Engineering Antioxidant Surfaces for Titanium-Based Metallic Biomaterials
Abstract
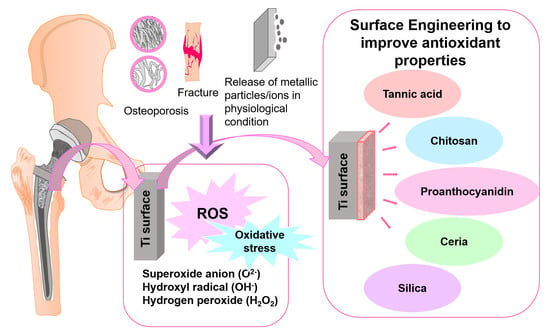
:1. Introduction
2. Titanium Alloys and Reactive Oxygen Species Metabolism
3. Surface Modification for Antioxidant Ti Surfaces
3.1. Organic Materials for Surface Modification
3.1.1. Tannic Acid
3.1.2. Chitosan
3.1.3. Proanthocyanidin
4. Inorganic Materials for Surface Engineering Antioxidative Properties
4.1. Ceria Based Coatings
4.2. Silica
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef] [PubMed]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Al Anouti, F.; Taha, Z.; Shamim, S.; Khalaf, K.; Al Kaabi, L.; Alsafar, H. An insight into the paradigms of osteoporosis: From genetics to biomechanics. Bone Rep. 2019, 11, 100216. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.-H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Schell, H.; Duda, G.N.; Peters, A.; Tsitsilonis, S.; Johnson, K.A.; Schmidt-Bleek, K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Walters, G.; Pountos, I.; Giannoudis, P.V. The cytokines and micro-environment of fracture haematoma: Current evidence. J. Tissue Eng. Regen. Med. 2018, 12, e1662–e1677. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [Green Version]
- Bellido, T. Osteocyte-Driven Bone Remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.; O’Brien, C.A. Osteocyte RANKL: New insights into the control of bone remodeling. J. Bone Miner. Res. 2012, 27, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Bădilă, A.E.; Rădulescu, D.M.; Ilie, A.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants 2022, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- León-Reyes, G.; Argoty-Pantoja, A.D.; Becerra-Cervera, A.; López-Montoya, P.; Rivera-Paredez, B.; Velázquez-Cruz, R. Oxidative-Stress-Related Genes in Osteoporosis: A Systematic Review. Antioxidants 2023, 12, 915. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. 2.5.4—Orthopedic Applications. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1079–1118. [Google Scholar] [CrossRef]
- Ratner, B.D.; Zhang, G. 1.1.2—A History of Biomaterials. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 21–34. [Google Scholar] [CrossRef]
- Wagner, W.R. 1.3.1—The Materials Side of the Biomaterials Relationship. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 83–84. [Google Scholar] [CrossRef]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef] [Green Version]
- Pilliar, R.M. Metallic Biomaterials. In Biomedical Materials; Narayan, R., Ed.; Springer International Publishing: Cham, Germany, 2021; pp. 1–47. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Villanueva, J.; Trino, L.; Thomas, J.; Bijukumar, D.; Royhman, D.; Stack, M.M.; Mathew, M.T. Corrosion, Tribology, and Tribocorrosion Research in Biomedical Implants: Progressive Trend in the Published Literature. J. Bio-Tribo-Corros. 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Steinbeck, M.J.; Jablonowski, L.J.; Parvizi, J.; Freeman, T.A. The Role of Oxidative Stress in Aseptic Loosening of Total Hip Arthroplasties. J. Arthroplast. 2014, 29, 843–849. [Google Scholar] [CrossRef] [Green Version]
- Primožič, J.; Poljšak, B.; Jamnik, P.; Kovač, V.; Čanadi Jurešić, G.; Spalj, S. Risk Assessment of Oxidative Stress Induced by Metal Ions Released from Fixed Orthodontic Appliances during Treatment and Indications for Supportive Antioxidant Therapy: A Narrative Review. Antioxidants 2021, 10, 1359. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin.Med. 2019, 8, 2091. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [Green Version]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [Green Version]
- Cerqueni, G.; Scalzone, A.; Licini, C.; Gentile, P.; Mattioli-Belmonte, M. Insights into oxidative stress in bone tissue and novel challenges for biomaterials. Mater. Sci. Eng. C 2021, 130, 112433. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol.Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Andrés, C.M.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol.Sci. 2023, 24, 1841. [Google Scholar]
