Effect of Exposure Concentration and Growth Conditions on the Association of Cerium Oxide Nanoparticles with Green Algae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Algal Growth Conditions
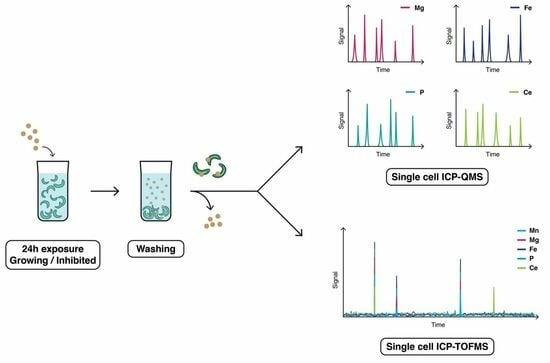
2.3. Algal–CeO2 Interaction Tests
2.4. ICP-MS Instrumentation
3. Results and Discussion
3.1. Selection of Cellular Tracer for R. subcapitata
3.2. Cellular Elemental Profile for R. subcapitata Exposed to CeO2 NPs
3.3. CeO2 Nanoparticle Association with Algal Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petersen, E.J.; Mortimer, M.; Burgess, R.M.; Handy, R.; Hanna, S.; Ho, K.T.; Johnson, M.; Loureiro, S.; Selck, H.; Scott-Fordsmand, J.J.; et al. Strategies for robust and accurate experimental approaches to quantify nanomaterial bioaccumulation across a broad range of organisms. Environ. Sci. Nano 2019, 6, 1619–1656. [Google Scholar] [CrossRef]
- Pang, C.; Skjolding, L.M.; Selck, H.; Baun, A. A “point-of-entry” bioaccumulation study of nanoscale pigment copper phthalocyanine in aquatic organisms. Environ. Sci. Nano 2021, 8, 554–564. [Google Scholar] [CrossRef]
- Zheng, Y.; Nowack, B. Meta-analysis of Bioaccumulation Data for Non-dissolvable Engineered Nanomaterials in Freshwater Aquatic Organisms. Environ. Toxicol. Chem. 2022, 41, 1202–1214. [Google Scholar] [CrossRef]
- Merrifield, R.C.; Stephan, C.; Lead, J.R. Quantification of Au Nanoparticle Biouptake and Distribution to Freshwater Algae Using Single Cell—ICP-MS. Environ. Sci. Technol. 2018, 52, 2271–2277. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Monikh, F.A.; Vijver, M.G.; Peijnenburg, W.J.G.M. Interaction of zero valent copper nanoparticles with algal cells under simulated natural conditions: Particle dissolution kinetics, uptake and heteroaggregation. Sci. Total Environ. 2019, 689, 133–140. [Google Scholar] [CrossRef]
- Monikh, F.A.; Fryer, B.; Arenas-Lago, D.; Vijver, M.G.; Krishna Darbha, G.; Valsami-Jones, E.; Peijnenburg, W.J.G.M. A Dose Metrics Perspective on the Association of Gold Nanomaterials with Algal Cells. Environ. Sci. Technol. Lett. 2019, 6, 732–738. [Google Scholar] [CrossRef]
- Bouldin, J.L.; Ingle, T.M.; Sengupta, A.; Alexander, R.; Hannigan, R.E.; Buchanan, R.A. Aqueous toxicity and food chain transfer of quantum dots™ in freshwater algae and Ceriodaphnia dubia. Environ. Toxicol. Chem. 2008, 27, 1958–1963. [Google Scholar] [CrossRef]
- Bhuvaneshwari, M.; Kumar, D.; Roy, R.; Chakraborty, S.; Parashar, A.; Mukherjee, A.; Chandrasekaran, N.; Mukherjee, A. Toxicity, accumulation, and trophic transfer of chemically and biologically synthesized nano zero valent iron in a two species freshwater food chain. Aquat. Toxicol. 2017, 183, 63–75. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Mei, N.; Hedberg, J.; Ekvall, M.T.; Kelpsiene, E.; Hansson, L.-A.; Cedervall, T.; Blomberg, E.; Odnevall, I. Transfer of Cobalt Nanoparticles in a Simplified Food Web: From Algae to Zooplankton to Fish. Appl. Nano 2021, 2, 184–205. [Google Scholar] [CrossRef]
- Monikh, F.A.; Chupani, L.; Arenas-Lago, D.; Guo, Z.; Zhang, P.; Darbha, G.K.; Valsami-Jones, E.; Lynch, I.; Vijver, M.G.; van Bodegom, P.M.; et al. Particle number-based trophic transfer of gold nanomaterials in an aquatic food chain. Nat. Commun. 2021, 12, 899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, X.; Liu, X.; Wang, Z.; Zhang, C.; White, J.C.; Xing, B. Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: Adhesion, uptake, and toxicity. Nanotoxicology 2016, 10, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Ozkaleli, M.; Erdem, A. Biotoxicity of TiO2 Nanoparticles on Raphidocelis subcapitata Microalgae Exemplified by Membrane Deformation. Int. J. Environ. Res. Public Health 2018, 15, 416. [Google Scholar] [CrossRef]
