24, 100851. [Google Scholar] [CrossRef]
- Soroldoni, S.; Abreu, F.; Castro, Í.B.; Duarte, F.A.; Pinho, G.L.L. Are Antifouling Paint Particles a Continuous Source of Toxic Chemicals to the Marine Environment? J. Hazard. Mater. 2017, 330, 76–82. [Google Scholar] [CrossRef]
- Soroldoni, S.; Vieira da Silva, S.; Castro, Í.B.; de Martinez Gaspar Martins, C.; Leães Pinho, G.L. Antifouling Paint Particles Cause Toxicity to Benthic Organisms: Effects on Two Species with Different Feeding Modes. Chemosphere 2020, 238, 124610. [Google Scholar] [CrossRef] [PubMed]
- Uc-Peraza, R.G.; Delgado-Blas, V.H.; Rendón-von Osten, J.; Castro, Í.B.; Proietti, M.C.; Fillmann, G. Mexican Paradise under Threat: The Impact of Antifouling Biocides along the Yucatán Peninsula. J. Hazard. Mater. 2022, 427, 128162. [Google Scholar] [CrossRef]
- Muller-Karanassos, C.; Arundel, W.; Lindeque, P.K.; Vance, T.; Turner, A.; Cole, M. Environmental Concentrations of Antifouling Paint Particles Are Toxic to Sediment-Dwelling Invertebrates. Environ. Pollut. 2021, 268, 115754. [Google Scholar] [CrossRef]
- Khodaparast, Z.; van Gestel, C.A.M.; Papadiamantis, A.G.; Gonçalves, S.F.; Lynch, I.; Loureiro, S. Toxicokinetics of Silver Nanoparticles in the Mealworm Tenebrio Molitor Exposed via Soil or Food. Sci. Total Environ. 2021, 777, 146071. [Google Scholar] [CrossRef]
- Svendsen, C.; Walker, L.A.; Matzke, M.; Lahive, E.; Harrison, S.; Crossley, A.; Park, B.; Lofts, S.; Lynch, I.; Vázquez-Campos, S.; et al. Key Principles and Operational Practices for Improved Nanotechnology Environmental Exposure Assessment. Nat. Nanotechnol. 2020, 15, 731–742. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (PH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Env. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Burgess, R.M.; Cantwell, M.G.; Portis, L.M.; Perron, M.M.; Wu, F.; Ho, K.T. Stability and Aggregation of Silver and Titanium Dioxide Nanoparticles in Seawater: Role of Salinity and Dissolved Organic Carbon. Env. Toxicol. Chem. 2014, 33, 1023–1029. [Google Scholar] [CrossRef]
- Praetorius, A.; Labille, J.; Scheringer, M.; Thill, A.; Hungerbühler, K.; Bottero, J.-Y. Heteroaggregation of Titanium Dioxide Nanoparticles with Model Natural Colloids under Environmentally Relevant Conditions. Env. Sci. Technol. 2014, 48, 10690–10698. [Google Scholar] [CrossRef] [PubMed]
- Barreto, Â.; Luis, L.G.; Girão, A.V.; Trindade, T.; Soares, A.M.V.M.; Oliveira, M. Behavior of Colloidal Gold Nanoparticles in Different Ionic Strength Media. J. Nanoparticle Res. 2015, 17, 1–13. [Google Scholar] [CrossRef]
- Figueiredo, J.; Oliveira, T.; Ferreira, V.; Sushkova, A.; Silva, S.; Carneiro, D.; Cardoso, D.N.; Gonçalves, S.F.; Maia, F.; Rocha, C.; et al. Toxicity of Innovative Anti-Fouling Nano-Based Solutions to Marine Species. Env. Sci. Nano 2019, 6, 1418–1429. [Google Scholar] [CrossRef]
- Metin, C.O.; Lake, L.W.; Miranda, C.R.; Nguyen, Q.P. Stability of Aqueous Silica Nanoparticle Dispersions. J. Nanoparticle Res. 2011, 13, 839–850. [Google Scholar] [CrossRef]
- Johari, S.A.; Sarkheil, M.; Behzadi Tayemeh, M.; Veisi, S. Influence of Salinity on the Toxicity of Silver Nanoparticles (AgNPs) and Silver Nitrate (AgNO3) in Halophilic Microalgae, Dunaliella Salina. Chemosphere 2018, 209, 156–162. [Google Scholar] [CrossRef]
