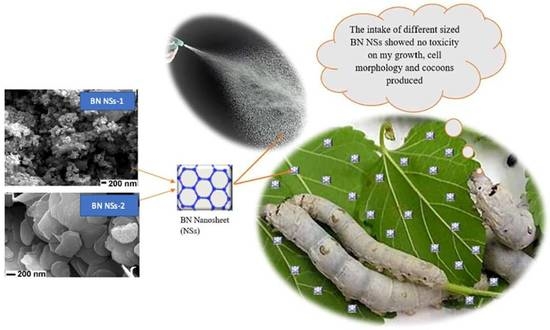
The Influence of the Size of BN NSs on Silkworm Development and Tissue Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Chemical Characterization
2.3. Preparation of BN NSs Solution
2.4. The Intake of BN NSs by Silkworms and Cocooning
2.5. Histopathological Evaluation of Silkworm Tissues
2.6. Statistical Analysis
3. Results and Discussions
3.1. Characteristics of the Two Distinct Sizes of BN NSs
3.2. The Effect of BN NSs on Larvae Growth and Mortality of Silkworm
3.3. The Influence of BN NSs on the Cocoons
3.4. Histopathological Evaluation of Silkworm Tissues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhi, C.; Weng, Q.; Bando, Y.; Golberg, D. Boron nitride nanosheets: Novel syntheses and applications in polymeric composites. J. Phys. Conf. Ser. 2013, 471, 012003. [Google Scholar] [CrossRef]
- Alansy, A.S.; Saeed, T.A.; Al-Attab, R.; Guo, Y.; Yang, Y.; Liu, B.; Fan, Z. Boron nitride nanosheets modified with zinc oxide nanoparticles as novel fillers of dental resin composite. Dent. Mater. 2022, 38, e266–e274. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Istrate, O.M.; May, P.; Barwich, S.; Bell, A.P.; Khan, U.; Coleman, J.N. Boron nitride nanosheets as barrier enhancing fillers in melt processed composites. Nanoscale 2015, 7, 4443–4450. [Google Scholar] [CrossRef]
- Qian, Y.; Xu, Y.; Yan, Z.; Jin, Y.; Chen, X.; Yuan, W.E.; Fan, C. Boron nitride nanosheets functionalized channel scaffold favors microenvironment rebalance cocktail therapy for piezocatalytic neuronal repair. Nano Energy 2021, 83, 105779. [Google Scholar] [CrossRef]
- Zheng, Z.; Cox, M.; Li, B. Surface modification of hexagonal boron nitride nanomaterials: A review. J. Mater. Sci. 2018, 53, 66–99. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Liang, L.; Guan, Z.; Ren, J. Synergistic Enhanced Thermal Conductivity and Crack Resistance of Reactor Epoxy Insulation with Boron Nitride Nanosheets and Multiwalled Carbon Nanotubes. Nanomaterials 2022, 12, 3235. [Google Scholar] [CrossRef]
- Khan, A.; Puttegowda, M.; Jagadeesh, P.; Marwani, H.M.; Asiri, A.M.; Manikandan, A.; Khan, A.A.P.; Ashraf, G.M.; Rangappa, S.M.; Siengchin, S. Review on Nitride compounds and its polymer composites: A multifunctional material. J. Mater. Res. Technol. 2022, 18, 2175–2193. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, H.; Wang, H.; Zhu, X.; Chen, L.; Gao, W.; Liu, C.; Yin, H. Defect engineering of hexagonal boron nitride nanosheets via hydrogen plasma irradiation. Appl. Surf. Sci. 2022, 593, 153386. [Google Scholar] [CrossRef]
- Pandit, S.; Gaska, K.; Mokkapati, V.R.S.S.; Forsberg, S.; Svensson, M.; Kádár, R.; Mijakovic, I. Antibacterial effect of boron nitride flakes with controlled orientation in polymer composites. RSC Adv. 2019, 9, 33454–33459. [Google Scholar] [CrossRef]
- Doğan, D.; Metin, A.Ü. Physicochemical and biological assessment of boron nitride nanosheets-reinforced poly (2-hydroxyethylmethacrylate) composite for biomedical applications. Mater. Today Commun. 2022, 33, 104807. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, H.; Li, G.; Li, Y.; Jiang, J.; Chen, J.; Xie, Q.; Wu, D.; Ma, R.; Liu, X.; et al. Antibiotic-Like Activity of Atomic Layer Boron Nitride for Combating Resistant Bacteria. ACS Nano 2022, 16, 7674–7688. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, Y.; Zhang, W.; Zeng, L.; Long, Y.; Wang, S.; Weng, Q. Gram-scale synthesis of boron nitride nanosheets by salt-template method for anticancer drug delivery. Chem. Eng. J. 2022, 437, 135304. [Google Scholar] [CrossRef]
