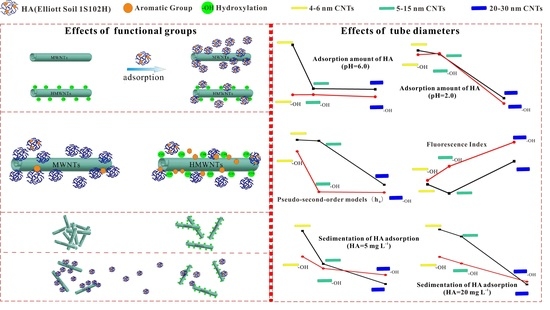
Effect of Tube Diameters and Functional Groups on Adsorption and Suspension Behaviors of Carbon Nanotubes in Presence of Humic Acid
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials and Reagents
2.2. Preparation of HA Stock Solution and Determination of Standard Curve for HA Concentration
2.3. Characterization of CNTs
2.4. Effect of Solution pH
2.5. Adsorption Isotherm
2.6. Adsorption Kinetics
2.7. Sedimentation Test of CNTs
2.8. Fluorescence Spectral Analysis
2.9. Raman and FTIR Spectroscopy Characterization of CNTs
2.10. Atomic Adsorption Theory Analysis Based on Density Functional Theory (DFT) Calculation
3. Results and Analysis
3.1. Characterization of CNTs
3.2. Effect of pH on the Adsorption of HA
3.3. Adsorption Isotherms
3.4. Adsorption Kinetics
3.5. Effect of HA on CNT Suspension/Sedimentation
3.6. Fluorescence Spectral Analysis
3.7. Raman Spectroscopy and FTIR Study
3.8. Atomic Adsorption Theory Analysis Based on DFT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNTs | carbon nanotubes |
| MWNTs | multiwall CNTs |
| HMWNTs | hydroxylated MWNTs |
| HA | humic acid |
| MWNT-1 | MWNTs with outer tube diameters of 4–6 nm |
| MWNT-2 | MWNTs with outer tube diameters of 5–15 nm |
| MWNT-3 | MWNTs with outer tube diameters of 20–30 nm |
| HMWNT-1 | HMWNTs with outer tube diameters of 4–6 nm |
| HMWNT-2 | HMWNTs with outer tube diameters of 5–15 nm |
| HMWNT-3 | HMWNTs with outer tube diameters of 20–30 nm |
| Q1 and Q2 in Table 1 | the maximum charge densities of the two classes of binding sites |
| LogK1 and LogK2 in Table 1 | logK values for proton binding by the two classes of sites |
| N in Table 1 | the number of fitted titration data points |
| TEM | transmission electron microscopy |
| Ce in sedimentation test | absorbance at different times in sedimentation test of CNTs |
| C0 in sedimentation test | initial absorbance in sedimentation test of CNTs |
| R2 | correlation coefficient |
| Em | scanning emission of fluorescence spectral analysis |
| Ex | scanning excitation of fluorescence spectral analysis |
| FI | fluorescence index of fluorescence spectral analysis |
| FTIR | Fourier-transform infrared spectroscopy |
| DFT | density functional theory |
| BET (m2 g−1) | specific surface area |
| OD (nm) | outer tube diameter |
| qe (mg g−1) | amount of HA adsorbed at equilibrium |
| Ce (mg L−1 | concentration of HA in solution at equilibrium |
| qm (mg g−1) | maximum adsorption capacity |
| kL (L g−1) | Langmuir equilibrium constant |
| kF (mg1−(1/n)L1/n g−1) | Freundlich parameters |
| n in Table 2 and Equation (2) | Freundlich parameters |
| k1 (min−1) | the first-order adsorption rate constant |
| k2 (g mg−1 min−1) the second-order adsorption rate constant; | |
| t (min) | adsorption time |
| qt (mg g−1) | the amount of HA adsorbed by CNTs at time t |
| ID | D-band intensity in Raman spectroscopy |
| IG | G-band intensity in Raman spectroscopy |
| k(mg2 mg−2) | a constant related to the adsorption amount |
| at | constant of Equation S(1) |
| bt | constant of Equation S(1) |
| R (8.314 J (mol K)−1) in Equation S(1) and Equation S(3) | the ideal gas constant |
| T (K) | the thermodynamic temperature |
| ε | the adsorption potential |