- Cupi, D.; Hartmann, N.B.; Baun, A. Influence of PH and Media Composition on Suspension Stability of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles and Immobilization of Daphnia Magna under Guideline Testing Conditions. Ecotoxicol. Environ. Saf. 2016, 127, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, S.; Xu, G.; Liu, R.; Xu, A.; Chen, S.; Wu, L. Effects of Ionic Strength on Physicochemical Properties and Toxicity of Silver Nanoparticles. Sci. Total. Environ. 2019, 647, 1088–1096. [Google Scholar] [CrossRef]
- Gondikas, A.; Gallego-Urrea, J.; Halbach, M.; Derrien, N.; Hassellöv, M. Nanomaterial Fate in Seawater: A Rapid Sink or Intermittent Stabilization? Front. Env. Sci. 2020, 8, 151. [Google Scholar] [CrossRef]
- Corsi, I.; Bergami, E.; Grassi, G. Behavior and Bio-Interactions of Anthropogenic Particles in Marine Environment for a More Realistic Ecological Risk Assessment. Front. Env. Sci. 2020, 8, 60. [Google Scholar] [CrossRef]
- Arienzo, M.; Ferrara, L. Environmental Fate of Metal Nanoparticles in Estuarine Environments. Water 2022, 14, 1297. [Google Scholar] [CrossRef]
- Corsi, I.; Desimone, M.F.; Cazenave, J. Building the Bridge From Aquatic Nanotoxicology to Safety by Design Silver Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 298. [Google Scholar] [CrossRef]
- Manzo, S.; Buono, S.; Rametta, G.; Miglietta, M.; Schiavo, S.; di Francia, G. The Diverse Toxic Effect of SiO2 and TiO2 nanoparticles toward the Marine Microalgae Dunaliella Tertiolecta. Environ. Sci. Pollut. Res. 2015, 22, 15941–15951. [Google Scholar] [CrossRef]
- Katsumiti, A.; Arostegui, I.; Oron, M.; Gilliland, D.; Valsami-Jones, E.; Cajaraville, M.P. Cytotoxicity of Au, ZnO and SiO2 NPs Using in Vitro Assays with Mussel Hemocytes and Gill Cells: Relevance of Size, Shape and Additives. Nanotoxicology 2016, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Ciacci, C.; Vallotto, D.; Gallo, G.; Marcomini, A.; Pojana, G. In Vitro Effects of Suspensions of Selected Nanoparticles (C60 Fullerene, TiO2, SiO2) on Mytilus Hemocytes. Aquat. Toxicol. 2010, 96, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Morgana, S.; di Bari, G.; Ramoino, P.; Bramini, M.; Diaspro, A.; Falugi, C.; Faimali, M. Multidisciplinary Screening of Toxicity Induced by Silica Nanoparticles during Sea Urchin Development. Chemosphere 2015, 139, 486–495. [Google Scholar] [CrossRef]
- Garner, K.L.; Keller, A.A. Emerging Patterns for Engineered Nanomaterials in the Environment: A Review of Fate and Toxicity Studies. J. Nanoparticle Res. 2014, 16, 2503. [Google Scholar] [CrossRef]
- Bondarenko, O.M.; Heinlaan, M.; Sihtmäe, M.; Ivask, A.; Kurvet, I.; Joonas, E.; Jemec, A.; Mannerström, M.; Heinonen, T.; Rekulapelly, R.; et al. Multilaboratory Evaluation of 15 Bioassays for (Eco)Toxicity Screening and Hazard Ranking of Engineered Nanomaterials: FP7 Project NANOVALID. Nanotoxicology 2016, 10, 1229–1242. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Tang, H.; Yan, C. Porous Silica Nanocapsules and Nanospheres: Dynamic Self-Assembly Synthesis and Application in Controlled Release. Chem. Mater. 2008, 20, 5894–5900. [Google Scholar] [CrossRef]
- Guillard, R.R. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Springer: Boston, UK, 1975; pp. 29–60. [Google Scholar]
- OECD. Guidance Document on Aquatic and Sediment Toxicological Testing of Nanomaterials; Series on Testing and Assessment No. 317; OECD: Paris, France, 2021. [Google Scholar]
- ISO 26824:2022; Particle Characterization of Particulate Systems (Vocabulary). International Organization for Standardization: Geneva, Switzerland, 2022; p. 26824.
