Graphene-Based Temperature Sensors–Comparison of the Temperature and Humidity Dependences
Abstract
:1. Introduction
2. Materials and Methods
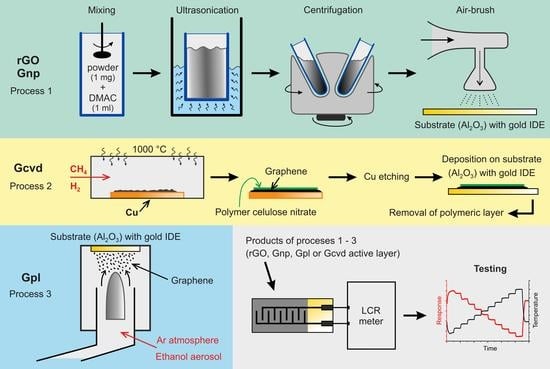
2.1. Sensor Fabrication
2.2. Characterization of Graphene Sensors
2.3. Temperature and Humidity Measurement
3. Results and Discussion
3.1. Material Properties
3.2. Temperature Dependences of Graphene Sensors
3.3. Humidity Dependences of Graphene Sensors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudrapati, R. Graphene: Fabrication Methods, Properties, and Applications in Modern Industries. In Graphene Production and Application; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, M.; Zhang, M.; Xu, G.; Yao, B.; Li, C.; Shi, G. Graphene-based electrochemical capacitors with integrated high-performance. Mater. Today Energy 2017, 6, 181–188. [Google Scholar] [CrossRef]
- Neella, N.; Gaddam, V.; Nayak, M.M.; Dinesh, N.S.; Rajanna, K. Scalable fabrication of highly sensitive flexible temperature sensors based on silver nanoparticles coated reduced graphene oxide nanocomposite thin films. Sens. Actuators A Phys. 2017, 268, 173–182. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, X.; He, Y.; Zhu, H. Graphene-Based Sensors; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128126516. [Google Scholar]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Coroş, M.; Pruneanu, S.; Stefan-van Staden, R.-I. Review—Recent Progress in the Graphene-Based Electrochemical Sensors and Biosensors. J. Electrochem. Soc. 2020, 167, 037528. [Google Scholar] [CrossRef] [Green Version]
- Shimoi, N.; Komatsu, M. Application of exfoliated graphene as conductive additive for lithium-ion secondary batteries. Powder Technol. 2021, 390, 268–272. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, Z.; Xiao, H.; Chen, S.; Fu, J. An eco-friendly imprinted polymer based on graphene quantum dots for fluorescent detection of: P -nitroaniline. RSC Adv. 2019, 9, 41383–41391. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Youji, L.; Feitai, C.; Peng, X.; Ming, L. Facile synthesis of mesoporous titanium dioxide doped by Ag-coated graphene with enhanced visible-light photocatalytic performance for methylene blue degradation. RSC Adv. 2017, 7, 25314–25324. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Zhang, X.; Wang, P.; Dai, J.; Guo, G.; Meng, Q.; Ma, J. Mechanically robust, highly sensitive and superior cycling performance nanocomposite strain sensors using 3-nm thick graphene platelets. Polym. Test. 2021, 98, 107178. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Romero, F.J.; Rivadeneyra, A.; Toral, V.; Castillo, E.; García-Ruiz, F.; Morales, D.P.; Rodriguez, N. Design guidelines of laser reduced graphene oxide conformal thermistor for IoT applications. Sens. Actuators A Phys. 2018, 274, 148–154. [Google Scholar] [CrossRef]
- Adetayo, A.; Runsewe, D. Synthesis and Fabrication of Graphene and Graphene Oxide: A Review. Open J. Compos. Mater. 2019, 9, 207–229. [Google Scholar] [CrossRef] [Green Version]
- Kairi, M.I.; Dayou, S.; Kairi, N.I.; Bakar, S.A.; Vigolo, B.; Mohamed, A.R. Toward high production of graphene flakes-a review on recent developments in their synthesis methods and scalability. J. Mater. Chem. A 2018, 6, 15010–15026. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Lee, J.; Shen, X.; Chen, X.; Kim, J.-K. Graphene-based wearable piezoresistive physical sensors. Mater. Today 2020, 36, 158–179. [Google Scholar] [CrossRef]
- Mahmoud, W.E.; Al-Bluwi, S.A. Development of highly sensitive temperature sensor made of graphene monolayers doped P(VDF-TrFE) nanocomposites. Sens. Actuators A Phys. 2020, 312, 112101. [Google Scholar] [CrossRef]