- Czarniewska, E.; Mrówczyńska, L.; Jędrzejczak-Silicka, M.; Nowicki, P.; Trukawka, M.; Mijowska, E. Non-cytotoxic hydroxyl-functionalized exfoliated boron nitride nanoflakes impair the immunological function of insect hemocytes in vivo. Sci. Rep. 2019, 9, 14027. [Google Scholar] [CrossRef]
- Mateti, S.; Wong, C.S.; Liu, Z.; Yang, W.; Li, Y.; Li, L.H.; Chen, Y. Biocompatibility of boron nitride nanosheets. Nano Res. 2018, 11, 334–342. [Google Scholar] [CrossRef]
- Fang, L.; Seifert, S.; Winans, R.E.; Li, T. Understanding synthesis and structural variation of nanomaterials through in situ/operando XAS and SAXS. Small 2022, 18, 2106017. [Google Scholar] [CrossRef]
- Li, M.; Huang, G.; Chen, X.; Yin, J.; Zhang, P.; Yao, Y.; Shen, J.; Wu, Y.; Huang, J. Perspectives on environmental applications of hexagonal boron nitride nanomaterials. Nano Today 2022, 44, 101486. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, G.; Singh, P.; Singh, K.; Kumar, B.; Vij, A.; Kumar, M.; Bala, R.; Meena, R.; Singh, A.; et al. Nanostructured boron nitride with high water dispersibility for boron neutron capture therapy. Sci. Rep. 2016, 6, 35535. [Google Scholar] [CrossRef]
- Liu, S.; Shen, Z.; Wu, B.; Yu, Y.; Hou, H.; Zhang, X.X.; Ren, H.Q. Cytotoxicity and efflux pump inhibition induced by molybdenum disulfide and boron nitride nanomaterials with sheetlike structure. Environ. Sci. Technol. 2017, 51, 10834–10842. [Google Scholar] [CrossRef]
- An, W.; Han, B.; Li, K.; Akhtar, S.; Zhang, Y.; Zhang, X.; Sha, X.; Gao, L. The protective study about alleviation of simvastatin on the damages of PEG-BNs in mice. Environ. Toxicol. Pharmacol. 2017, 53, 64–73. [Google Scholar] [CrossRef]
- Ma, L.; Andoh, V.; Adjei, M.O.; Liu, H.; Shen, Z.; Li, L.; Song, J.; Zhao, W.; Wu, G. In vivo toxicity evaluation of boron nitride nanosheets in Bombyx mori silkworm model. Chemosphere 2020, 247, 125877. [Google Scholar] [CrossRef] [PubMed]
- Panthee, S.; Paudel, A.; Hamamoto, H.; Sekimizu, K. Advantages of the silkworm as an animal model for developing novel antimicrobial agents. Front. Microbiol. 2017, 8, 373. [Google Scholar] [CrossRef]
- Abdelli, N.; Peng, L.; Keping, C. Silkworm, Bombyx mori, as an alternative model organism in toxicological research. Environ. Sci. Pollut. Res. 2018, 25, 35048–35054. [Google Scholar] [CrossRef] [PubMed]
- Andoh, V.; Chen, L.; Zhu, F.; Ge, Q.; Ma, L.; Wang, Q.; Chen, K. The Evaluation of the Biological Effects of Melanin by Using Silkworm as a Model Animal. Toxins 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Andoh, V.; Shen, Z.; Liu, H.; Li, L.; Chen, K. Subchronic toxicity of magnesium oxide nanoparticles to Bombyx mori silkworm. RSC Adv. 2022, 12, 17276–17284. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Huang, H.; Chen, S.; Wang, W.; Dai, F.; Zhao, H. Characterization of silkworm larvae growth and properties of silk fibres after direct feeding of copper or silver nanoparticles. Mater. Des. 2017, 129, 125–134. [Google Scholar] [CrossRef]
- Hatem, G.; Zeidan, J.; Goossens, M.; Moreira, C. Normality testing methods and the importance of skewness and kurtosis in statistical analysis. BAU J. Sci. Technol. 2022, 3, 7. [Google Scholar] [CrossRef]