- Michailidis, M.; Sorzabal-bellido, I.; Adamidou, E.A.; Diaz-fernandez, Y.A.; Aveyard, J.; Wengier, R.; Grigoriev, D.; Raval, R.; Benayahu, Y.; Sa, R.A.D.; et al. Modified Mesoporous Silica Nanoparticles with a Dual Synergetic Antibacterial Effect. ACS Appl. Mater. Interfaces 2017, 9, 38364–38372. [Google Scholar] [CrossRef]
- Book, F.; Backhaus, T. Aquatic Ecotoxicity of Manufactured Silica Nanoparticles: A Systematic Review and Meta-Analysis. Sci. Total. Environ. 2022, 806, 150893. [Google Scholar] [CrossRef] [PubMed]
- OECD Test. No. 318: Dispersion Stability of Nanomaterials in Simulated Environmental Media, OECD Guidelines for the Testing of Chemicals; Section 3; OECD: Paris, France, 2017; pp. 1–32. [Google Scholar] [CrossRef]
- Sousa, I.; Maia, F.; Silva, A.; Cunha, Â.; Almeida, A.; Evtyugin, D.V.; Tedim, J.; Ferreira, M.G. A Novel Approach for Immobilization of Polyhexamethylene Biguanide within Silica Capsules. RSC Adv. 2015, 5, 92656–92663. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Sousa, I.; Martins, R.; Figueiredo, J.; Loureiro, S. Gemini Surfactant as a Template Agent for the Synthesis of More Eco-Friendly Silica Nanocapsules. Appl. Sci. 2020, 10, 8085. [Google Scholar] [CrossRef]
- Ambrosone, A.; Scotto di Vettimo, M.R.; Malvindi, M.A.; Roopin, M.; Levy, O.; Marchesano, V.; Pompa, P.P.; Tortiglione, C.; Tino, A. Impact of Amorphous SiO2 Nanoparticles on a Living Organism: Morphological, Behavioral, and Molecular Biology Implications. Front. Bioeng. Biotechnol. 2014, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.M.; Yassin, F.M.; Elshemey, W.M.; Fahmy, H.M. Insight on the Dependence of the Drug Delivery Applications of Mesoporous Silica Nanoparticles on Their Physical Properties. Silicon 2022, 15, 61–70. [Google Scholar] [CrossRef]
- Zhang, X.; Du, X.; Wang, M.; Li, Z.; Zhang, Z.; Tan, C.; Liu, J.; Li, H. Stability of SiO2 Nanoparticles with Complex Environmental Conditions with the Presence of Electrolyte and NOM. J. Nanoparticle Res. 2022, 24, 187. [Google Scholar] [CrossRef]
- Cupi, D.; Hartmann, N.B.; Baun, A. The Influence of Natural Organic Matter and Aging on Suspension Stability in Guideline Toxicity Testing of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles with Daphnia Magna. Env. Toxicol. Chem. 2015, 34, 497–506. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Pavani, M.; Archanaa, S.; Seenivasan, R.; Chandrasekaran, N.; Suraishkumar, G.K.; Mukherjee, A. Diminishing Bioavailability and Toxicity of P25 TiO2 NPs during Continuous Exposure to Marine Algae Chlorella sp. Chemosphere 2019, 233, 363–372. [Google Scholar] [CrossRef]