| C in Table 3 and Equation (6) | the intercept in the intraparticle diffusion rate |
| kint (mg g−1 min−1/2) | the intraparticle diffusion rate constant |
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.Z.; Chen, X.Y.; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestl, G.; Maksimova, N.I.; Keller, N.; Roddatis, V.; Schlogl, R. Carbon nanofilaments in heterogeneous catalysis: An industrial application for new carbon materials? Angew. Chem. Int. Ed. 2001, 40, 2066–2068. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Khang, D.Y.; Xiao, J.L.; Kocabas, C.; MacLaren, S.; Banks, T.; Jiang, H.Q.; Huang, Y.G.Y.; Rogers, J.A. Molecular scale buckling mechanics in individual aligned single-wall carbon nanotubes on elastomeric substrates. Nano Lett. 2008, 8, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvidsson, R.; Sanden, B.A. Carbon nanomaterials as potential substitutes for scarce metals. J. Clean. Prod. 2017, 156, 253–261. [Google Scholar] [CrossRef]
- Alloy, M.M.; Roberts, A.P. Effects of suspended multi-walled carbon nanotubes on daphnid growth and reproduction. Ecotoxicol. Environ. Saf. 2011, 74, 1839–1843. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Chang, D.W.; Dai, L.M.; Hong, Y.L. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007, 7, 3592–3597. [Google Scholar] [CrossRef]
- Lam, C.W. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2003, 77, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Qin, X.S.; Li, J.; Zeng, G.M. Probing molecular basis of single-walled carbon nanotube degradation and nondegradation by enzymes based on manganese peroxidase and lignin peroxidase. RSC Adv. 2016, 6, 3592–3599. [Google Scholar] [CrossRef]
- Lin, D.H.; Li, T.T.; Yang, K.; Wu, F.C. The relationship between humic acid (HA) adsorption on and stabilizing multiwalled carbon nanotubes (MWNTs) in water: Effects of HA, MWNT and solution properties. J. Hazard. Mater. 2012, 241, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Alpatova, A.L.; Shan, W.Q.; Babica, P.; Upham, B.L.; Rogensues, A.R.; Masten, S.J.; Drown, E.; Mohanty, A.K.; Alocilja, E.C.; Tarabara, V.V. Single-walled carbon nanotubes dispersed in aqueous media via non-covalent functionalization: Effect of dispersant on the stability, cytotoxicity, and epigenetic toxicity of nanotube suspensions. Water Res. 2010, 44, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Liu, N.; Yang, K.; Xing, B.S.; Wu, F.C. Different stabilities of multiwalled carbon nanotubes in fresh surface water samples. Environ. Pollut. 2010, 158, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Tian, X.L.; Li, T.T.; Zhang, Z.Y.; He, X.; Xing, B.S. Surface-bound humic acid increased Pb2+ sorption on carbon nanotubes. Environ. Pollut. 2012, 167, 138–147. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, B.; Cook, R.L.; Xing, B.S. Multi-walled carbon nanotube dispersion by the adsorbed humic acids with different chemical structures. Environ. Pollut. 2015, 196, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Hyung, H.; Kim, J.H. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: Effect of NOM characteristics and water quality parameters. Environ. Sci. Technol. 2008, 42, 4416–4421. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B.S. Adsorption of fulvic acid by carbon nanotubes from water. Environ. Pollut. 2009, 157, 1095–1100. [Google Scholar] [CrossRef]