- Kim, Y.J.; Le, T.S.D.; Nam, H.K.; Yang, D.; Kim, B. Wood-based flexible graphene thermistor with an ultra-high sensitivity enabled by ultraviolet femtosecond laser pulses. CIRP Ann. 2021, 70, 443–446. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Hone, J.; Stormer, H.L.; Kim, P. Temperature-dependent transport in suspended graphene. Phys. Rev. Lett. 2008, 101, 096802. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Tao, L.Q.; Wang, Y.; Zheng, K.; Yu, J.; Li, X.; Chen, X.; Huang, Y. Graphene oxide humidity sensor with laser-induced graphene porous electrodes. Sens. Actuators B Chem. 2020, 325, 128790. [Google Scholar] [CrossRef]
- Salvo, P.; Calisi, N.; Melai, B.; Cortigiani, B.; Mannini, M.; Caneschi, A.; Lorenzetti, G.; Paoletti, C.; Lomonaco, T.; Paolicchi, A.; et al. Temperature and pH sensors based on graphenic materials. Biosens. Bioelectron. 2017, 91, 870–877. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent advances in graphene-based humidity sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Sagade, A.A.; Neumaier, D.; Schall, D.; Otto, M.; Pesquera, A.; Centeno, A.; Elorza, A.Z.; Kurz, H. Highly air stable passivation of graphene based field effect devices. Nanoscale 2015, 7, 3558–3564. [Google Scholar] [CrossRef]
- Toman, J.; Jasek, O.; Snirer, M.; Kudrle, V.; Jurmanova, J. On the interplay between plasma discharge instability and formation of free-standing graphene nanosheets in a dual-channel microwave plasma torch at atmospheric pressure. J. Phys. D Appl. Phys. 2019, 52, 265205. [Google Scholar] [CrossRef]
- Kovaříček, P.; Drogowska, K.; Melníková Komínková, Z.; Blechta, V.; Bastl, Z.; Gromadzki, D.; Fridrichová, M.; Kalbáč, M. EDOT polymerization at photolithographically patterned functionalized graphene. Carbon N. Y. 2017, 113, 33–39. [Google Scholar] [CrossRef]
- Hallam, T.; Berner, N.C.; Yim, C.; Duesberg, G.S. Strain, Bubbles, Dirt, and Folds: A Study of Graphene Polymer-Assisted Transfer. Adv. Mater. Interfaces 2014, 1, 1400115. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Sehrawat, P.; Abid; Islam, S.S.; Mishra, P. Reduced graphene oxide based temperature sensor: Extraordinary performance governed by lattice dynamics assisted carrier transport. Sens. Actuators B Chem. 2018, 258, 424–435. [Google Scholar] [CrossRef]
- Liu, G.; Tan, Q.; Kou, H.; Zhang, L.; Wang, J.; Lv, W.; Dong, H.; Xiong, J. A flexible temperature sensor based on reduced graphene oxide for robot skin used in internet of things. Sensors 2018, 18, 1400. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Wang, J.; Lee, P.S. Stretchable graphene thermistor with tunable thermal index. ACS Nano 2015, 9, 2130–2137. [Google Scholar] [CrossRef]
- Muchharla, B.; Narayanan, T.N.; Balakrishnan, K.; Ajayan, P.M.; Talapatra, S. Temperature dependent electrical transport of disordered reduced graphene oxide. 2D Mater. 2014, 1, 011008. [Google Scholar] [CrossRef]
- Liang, R.; Luo, A.; Zhang, Z.; Li, Z.; Han, C.; Wu, W. Research progress of graphene-based flexible humidity sensor. Sensors 2020, 20, 5601. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Ji, J.; Wan, S.; Sun, L.; Terrones, M.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3, 2714. [Google Scholar] [CrossRef] [Green Version]
- Popov, V.I.; Nikolaev, D.V.; Timofeev, V.B.; Smagulova, S.A.; Antonova, I.V. Graphene Based Humidity Sensors: The Origin of Resistance Change. Nanotechnology 2017, 28, 355501. [Google Scholar] [CrossRef] [PubMed]
- Kula, P.; Szymanski, W.; Kolodziejczyk, L.; Atraszkiewicz, R.; Dybowski, K.; Grabarczyk, J.; Pietrasik, R.; Niedzielski, P.; Kaczmarek, L.; Clapa, M. High strength metallurgical graphene—Mechanisms of growth and properties. Arch. Metall. Mater. 2015, 60, 2535–2541. [Google Scholar] [CrossRef] [Green Version]