- Morelli, E.; Gabellieri, E.; Bonomini, A.; Tognotti, D.; Grassi, G.; Corsi, I. TiO2 Nanoparticles in Seawater: Aggregation and Interactions with the Green Alga Dunaliella Tertiolecta. Ecotoxicol. Env. Saf. 2018, 148, 184–193. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Miller, R.J. Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices. Env. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Chinnapongse, S.L.; MacCuspie, R.I.; Hackley, V.A. Persistence of Singly Dispersed Silver Nanoparticles in Natural Freshwaters, Synthetic Seawater, and Simulated Estuarine Waters. Sci. Total. Environ. 2011, 409, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, M.; Wang, X.; Zou, X.; Sun, X.; Shi, J.; Zhang, H. Environmental Fate and Behavior of Silver Nanoparticles in Natural Estuarine Systems. J. Env. Sci. 2020, 88, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Kalarikkal, N.; Stephan, A.M.; Raneesh, B.; Haghi, A.K. Advanced Nanomaterials: Synthesis, Properties, and Applications; CRC Press: Oakville, ON, Canada, 2014. [Google Scholar]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J. Impact of Natural Organic Matter and Divalent Cations on the Stability of Aqueous Nanoparticles. Water Res. 2009, 43, 4249–4257. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.B.; de Cortalezzi, M.M.F. An Experimental Study on the Aggregation of TiO2 Nanoparticles under Environmentally Relevant Conditions. Water Res. 2013, 47, 3887–3898. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidance Document for the Testing of Dissolution and Dispersion Stability of Nanomaterials and the Use of the Data for Further Environmental Testing and Assessment Strategies; Series on Testing and Assessment No. 318; OECD: Paris, France, 2020. [Google Scholar]
- Domingos, R.F.; Wilkinson, K.J. Aggregation of Titanium Dioxide Nanoparticles: Role of a Fulvic Acid. Environ. Sci. Technol. 2009, 43, 1282–1286. [Google Scholar] [CrossRef]
- Lee, B.T.; Ranville, J.F. The Effect of Hardness on the Stability of Citrate-Stabilized Gold Nanoparticles and Their Uptake by Daphnia Magna. J. Hazard. Mater. 2012, 213–214, 434–439. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Aseyev, V.; von Haartman, E.; Karaman, D.Ş.; Mäkilä, E.; Tenhu, H.; Rosenholm, J.; Salonen, J. Size, Stability, and Porosity of Mesoporous Nanoparticles Characterized with Light Scattering. Nanoscale Res. Lett. 2017, 12, 74. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption Surface Area and Porosity. J. Electrochem. Soc. 1967, 114, 279C. [Google Scholar] [CrossRef]

| Nanomaterial | Concentration (mg/L) | Time (h) | pH | ζP (Mean ± SD, mV) | |
|---|---|---|---|---|---|
| SiNC | 0.01 | 0 | 6.4 | −8.7 ± 0.2 | (a,*) |
| 24 | 6.6 | −28.3 ± 3.0 | (b) | ||
| 48 | 6.7 | −23.9 ± 2.0 | (b,*) | ||
| 0.5 | 0 | 6.8 | −11.6 ± 0.6 | (a,*#) | |