- Summers, R.S.; Roberts, P.V. Activated carbon adsorption of humic substances: I. Heterodisperse mixtures and desorption. J. Colloid Interface Sci. 1988, 122, 367–381. [Google Scholar] [CrossRef]
- Wang, L.X.; Yang, X.Z.; Wang, Q.; Zeng, Y.X.; Jiang, W. Effects of ionic strength and temperature on the aggregation and deposition of multi-walled carbon nanotubes. J. Environ. Sci. 2016, 51, 248–255. [Google Scholar] [CrossRef]
- Melo, B.A.G.D.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Polak, J.; Bartoszek, M.; Sułkowski, W.W. Comparison of some spectroscopic and physico-chemical properties of humic acids extracted from sewage sludge and bottom sediments. J. Mol. Struct. 2009, 924, 309–312. [Google Scholar] [CrossRef]
- Chen, G.C.; Shan, X.Q.; Zhou, Y.Q.; Shen, X.E.; Huang, H.L.; Khan, S.U. Adsorption kinetics, isotherms and thermodynamics of atrazine onsurface oxidized multi-walled carbon nanotubes. J. Hazard. Mater. 2009, 169, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Xing, B.S. Adsorption of phenolic compounds by carbon nanotubes: Role of aromaticity and substitution of hydroxyl groups. Environ. Sci. Technol. 2008, 42, 7254–7259. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Wepasnick, K.; Schrote, K.E.; Cho, H.; Ball, W.P.; Fairbrother, D.H. Influence of surface oxides on the colloidal stability of multi-walled carbon nanotubes: A structureproperty relationship. Langmuir 2009, 25, 9767–9776. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Shu, L.; Wang, Y.Q.; Xu, B.B.; Bai, Y.C.; Tao, S.; Xing, B.S. Sorption of peat humic acids to multi-walled carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9276–9283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Z.; Shu, L.; Zhao, H.B.; Guo, X.Y.; Wang, X.L.; Tao, S.; Xing, B.S. Suspending multi-walled carbon nanotubes by humic acids from a peat soil. Environ. Sci. Technol. 2012, 46, 3891–3897. [Google Scholar] [CrossRef] [PubMed]
- International Humic Substances Society. Chemical Properties of IHSS Samples[EB/OL]. Available online: http://www.humicsubstances.org (accessed on 9 August 2019).
- Tang, Z.; Zhao, X.L.; Zhao, T.H.; Wang, H.; Wang, P.F.; Wu, F.C.; Giesy, J.P. Magnetic nanoparticles interaction with humic acid: In the presence of surfactants. Environ. Sci. Technol. 2016, 50, 8640–8648. [Google Scholar] [CrossRef]
- Ateia, M.; Apul, O.G.; Shimizu, Y.; Muflihah, A.; Yoshimura, C.; Karanfil, T. Elucidating adsorptive fractions of natural organic matter on carbon nanotubes. Environ. Sci. Technol. 2017, 51, 7101–7110. [Google Scholar] [CrossRef]
- Hutter, J.; Iannuzzi, M.; Schiffmann, F.; VandeVondele, J. Cp2k: Atomistic simulations of condensed matter systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 7, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- VandeVondele, J.; Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 2007, 127, 105–114. [Google Scholar] [CrossRef] [Green Version]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef] [Green Version]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef] [Green Version]