- Lin, S.; Bhattacharya, P.; Rajapakse, N.C.; Brune, D.E.; Ke, P.C. Effects of Quantum Dots Adsorption on Algal Photosynthesis. J. Phys. Chem. C 2009, 113, 10962–10966. [Google Scholar] [CrossRef]
- Cherchi, C.; Chernenko, T.; Diem, M.; Gu, A.Z. Impact of nano titanium dioxide exposure on cellular structure of Anabaena variabilis and evidence of internalization. Environ. Toxicol. Chem. 2011, 30, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Sekine, R.; Moore, K.L.; Matzke, M.; Vallotton, P.; Jiang, H.; Hughes, G.M.; Kirby, J.K.; Donner, E.; Grovenor, C.R.M.; Svendsen, C.; et al. Complementary Imaging of Silver Nanoparticle Interactions with Green Algae: Dark-Field Microscopy, Electron Microscopy, and Nanoscale Secondary Ion Mass Spectrometry. ACS Nano 2017, 11, 10894–10902. [Google Scholar] [CrossRef]
- Montaño, M.D.; Badiei, H.R.; Bazargan, S.; Ranville, J.F. Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times. Environ. Sci. Nano 2014, 1, 338–346. [Google Scholar] [CrossRef]
- Theiner, S.; Loehr, K.; Koellensperger, G.; Mueller, L.; Jakubowski, N. Single-cell analysis by use of ICP-MS. J. Anal. At. Spectrom. 2020, 35, 1784–1813. [Google Scholar] [CrossRef]
- Hendriks, L.; Skjolding, L.M. Single-cell analysis by inductively coupled plasma-time-of-flight mass spectrometry to quantify algal cell interaction with nanoparticles by their elemental fingerprint. Spectroscopy 2020, 35, 9–16. [Google Scholar]
- Oliver, A.L.; Baumgart, S.; Bremser, W.; Flemig, S.; Wittke, D.; Grützkau, A.; Luch, A.; Haase, A.; Jakubowski, N. Quantification of silver nanoparticles taken up by single cells using inductively coupled plasma mass spectrometry in the single cell measurement mode. J. Anal. At. Spectrom. 2018, 33, 1256–1263. [Google Scholar] [CrossRef]
- Hsiao, I.-L.; Bierkandt, F.S.; Reichardt, P.; Luch, A.; Huang, Y.-J.; Jakubowski, N.; Tentschert, J.; Haase, A. Quantification and visualization of cellular uptake of TiO2 and Ag nanoparticles: Comparison of different ICP-MS techniques. J. Nanobiotechnol. 2016, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.-S.; Chan, W.-T. Time-resolved ICP-MS measurement for single-cell analysis and on-line cytometry. J. Anal. At. Spectrom. 2010, 25, 1114–1122. [Google Scholar] [CrossRef]
- von der Au, M.; Borovinskaya, O.; Flamigni, L.; Kuhlmeier, K.; Büchel, C.; Meermann, B. Single cell-inductively coupled plasma-time of flight-mass spectrometry approach for ecotoxicological testing. Algal Res. 2020, 49, 101964. [Google Scholar] [CrossRef]
- Ivanov, V.K.; Shcherbakov, A.B.; Usatenko, A.V. Structure-sensitive properties and biomedical applications of nanodispersed cerium dioxide. Russ. Chem. Rev. 2009, 78, 855–871. [Google Scholar]
- Corma, A.; Atienzar, P.; García, H.; Chane-Ching, J.-Y. Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat. Mater. 2004, 3, 394–397. [Google Scholar] [CrossRef]
- Jung, H.; Kittelson, D.B.; Zachariah, M.R. The influence of a cerium additive on ultrafine diesel particle emissions and kinetics of oxidation. Combust. Flame 2005, 142, 276–288. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H.F. Oxygen Vacancies and Catalysis on Ceria Surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef]
- Pulido-Reyes, G.; Briffa, S.M.; Hurtado-Gallego, J.; Yudina, T.; Leganés, F.; Puntes, V.; Valsami-Jones, E.; Rosal, R.; Fernández-Piñas, F. Internalization and toxicological mechanisms of uncoated and PVP-coated cerium oxide nanoparticles in the freshwater alga Chlamydomonas reinhardtii. Environ. Sci. Nano 2019, 6, 1959–1972. [Google Scholar] [CrossRef]