- Johnson, R.W. Alternate Forms of the One-Way ANOVA F and Kruskal–Wallis Test Statistics. J. Stat. Data Sci. Educ. 2022, 30, 82–85. [Google Scholar] [CrossRef]
- Siegel, S. 1957. Nonparametric statistics. Am. Stat. 1957, 11, 13–19. [Google Scholar]
- Li, K.L.; Zhang, Y.H.; Xing, R.; Zhou, Y.F.; Chen, X.D.; Wang, H.; Song, B.; Sima, Y.H.; He, Y.; Xu, S.Q. Different toxicity of cadmium telluride, silicon, and carbon nanomaterials against hemocytes in silkworm, Bombyx mori. RSC Adv. 2017, 7, 50317–50327. [Google Scholar] [CrossRef]
- Gao, K.; Li, B.; Chen, R.; Qian, P.; Dong, J.; Xue, C.; Guo, X.; Deng, X. A feasibility study of using silkworm larvae as a novel in vivo model to evaluate the biotoxicity of ionic liquids. Ecotoxicol. Environ. Saf. 2021, 209, 111759. [Google Scholar] [CrossRef]
- Li, L.H.; Cervenka, J.; Watanabe, K.; Taniguchi, T.; Chen, Y. Strong oxidation resistance of atomically thin boron nitride nanosheets. ACS Nano 2014, 8, 1457–1462. [Google Scholar] [CrossRef]
- Cho, H.B.; Tokoi, Y.; Tanaka, S.; Suematsu, H.; Suzuki, T.; Jiang, W.; Niihara, K.; Nakayama, T. Modification of BN nanosheets and their thermal conducting properties in nanocomposite film with polysiloxane according to the orientation of BN. Compos. Sci. Technol. 2011, 71, 1046–1052. [Google Scholar] [CrossRef]
- Tabunoki, H.; Higurashi, S.; Ninagi, O.; Fujii, H.; Banno, Y.; Nozaki, M.; Kitajima, M.; Miura, N.; Atsumi, S.; Tsuchida, K.; et al. A carotenoid-binding protein (CBP) plays a crucial role in cocoon pigmentation of silkworm (Bombyx mori) larvae. FEBS Lett. 2004, 567, 175–178. [Google Scholar] [CrossRef]
- Sakudoh, T.; Sezutsu, H.; Nakashima, T.; Kobayashi, I.; Fujimoto, H.; Uchino, K.; Banno, Y.; Iwano, H.; Maekawa, H.; Tamura, T.; et al. Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the yellow blood gene. Proc. Natl. Acad. Sci. USA 2007, 104, 8941–8946. [Google Scholar] [CrossRef]
- Sluchanko, N.N.; Slonimskiy, Y.B.; Egorkin, N.A.; Varfolomeeva, L.A.; Faletrov, Y.V.; Moysenovich, A.M.; Parshina, E.Y.; Friedrich, T.; Maksimov, E.G.; Boyko, K.M.; et al. Silkworm carotenoprotein as an efficient carotenoid extractor, solubilizer and transporter. Int. J. Biol. Macromol. 2022, 223, 1381–1393. [Google Scholar] [CrossRef]
- Tabunoki, H.; Sugiyama, H.; Tanaka, Y.; Fujii, H.; Banno, Y.; Jouni, Z.E.; Kobayashi, M.; Sato, R.; Maekawa, H.; Tsuchida, K. Isolation, characterization, and cDNA sequence of a carotenoid binding protein from the silk gland of Bombyx mori larvae. J. Biol. Chem. 2002, 277, 32133–32140. [Google Scholar] [CrossRef]
- Sun, L.X. Research on the Structure and Properties of a Natural Yellow Mulberry Silk; Soochow University: Suzhou, China, 2007; pp. 3–49. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andoh, V.; Liu, H.; Chen, L.; Ma, L.; Chen, K. The Influence of the Size of BN NSs on Silkworm Development and Tissue Microstructure. Nanomaterials 2023, 13, 1502. https://doi.org/10.3390/nano13091502
Andoh V, Liu H, Chen L, Ma L, Chen K. The Influence of the Size of BN NSs on Silkworm Development and Tissue Microstructure. Nanomaterials. 2023; 13(9):1502. https://doi.org/10.3390/nano13091502
Chicago/Turabian StyleAndoh, Vivian, Haiyan Liu, Liang Chen, Lin Ma, and Keping Chen. 2023. "The Influence of the Size of BN NSs on Silkworm Development and Tissue Microstructure" Nanomaterials 13, no. 9: 1502. https://doi.org/10.3390/nano13091502