| 24 | 6.7 | −31.8 ± 3.7 | (b) | ||
| 48 | 6.7 | −32.6 ± 2.4 | (b,#) | ||
| 1.0 | 0 | 6.6 | −16.6 ± 1.5 | (a,#) | |
| 24 | 6.7 | −31.7 ± 2.5 | (b) | ||
| 48 | 6.8 | −29.8 ± 5.5 | (b,#) | ||
| SiNC-DCOIT | 0.01 | 0 | 6.2 | −12.2 ± 0.6 | (a,*) |
| 24 | 6.4 | −16.3 ± 1.5 | (b) | ||
| 48 | 6.7 | −15.1 ± 1.1 | (b,*#) | ||
| 0.5 | 0 | 6.0 | −15.3 ± 0.9 | (b,#) | |
| 24 | 6.0 | −14.3 ± 1.7 | (ab) | ||
| 48 | 6.3 | −13.2 ± 0.4 | (a,*) | ||
| 1.0 | 0 | 6.2 | −17.1 ± 0.1 | ($) | |
| 24 | 6.2 | −16 ± 0.8 | |||
| 48 | 6.4 | −15.4 ± 0.3 | (#) |
| Nanomaterial | Concentration (mg/L) | Time (h) | Size (nm) | |||||
|---|---|---|---|---|---|---|---|---|
| UP | ASW | f/2 Medium | ||||||
| SiNC | 0.01 | 0 | 148 ± 11.0 | (a,*) | 161 ± 36.6 | (a,*) | 365 ± 43.9 | (b,#) |
| 24 | 272 ± 51.2 | (#) | 269 ± 70.7 | (#) | 279 ± 27.4 | (*) | ||
| 48 | 451 ± 40.1 | (b,$) | 262 ± 51.9 | (a,#) | 276 ± 21.6 | (a,*) | ||
| 0.5 | 0 | 103 ± 44.4 | (a,*) | 257 ± 103.2 | (ab) | 374 ± 55.5 | (b) | |
| 24 | 320 ± 112.0 | (#) | 223 ± 22.5 | 312 ± 30.2 | ||||
| 48 | 318 ± 119.3 | (#) | 287 ± 12.9 | 310 ± 60.5 | ||||
| 1.0 | 0 | 235 ± 113.7 | 176 ± 122.6 | 336 ± 47.0 | ||||
| 24 | 456 ± 203.6 | 224 ± 57.7 | 334 ± 14.5 | |||||
| 48 | 264 ± 63.8 | 235 ± 29.9 | 363 ± 13.8 | |||||
| SiNC-DCOIT | 0.01 | 0 | 169 ± 53.5 | (a,*) | 233 ± 26.0 | (b) | 196 ± 94.8 | (b) |
| 24 | 262 ± 18.6 | (b,#) | 200 ± 25.4 | (a) | 190 ± 5.5 | (a) | ||
| 48 | 209 ± 15.6 | (ab,*#) | 266 ± 9.1 | (b) | 203 ± 45.2 | (a) | ||
| 0.5 | 0 | 194 ± 6.1 | 195 ± 56.6 | 180 ± 32.2 | ||||
| 24 | 246 ± 29.5 | 201 ± 3.6 | 199 ± 58.8 | |||||
| 48 | 212 ± 9.1 | 192 ± 55.1 | 169 ± 24.6 | |||||
| 1.0 | 0 | 152 ± 20.3 | (*) | 189 ± 69.0 | 254 ± 90.2 | (*) | ||
| 24 | 241 ± 23.5 | (#) | 220 ± 69.6 | 344 ± 61.0 | (#) | |||
| 48 | 237 ± 43.2 | (#) | 240 ± 104.0 | 310 ± 84.6 | (*#) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, V.; Figueiredo, J.; Martins, R.; Sushkova, A.; Maia, F.; Calado, R.; Tedim, J.; Loureiro, S. Characterization and Behaviour of Silica Engineered Nanocontainers in Low and High Ionic Strength Media. Nanomaterials 2023, 13, 1738. https://doi.org/10.3390/nano13111738
Ferreira V, Figueiredo J, Martins R, Sushkova A, Maia F, Calado R, Tedim J, Loureiro S. Characterization and Behaviour of Silica Engineered Nanocontainers in Low and High Ionic Strength Media. Nanomaterials. 2023; 13(11):1738. https://doi.org/10.3390/nano13111738
Chicago/Turabian StyleFerreira, Violeta, Joana Figueiredo, Roberto Martins, Alesia Sushkova, Frederico Maia, Ricardo Calado, João Tedim, and Susana Loureiro. 2023. "Characterization and Behaviour of Silica Engineered Nanocontainers in Low and High Ionic Strength Media" Nanomaterials 13, no. 11: 1738. https://doi.org/10.3390/nano13111738