- Pervaiz, S.; Clement, M.-V. Superoxide anion: Oncogenic reactive oxygen species? Int. J. Biochem. Cell Biol. 2007, 39, 1297–1304. [Google Scholar] [CrossRef]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media&Mdash;A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants&Mdash; A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Wang, G.; Li, J.J. Advances in implant surface modifications to improve osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Ghosh, S.; Abanteriba, S. Status of surface modification techniques for artificial hip implants. Sci. Technol. Adv. Mater. 2016, 17, 715–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef]
- Dumitriu, C.; Ungureanu, C.; Popescu, S.; Tofan, V.; Popescu, M.; Pirvu, C. Ti surface modification with a natural antioxidant and antimicrobial agent. Surf. Coat. Technol. 2015, 276, 175–185. [Google Scholar] [CrossRef]
- Di, H.; Qiaoxia, L.; Yujie, Z.; Jingxuan, L.; Yan, W.; Yinchun, H.; Xiaojie, L.; Song, C.; Weiyi, C. Ag nanoparticles incorporated tannic acid/nanoapatite composite coating on Ti implant surfaces for enhancement of antibacterial and antioxidant properties. Surf. Coat. Technol. 2020, 399, 126169. [Google Scholar] [CrossRef]
- Clanton, T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. 2007, 102, 2379–2388. [Google Scholar] [CrossRef] [Green Version]
- Chapple, I.L.C. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef]
- Han, X.; Ma, J.; Tian, A.; Wang, Y.; Li, Y.; Dong, B.; Tong, X.; Ma, X. Surface modification techniques of titanium and titanium alloys for biomedical orthopaedics applications: A review. Colloids Surf. B Biointerfaces 2023, 227, 113339. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; McFadden, R.; Chan, C.-W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Kolli, R.P.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Abdel-Hady Gepreel, M.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Vishnu, J.; Manivasagam, G. Surface Modification and Biological Approaches for Tackling Titanium Wear-Induced Aseptic Loosening. J. Bio-Tribo-Corros. 2021, 7, 32. [Google Scholar] [CrossRef]
- Peng, K.T.; Hsu, W.H.; Shih, H.N.; Hsieh, C.W.; Huang, T.W.; Hsu, R.W.W.; Chang, P.J. The role of reactive oxygen species scavenging enzymes in the development of septic loosening after total hip replacement. J. Bone Jt. Surg. Br. Vol. 2011, 93-B, 1201–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prestat, M.; Thierry, D. Corrosion of titanium under simulated inflammation conditions: Clinical context and in vitro investigations. Acta Biomater. 2021, 136, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groemping, Y.; Rittinger, K. Activation and assembly of the NADPH oxidase: A structural perspective. Biochem. J. 2005, 386, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Getzoff, E.D.; Tainer, J.A.; Weiner, P.K.; Kollman, P.A.; Richardson, J.S.; Richardson, D.C. Electrostatic recognition between superoxide and copper, zinc superoxide dismutase. Nature 1983, 306, 287–290. [Google Scholar] [CrossRef]
- Paumann-Page, M.; Furtmüller, P.G.; Hofbauer, S.; Paton, L.N.; Obinger, C.; Kettle, A.J. Inactivation of human myeloperoxidase by hydrogen peroxide. Arch. Biochem. Biophys. 2013, 539, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Prousek, J. Fenton chemistry in biology and medicine. Pure Appl. Chem. 2007, 79, 2325–2338. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber—Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Peñarrieta-Juanito, G.; Sordi, M.B.; Henriques, B.; Dotto, M.E.R.; Teughels, W.; Silva, F.S.; Magini, R.S.; Souza, J.C.M. Surface damage of dental implant systems and ions release after exposure to fluoride and hydrogen peroxide. J. Periodontal Res. 2019, 54, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalbacova, M.; Roessler, S.; Hempel, U.; Tsaryk, R.; Peters, K.; Scharnweber, D.; Kirkpatrick, J.C.; Dieter, P. The effect of electrochemically simulated titanium cathodic corrosion products on ROS production and metabolic activity of osteoblasts and monocytes/macrophages. Biomaterials 2007, 28, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.S.; Bhushan, B. Mechanically durable, superomniphobic coatings prepared by layer-by-layer technique for self-cleaning and anti-smudge. J. Colloid Interface Sci. 2015, 456, 210–218. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Ji, Q. Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 2007, 9, 2319–2340. [Google Scholar] [CrossRef]
- Neacşu, I.A.; Nicoară, A.I.; Vasile, O.R.; Vasile, B.Ş. Chapter 9—Inorganic micro- and nanostructured implants for tissue engineering. In Nanobiomaterials in Hard Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 271–295. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Riccucci, G.; Ferraris, S.; Cochis, A.; Scalia, A.C.; Rimondini, L.; Panzella, L.; Spriano, S.; Napolitano, A. Deposition of Antioxidant and Cytocompatible Caffeic Acid-Based Thin Films onto Ti6Al4V Alloys through Hexamethylenediamine-Mediated Crosslinking. ACS Appl. Mater. Interfaces 2023, 15, 29618–29635. [Google Scholar] [CrossRef]
- Rased, N.H.; Vengadaesvaran, B.; Raihan, S.R.S.; Rahim, N.A. Chapter 6—Introduction to solar energy and its conversion into electrical energy by using dye-sensitized solar cells. In Energy Materials; Dhoble, S.J., Kalyani, N.T., Vengadaesvaran, B., Kariem Arof, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 139–178. [Google Scholar] [CrossRef]
- Sahu, N.; Parija, B.; Panigrahi, S. Fundamental understanding and modeling of spin coating process: A review. Indian J. Phys. 2009, 83, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, M.; Kunitake, T. Preparation of Self-Supporting Ultrathin Films of Titania by Spin Coating. Langmuir 2003, 19, 10172–10178. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Wang, C.-H.; Bilek, M.M.M.; Cheng, X. An effective method to optimise plasma immersion ion implantation: Sensitivity analysis and design based on low-density polyethylene. Plasma Process. Polym. 2022, 19, 2100199. [Google Scholar] [CrossRef]
- Sotoudeh Bagha, P.; Paternoster, C.; Khakbiz, M.; Sheibani, S.; Gholami, N.; Mantovani, D. Surface Modification of an Absorbable Bimodal Fe-Mn-Ag Alloy by Nitrogen Plasma Immersion Ion Implantation. Materials 2023, 16, 1048. [Google Scholar] [CrossRef]
- Walschus, U.; Hoene, A.; Patrzyk, M.; Lucke, S.; Finke, B.; Polak, M.; Lukowski, G.; Bader, R.; Zietz, C.; Podbielski, A.; et al. A Cell-Adhesive Plasma Polymerized Allylamine Coating Reduces the In Vivo Inflammatory Response Induced by Ti6Al4V Modified with Plasma Immersion Ion Implantation of Copper. J. Funct. Biomater. 2017, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Terasawa, T.-O.; Saiki, K. Growth of graphene on Cu by plasma enhanced chemical vapor deposition. Carbon 2012, 50, 869–874. [Google Scholar] [CrossRef]
- Gan, Z.; Wang, C.; Chen, Z. Material Structure and Mechanical Properties of Silicon Nitride and Silicon Oxynitride Thin Films Deposited by Plasma Enhanced Chemical Vapor Deposition. Surfaces 2018, 1, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of Plasma-Enhanced Chemical Vapor Deposition as a Method for Thin-Film Fabrication with Biological Applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Lopes, G.K.B.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions1This study is dedicated to the memory of Botany Professor Luiz F.G. Labouriau (1921–1996).1. Biochim. Et Biophys. Acta BBA-Gen. Subj. 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gopinath, K.; Zhang, K.; Kang, E.-T.; Xu, L.; Yu, Y. Recent progress in tannic acid-driven antibacterial/antifouling surface coating strategies. J. Mater. Chem. B 2022, 10, 2296–2315. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Zhu, L.; Meng, J.; He, F. Tannic acid-based functional coating: Surface engineering of membranes for oil-in-water emulsion separation. Chem. Commun. 2022, 58, 12629–12641. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, D.; Zan, X.; Ye, Q.; Sheng, S. Engineering the surfaces of orthopedic implants with osteogenesis and antioxidants to enhance bone formation in vitro and in vivo. Colloids Surf. B Biointerfaces 2022, 212, 112319. [Google Scholar] [CrossRef]