- Hartwigsen, C.; Gœdecker, S.; Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B 1998, 58, 3641–3662. [Google Scholar] [CrossRef] [Green Version]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, M.; Kranan, M. Adsorption of two cationic textile dyes from water with modified nanoclay: A comparative study by using central composite design. J. Environ. Chem. Eng. 2015, 3, 2738–2749. [Google Scholar] [CrossRef]
- Karaca, S.; Gürsesa, A.; Açışlıö, O.; Hassania, A.; Kiransan, M.; Yikilmaz, K. Modeling of adsorption isotherms and kinetics of Remazol Red RB adsorption from aqueous solution by modified clay. Desalin. Water Treat. 2013, 51, 2726–2739. [Google Scholar] [CrossRef]
- Chung, H.K.; Kim, W.H.; Park, J.; Cho, J.; Jeong, T.Y.; Park, P.K. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Kumar, B.G.P.; Shivakamy, K.; Miranda, L.R.; Velan, M. Preparation of steam activated carbon from rubberwood sawdust (Hevea brasiliensis) and its adsorption kinetics. J. Hazard. Mater. 2006, 136, 922–929. [Google Scholar]
- Ateia, M.; Ran, J.; Fujii, M.; Yoshimura, C. The relationship between molecular composition and fluorescence properties of humic substances. Int. J. Environ. Sci. Technol. 2017, 14, 867–880. [Google Scholar] [CrossRef]
- Shimabuk, K.K.; Kennedy, A.M.; Mulher, R.E.; Summers, R.S. Evaluating activated carbon adsorption of dissolved organic matter and micropollutants using fluorescence spectroscopy. Environ. Sci. Technol. 2017, 51, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.L.; Corrado, G.; Francioso, O.; Sanchez-Cortes, S. Interaction of soil humic acids with herbicide paraquat analyzed by surface-enhanced Raman scattering and fluorescence spectroscopy on silver plasmonic nanoparticles. Anal. Chim. Acta 2011, 699, 87–95. [Google Scholar] [CrossRef]
- Martínez, M.T.; Callejas, M.A.; Benito, A.M.; Cochet, M.; Maser, W.K. Sensitivity of single wall carbon nanotubes to oxidative processing: Structural modification, intercalation and functionalisation. Carbon 2003, 41, 2247–2256. [Google Scholar] [CrossRef]
- Qian, W.Z.; Wei, F.; Liu, T.; Wang, Z.W.; Li, Y.D. What causes the carbon nanotubes collapse in a chemical vapor deposition process. J. Chem. Phys. 2003, 118, 878–882. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Kovér, L.; Tóth, J.; Biniak, S.; Trykowski, G.; Judek, J. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. J. Alloys Compd. 2010, 501, 77–84. [Google Scholar] [CrossRef]
- Yang, K.; Lin, D.H.; Xing, B.S. Interactions of humic acid with nanosized inorganic oxides. Langmuir ACS J. Surf. Colloids 2009, 25, 3571–3576. [Google Scholar] [CrossRef]
- Wu, W.; Shan, G.Q.; Xiang, Q.; Zhang, Y.Q.; Yi, S.J.; Zhu, L.Y. Effects of humic acids with different polarities on the photocatalytic activity of nano-TiO2 at environment relevant concentration. Water Res. 2017, 122, 78–85. [Google Scholar] [CrossRef]
- Chen, H.F.; Li, Q.; Wang, M.X.; Ji, D.B.; Tan, W.F. XPS and two-dimensional FTIR correlation analysis on the binding characteristics of humic acid onto kaolinite surface. Sci. Total Environ. 2020, 724, 138–154. [Google Scholar] [CrossRef]












| Carbon Distribution (mg L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Carbonyl 220–190 | Carboxyl 190–165 | Aromatic 165–110 | Acetal 110–90 | Hetero Aliphatic 90–60 | Aliphatic 60–0 | Aromatic/Aliphatic | |
| HA | 6 | 18 | 50 | 4 | 6 | 16 | 3.125 | |