- Kosak née Röhder, L.A.; Brandt, T.; Sigg, L.; Behra, R. Uptake and effects of cerium(III) and cerium oxide nanoparticles to Chlamydomonas reinhardtii. Aquat. Toxicol. 2018, 197, 41–46. [Google Scholar] [CrossRef]
- Booth, A.; Størseth, T.; Altin, D.; Fornara, A.; Ahniyaz, A.; Jungnickel, H.; Laux, P.; Luch, A.; Sørensen, L. Freshwater dispersion stability of PAA-stabilised cerium oxide nanoparticles and toxicity towards Pseudokirchneriella subcapitata. Sci. Total Environ. 2015, 505, 596–605. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Z.; Zhai, Y.; Zhang, F.; Vijver, M.G.; Peijnenburg, W.J.G.M. Effects of humic substances on the aqueous stability of cerium dioxide nanoparticles and their toxicity to aquatic organisms. Sci. Total Environ. 2021, 781, 146583. [Google Scholar] [CrossRef] [PubMed]
- Mahaye, N.; Musee, N. Evaluation of Apical and Molecular Effects of Algae Pseudokirchneriella subcapitata to Cerium Oxide Nanoparticles. Toxics 2023, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Test No. 318: Dispersion Stability of Nanomaterials in Simulated Environmental Media; OECD: Paris, France, 2018. [Google Scholar]
- ISO 8692:2012; Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. nternational Organization for Standardization: Geneva, Switzerland, 2012.
- Skjolding, L.M.; Kruse, S.; Sørensen, S.N.; Hjorth, R.; Baun, A. A Small-Scale Setup for Algal Toxicity Testing of Nanomaterials and Other Difficult Substances. JoVE 2020, 164, e61209. [Google Scholar]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar]
- Lekamge, S.; Miranda, A.F.; Ball, A.S.; Shukla, R.; Nugegoda, D. The toxicity of coated silver nanoparticles to Daphnia carinata and trophic transfer from alga Raphidocelis subcapitata. PLoS ONE 2019, 14, e0214398. [Google Scholar] [CrossRef] [PubMed]
- Rivero Arze, A.; Manier, N.; Chatel, A.; Mouneyrac, C. Characterization of the nano–bio interaction between metallic oxide nanomaterials and freshwater microalgae using flow cytometry. Nanotoxicology 2020, 14, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Joonas, E.; Aruoja, V.; Olli, K.; Kahru, A. Environmental safety data on CuO and TiO2 nanoparticles for multiple algal species in natural water: Filling the data gaps for risk assessment. Sci. Total Environ. 2019, 647, 973–980. [Google Scholar] [CrossRef]
- Arvidsson, R.; Hansen, S.F.; Baun, A. Influence of natural organic matter on the aquatic ecotoxicity of engineered nanoparticles: Recommendations for environmental risk assessment. NanoImpact 2020, 20, 100263. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackevica, A.; Hendriks, L.; Meili-Borovinskaya, O.; Baun, A.; Skjolding, L.M. Effect of Exposure Concentration and Growth Conditions on the Association of Cerium Oxide Nanoparticles with Green Algae. Nanomaterials 2023, 13, 2468. https://doi.org/10.3390/nano13172468
Mackevica A, Hendriks L, Meili-Borovinskaya O, Baun A, Skjolding LM. Effect of Exposure Concentration and Growth Conditions on the Association of Cerium Oxide Nanoparticles with Green Algae. Nanomaterials. 2023; 13(17):2468. https://doi.org/10.3390/nano13172468
Chicago/Turabian StyleMackevica, Aiga, Lyndsey Hendriks, Olga Meili-Borovinskaya, Anders Baun, and Lars Michael Skjolding. 2023. "Effect of Exposure Concentration and Growth Conditions on the Association of Cerium Oxide Nanoparticles with Green Algae" Nanomaterials 13, no. 17: 2468. https://doi.org/10.3390/nano13172468