- Iqbal, M.H.; Schroder, A.; Kerdjoudj, H.; Njel, C.; Senger, B.; Ball, V.; Meyer, F.; Boulmedais, F. Effect of the Buffer on the Buildup and Stability of Tannic Acid/Collagen Multilayer Films Applied as Antibacterial Coatings. ACS Appl. Mater. Interfaces 2020, 12, 22601–22612. [Google Scholar] [CrossRef]
- He, X.; Gopinath, K.; Sathishkumar, G.; Guo, L.; Zhang, K.; Lu, Z.; Li, C.; Kang, E.-T.; Xu, L. UV-Assisted Deposition of Antibacterial Ag–Tannic Acid Nanocomposite Coating. ACS Appl. Mater. Interfaces 2021, 13, 20708–20717. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Geißler, S.; Barrantes, A.; Tengvall, P.; Messersmith, P.B.; Tiainen, H. Deposition Kinetics of Bioinspired Phenolic Coatings on Titanium Surfaces. Langmuir 2016, 32, 8050–8060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiaoxia, L.; Yujie, Z.; Meng, Y.; Yizhu, C.; Yan, W.; Yinchun, H.; Xiaojie, L.; Weiyi, C.; Di, H. Hydroxyapatite/tannic acid composite coating formation based on Ti modified by TiO2 nanotubes. Colloids Surf. B Biointerfaces 2020, 196, 111304. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, P.; Wang, H.; Cai, S.; Liao, Y.; Mo, Z.; Xu, X.; Ding, C.; Zhao, C.; Li, J. Antibacterial and anti-biofouling coating on hydroxyapatite surface based on peptide-modified tannic acid. Colloids Surf. B Biointerfaces 2017, 160, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Camós Noguer, A.; Olsen, S.M.; Hvilsted, S.; Kiil, S. Long-term stability of PEG-based antifouling surfaces in seawater. J. Coat. Technol. Res. 2016, 13, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.L.; Cheng, Y.F.; Ren, X.; Gopinath, K.; Lu, Z.S.; Li, C.M.; Xu, L.Q. Simultaneous deposition of tannic acid and poly(ethylene glycol) to construct the antifouling polymeric coating on Titanium surface. Colloids Surf. B Biointerfaces 2021, 200, 111592. [Google Scholar] [CrossRef]
- Zhuk, I.; Jariwala, F.; Attygalle, A.B.; Wu, Y.; Libera, M.R.; Sukhishvili, S.A. Self-Defensive Layer-by-Layer Films with Bacteria-Triggered Antibiotic Release. ACS Nano 2014, 8, 7733–7745. [Google Scholar] [CrossRef]
- Hizal, F.; Zhuk, I.; Sukhishvili, S.; Busscher, H.J.; van der Mei, H.C.; Choi, C.-H. Impact of 3D Hierarchical Nanostructures on the Antibacterial Efficacy of a Bacteria-Triggered Self-Defensive Antibiotic Coating. ACS Appl. Mater. Interfaces 2015, 7, 20304–20313. [Google Scholar] [CrossRef] [PubMed]
- Steffi, C.; Shi, Z.; Kong, C.H.; Chong, S.W.; Wang, D.; Wang, W. Use of Polyphenol Tannic Acid to Functionalize Titanium with Strontium for Enhancement of Osteoblast Differentiation and Reduction of Osteoclast Activity. Polymers 2019, 11, 1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Qi, X.; Guo, W.; Liang, D.; Chen, H.; Lin, B.; Deng, X. A barrier against reactive oxygen species: Chitosan/acellular dermal matrix scaffold enhances stem cell retention and improves cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 383. [Google Scholar] [CrossRef]
- Khokon, M.A.R.; Uraji, M.; Munemasa, S.; Okuma, E.; Nakamura, Y.; Mori, I.C.; Murata, Y. Chitosan-Induced Stomatal Closure Accompanied by Peroxidase-Mediated Reactive Oxygen Species Production in Arabidopsis. Biosci. Biotechnol. Biochem. 2010, 74, 2313–2315. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, M.; Mallick, S.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Heightened Reactive Oxygen Species Generation in the Antimicrobial Activity of a Three Component Iodinated Chitosan−Silver Nanoparticle Composite. Langmuir 2010, 26, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Kharaziha, M.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; RamaKrishna, S.; Berto, F. Antimicrobial Synthetic and Natural Polymeric Nanofibers as Wound Dressing: A Review. Adv. Eng. Mater. 2022, 24, 2101460. [Google Scholar] [CrossRef]