| Element Constitution %(w·w−1) | ||||||||
| H2O | Ash | C | H | O | N | S | P | |
| HA | 8.2 | 0.88 | 58.13 | 3.68 | 34.08 | 4.14 | 0.44 | 0.24 |
| Acid Functional Groups(m mol·g−1) | ||||||||
| Carboxyl | Phenolic | Q1 | LogK1 | N1 | Q2 | LogK2 | N2 | |
| HA | 8.28 | 1.87 | 8.90 | 4.36 | 3.16 | 0.85 | 9.80 | 1.00 |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | KF (mg1−(1/n)L1/ng−1) | n | R2 | |
| MWNT-1 | 195.95 | 0.0142 | 0.975 | 4.50 | 1.341 | 0.974 |
| MWNT-2 | 89.73 | 0.0264 | 0.977 | 4.48 | 1.578 | 0.995 |
| MWNT-3 | 87.99 | 0.0137 | 0.934 | 2.08 | 1.362 | 0.998 |
| HMWNT-1 | 74.74 | 0.0161 | 0.991 | 2.04 | 1.383 | 0.996 |
| HMWNT-2 | 75.16 | 0.0136 | 0.985 | 1.74 | 1.362 | 0.977 |
| HMWNT-3 | 69.81 | 0.0172 | 0.985 | 1.92 | 1.368 | 0.971 |
| Samples | Pseudo-First-Order Models | Pseudo-Second-Order Models | Intraparticle Diffusion Equation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | h0 (mg g−1 min−1) | R2 | kint (mg g−1 min−1/2) | C (mg g−1) | R2 | |
| MWNT-1 | 0.0111 | 28.28 | 0.364 | 6.54 × 10−4 | 30.30 | 0.612 | 0.999 | 0.5329 | 11.907 | 0.945 |
| MWNT-2 | 0.0076 | 22.65 | 0.687 | 6.54 × 10−4 | 30.29 | 0.603 | 0.999 | 0.6508 | 8.476 | 0.881 |
| MWNT-3 | 0.0092 | 27.60 | 0.831 | 3.19 × 10−4 | 25.64 | 0.214 | 0.999 | 0.4424 | 7.095 | 0.944 |
| HMWNT-1 | 0.1258 | 18.11 | 0.210 | 10.65 × 10−4 | 21.88 | 0.510 | 0.999 | 0.2479 | 12.478 | 0.929 |
| HMWNT-2 | 0.0124 | 16.50 | 0.199 | 3.61 × 10−4 | 20.45 | 0.151 | 0.997 | 0.2256 | 8.289 | 0.973 |
| HMWNTs-3 | 0.0045 | 17.60 | 0.886 | 4.35 × 10−4 | 18.45 | 0.148 | 0.996 | 0.4662 | 2.203 | 0.905 |
| Samples | WG (cm−1) | WD (cm−1) | W2D (cm−1) | WD+G (cm−1) | ID/IG | I2D/ID+G |
|---|---|---|---|---|---|---|
| MWNT-1 | 1575 | 1340 | 2672 | 2895 | 1.17 | 2.24 |
| MWNT-1 + HA | 1573 | 1337 | 2675 | 2910 | 1.20 | 1.94 |
| MWNT-2 | 1573 | 1339 | 2672 | 2893 | 1.01 | 2.55 |
| MWNT-2 + HA | 1576 | 1341 | 2675 | 2895 | 0.96 | 2.25 |
| MWNT-3 | 1568 | 1340 | 2673 | 2922 | 1.08 | 2.03 |
| MWNT-3 + HA | 1577 | 1344 | 2683 | 2928 | 1.03 | 1.72 |
| HMWNT-1 | 1571 | 1341 | 2673 | 2929 | 1.10 | 2.54 |
| HMWNT-1 + HA | 1570 | 1335 | 2667 | 2895 | 1.24 | 1.72 |
| HMWNT-2 | 1571 | 1336 | 2671 | 2895 | 1.07 | 2.18 |
| HMWNT-2 + HA | 1575 | 1341 | 2673 | 2916 | 0.99 | 1.97 |
| HMWNT-3 | 1570 | 1340 | 2674 | 2910 | 1.12 | 2.39 |
| HMWNT-3 + HA | 1574 | 1342 | 2677 | 2922 | 1.06 | 2.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, M.; Zhao, T.; Zhao, X.; Tang, Z.; Liu, S.; Wang, J.; Niu, L.; Wu, F. Effect of Tube Diameters and Functional Groups on Adsorption and Suspension Behaviors of Carbon Nanotubes in Presence of Humic Acid. Nanomaterials 2022, 12, 1592. https://doi.org/10.3390/nano12091592
Fang M, Zhao T, Zhao X, Tang Z, Liu S, Wang J, Niu L, Wu F. Effect of Tube Diameters and Functional Groups on Adsorption and Suspension Behaviors of Carbon Nanotubes in Presence of Humic Acid. Nanomaterials. 2022; 12(9):1592. https://doi.org/10.3390/nano12091592
Chicago/Turabian StyleFang, Mengyuan, Tianhui Zhao, Xiaoli Zhao, Zhi Tang, Shasha Liu, Junyu Wang, Lin Niu, and Fengchang Wu. 2022. "Effect of Tube Diameters and Functional Groups on Adsorption and Suspension Behaviors of Carbon Nanotubes in Presence of Humic Acid" Nanomaterials 12, no. 9: 1592. https://doi.org/10.3390/nano12091592