- Vasu, K.S.; Chakraborty, B.; Sampath, S.; Sood, A.K. Probing top-gated field effect transistor of reduced graphene oxide monolayer made by dielectrophoresis. Solid State Commun. 2010, 150, 1295–1298. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Bhatt, K.; Kumar, P.; Sharma, S.; Kumar, A.; Tripathi, C.C. Laser patterned, high-power graphene paper resistor with dual temperature coefficient of resistance. RSC Adv. 2019, 9, 8262–8270. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Desai, J.A.; Biswas, C.; Vié, R.; Drahi, E.; Baudino, O.; Del, S.K.; Bornemann, R.; Bablich, A.; Michel, M.; et al. Graphene Resistor for Flexible Electronics. 2D Mater. 2017, 4, 025076. [Google Scholar] [CrossRef]
- Rajan, G.; Morgan, J.J.; Murphy, C.; Torres Alonso, E.; Wade, J.; Ott, A.K.; Russo, S.; Alves, H.; Craciun, M.F.; Neves, A.I.S. Low Operating Voltage Carbon-Graphene Hybrid E-textile for Temperature Sensing. ACS Appl. Mater. Interfaces 2020, 12, 29861–29867. [Google Scholar] [CrossRef]
- Sibilia, S.; Bertocchi, F.; Chiodini, S.; Cristiano, F.; Ferrigno, L.; Giovinco, G.; Maffucci, A. Temperature-dependent electrical resistivity of macroscopic graphene nanoplatelet strips. Nanotechnology 2021, 32, 275701. [Google Scholar] [CrossRef]
- Bae, J.J.; Yoon, J.H.; Jeong, S.; Moon, B.H.; Han, J.T.; Jeong, H.J.; Lee, G.W.; Hwang, H.R.; Lee, Y.H.; Jeong, S.Y.; et al. Sensitive photo-thermal response of graphene oxide for mid-infrared detection. Nanoscale 2015, 7, 15695–15700. [Google Scholar] [CrossRef]







| Sample | Method | Carbon | Oxygen | Manganese | Aluminum |
|---|---|---|---|---|---|
| At. Conc. (%) | At. Conc. (%) | At. Conc. (%) | At. Conc. (%) | ||
| rGO | EDS | 47.58 | 42.80 | 5.05 | 4.56 |
| Gnp | EDS | 61.64 | 38.36 | x | x |
| Gpl | EDS | 76.03 | 23.97 | x | x |
| Gcvd | XPS | 99.14 | 0.86 | x | x |
| Samples | T Response 0–100 °C (%) | TCR (K−1) | t90 (s) | Response to 20–90% RH at 20 °C (%) | Response to 20–90% RH at 50 °C (%) |
|---|---|---|---|---|---|
| rGO | −45.1 | −4.86 × 10−3 | 121 | 4.1 | 10.1 |
| Gnp | −52.0 | −5.16 × 10−3 | 89 | 6.9 | 9.2 |
| Gpl | −20.5 | −1.68 × 10−3 | 125 | −1.0 | 1.8 |
| Gcvd | 27.0 | 2.65 × 10−3 | 68 | −0.9 | |2.0| |
| Materials | Temperature Range (K) | TCR (K−1) | Reference |
|---|---|---|---|
| Graphene oxide | 303–373 | −5.74 × 10−3 | [27] |
| Conductive high strength metallurgical graphene | 253–333 | 3.5 × 10−4 to 4.1 × 10−4 | [31] |
| Semiconductive high strength metallurgical graphene | 253–333 | −1.7 × 10−3 to −4.0 × 10−4 | [31] |
| Chemical vapor deposition graphene | 253–333 | 1.5 × 10−4 to 4.0 × 10−4 | [31] |
| Reduced graphene oxide monolayer | 305–395 | –9.5 × 10−4 | [32] |
| Graphene ink | 300–374 | −1.77 × 10−3 ± 4.8 × 10−4 | [33] |
| 173–300 | −8 × 10−4 ± 5 × 10−5 | [33] | |
| Printed multilayer graphene film | 6–360 | −1.5 × 10−4 | [34] |
| Graphene | 303–373 | −0.0105 | [29] |
| TLG-coated fibers (trilayer graphene) | 303–343 | −0.0017 | [35] |
| graphene nanoplatelets | 213–333 | −1.55 × 10−3 to −1.02 × 10−3 | [36] |
| Graphene oxide | 200–350 | −0.024 to −0.04 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štulík, J.; Musil, O.; Josefík, F.; Kadlec, P. Graphene-Based Temperature Sensors–Comparison of the Temperature and Humidity Dependences. Nanomaterials 2022, 12, 1594. https://doi.org/10.3390/nano12091594
Štulík J, Musil O, Josefík F, Kadlec P. Graphene-Based Temperature Sensors–Comparison of the Temperature and Humidity Dependences. Nanomaterials. 2022; 12(9):1594. https://doi.org/10.3390/nano12091594
Chicago/Turabian StyleŠtulík, Jiří, Ondřej Musil, František Josefík, and Petr Kadlec. 2022. "Graphene-Based Temperature Sensors–Comparison of the Temperature and Humidity Dependences" Nanomaterials 12, no. 9: 1594. https://doi.org/10.3390/nano12091594