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.L.; Skirtach, A.G.; Mohan, M.K. Surface functionalization of chitosan as a coating material for orthopaedic applications: A comprehensive review. Carbohydr. Polym. 2021, 255, 117487. [Google Scholar] [CrossRef]
- Abinaya, B.; Prasith, T.P.; Ashwin, B.; Viji Chandran, S.; Selvamurugan, N. Chitosan in Surface Modification for Bone Tissue Engineering Applications. Biotechnol. J. 2019, 14, 1900171. [Google Scholar] [CrossRef]
- Lieder, R.; Darai, M.; Thor, M.B.; Ng, C.H.; Einarsson, J.M.; Gudmundsson, S.; Helgason, B.; Gaware, V.S.; Másson, M.; Gíslason, J.; et al. In vitro bioactivity of different degree of deacetylation chitosan, a potential coating material for titanium implants. J. Biomed. Mater. Res. Part A 2012, 100A, 3392–3399. [Google Scholar] [CrossRef]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef]
- Xue, C.; Yu, G.; Hirata, T.; Terao, J.; Lin, H. Antioxidative Activities of Several Marine Polysaccharides Evaluated in a Phosphatidylcholine-liposomal Suspension and Organic Solvents. Biosci. Biotechnol. Biochem. 1998, 62, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorganic Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X.-Y.; Feng, Y.-F.; Ma, Z.-S.; Wang, J.; Ma, T.-C.; Qi, W.; Lei, W.; Wang, L. Osseointegration of chitosan coated porous titanium alloy implant by reactive oxygen species-mediated activation of the PI3K/AKT pathway under diabetic conditions. Biomaterials 2015, 36, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, P.; Kyzioł, A.; Pawcenis, D.; Pucelik, B.; Hebda, M.; Migdalska, M.; Krawiec, H.; Arruebo, M.; Kyzioł, K. Electrostatic self-assembly approach in the deposition of bio-functional chitosan-based layers enriched with caffeic acid on Ti-6Al-7Nb alloys by alternate immersion. Biomater. Adv. 2022, 136, 212791. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, M.; Djošić, M.; Janković, A.; Kojić, V.; Stojanović, J.; Grujić, S.; Bujagić, I.M.; Rhee, K.Y.; Mišković-Stanković, V. The chitosan-based bioactive composite coating on titanium. J. Mater. Res. Technol. 2021, 15, 4461–4474. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. BioFactors 2010, 36, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Jeon, M.H.; Hwang, H.J.; Park, M.R.; Lee, S.H.; Kim, S.G.; Kim, M. Antioxidant activity and analysis of proanthocyanidins from pine (Pinus densiflora) needles. Nutr. Res. Pract. 2011, 5, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef] [Green Version]
- Andersen-Civil, A.I.S.; Leppä, M.M.; Thamsborg, S.M.; Salminen, J.-P.; Williams, A.R. Structure-function analysis of purified proanthocyanidins reveals a role for polymer size in suppressing inflammatory responses. Commun. Biol. 2021, 4, 896. [Google Scholar] [CrossRef]
- Tenkumo, T.; Aobulikasimu, A.; Asou, Y.; Shirato, M.; Shishido, S.; Kanno, T.; Niwano, Y.; Sasaki, K.; Nakamura, K. Proanthocyanidin-rich grape seed extract improves bone loss, bone healing, and implant osseointegration in ovariectomized animals. Sci. Rep. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.; Yan, D.; Shen, Z.; Wang, B.; Weng, S.; Wu, Z.; Xie, Z.; Shao, J.; Yang, L.; et al. Surface Functionalization with Proanthocyanidins Provides an Anti-Oxidant Defense Mechanism That Improves the Long-Term Stability and Osteogenesis of Titanium Implants. Int. J. Nanomed. 2020, 15, 1643–1659. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Hu, K.; Shou, Z.; Yu, J.; Meng, H.; Zhou, H.; Chen, L.; Yu, T.; Lu, R.; Li, N.; et al. Layer-by-layer assembly of procyanidin and collagen promotes mesenchymal stem cell proliferation and osteogenic differentiation in vitro and in vivo. Regen. Biomater. 2023, 10, rbac107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wu, T.; Bai, Z.; Hong, G.; Bian, J.; Xie, H.; Chen, C. A silane-based coupling strategy for enhancing the mechanical properties of proanthocyanidin nanocoatings on Ti dental implants. Appl. Surf. Sci. 2022, 602, 154400. [Google Scholar] [CrossRef]
- Maccarone, R.; Tisi, A.; Passacantando, M.; Ciancaglini, M. Ophthalmic Applications of Cerium Oxide Nanoparticles. J. Ocul. Pharmacol. Ther. 2019, 36, 376–383. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.; Jiang, X.; Yeung, K.W.K.; Chu, P.K. Valence State Manipulation of Cerium Oxide Nanoparticles on a Titanium Surface for Modulating Cell Fate and Bone Formation. Adv. Sci. 2018, 5, 1700678. [Google Scholar] [CrossRef] [PubMed]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron Localization Determines Defect Formation on Ceria Substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [Green Version]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 1056–1058. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Plasma sprayed cerium oxide coating inhibits H2O2-induced oxidative stress and supports cell viability. J. Mater. Sci. Mater. Med. 2016, 27, 100. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, C.; Singh, R.K.; Lee, J.-H.; Jung, J.; Hyun, J.K.; Kim, H.-W. Nano-shape varied cerium oxide nanomaterials rescue human dental stem cells from oxidative insult through intracellular or extracellular actions. Acta Biomater. 2017, 50, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, M.; Sun, X.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qiu, J.; Liu, X. Enhanced antioxidant capability and osteogenic property of medical titanium by cerium plasma immersion ion implantation. Surf. Interfaces 2021, 26, 101402. [Google Scholar] [CrossRef]
- Qi, S.; Wu, J.; Xu, Y.; Zhang, Y.; Wang, R.; Li, K.; Xu, Y. Chemical Stability and Antimicrobial Activity of Plasma-Sprayed Cerium Oxide–Incorporated Calcium Silicate Coating in Dental Implants. Implant Dent. 2019, 28, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, D.; Li, M.; He, Y.; He, T.; Chen, M.; Hu, Y.; Luo, Z.; Cai, K. Nanocatalytic Biofunctional MOF Coating on Titanium Implants Promotes Osteoporotic Bone Regeneration through Cooperative Pro-osteoblastogenesis MSC Reprogramming. ACS Nano 2022, 16, 15397–15412. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Ceruti, P.; Pirri, C.F.; Carossa, S. Reduction of bacterial adhesion on dental composite resins by silicon–oxygen thin film coatings. Biomed. Mater. 2015, 10, 015017. [Google Scholar] [CrossRef]
- Mandracci, P.; Ceruti, P.; Ricciardi, C.; Mussano, F.; Carossa, S. a-SiOx Coatings Grown on Dental Materials by PECVD: Compositional Analysis and Preliminary Investigation of Biocompatibility Improvements. Chem. Vap. Depos. 2010, 16, 29–34. [Google Scholar] [CrossRef]
- Alves Silva, E.C.; Tanomaru-Filho, M.; da Silva, G.F.; Delfino, M.M.; Cerri, P.S.; Guerreiro-Tanomaru, J.M. Biocompatibility and Bioactive Potential of New Calcium Silicate–based Endodontic Sealers: Bio-C Sealer and Sealer Plus BC. J. Endod. 2020, 46, 1470–1477. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Sol–gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Heimann, R.B. Silicon Nitride, a Close to Ideal Ceramic Material for Medical Application. Ceramics 2021, 4, 208–223. [Google Scholar] [CrossRef]
- Ilyas, A.; Odatsu, T.; Shah, A.; Monte, F.; Kim, H.K.W.; Kramer, P.; Aswath, P.B.; Varanasi, V.G. Amorphous Silica: A New Antioxidant Role for Rapid Critical-Sized Bone Defect Healing. Adv. Healthc. Mater. 2016, 5, 2199–2213. [Google Scholar] [CrossRef]
- Monte, F.A.D.; Awad, K.R.; Ahuja, N.; Kim, H.K.W.; Aswath, P.; Brotto, M.; Varanasi, V.G. Amorphous Silicon Oxynitrophosphide-Coated Implants Boost Angiogenic Activity of Endothelial Cells. Tissue Eng. Part A 2019, 26, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Laurenti, M.; Munaron, L.; Pirri, C.F.; Rivolo, P.; Carossa, S.; Mandracci, P. Hydrogenated amorphous silicon coatings may modulate gingival cell response. Appl. Surf. Sci. 2018, 436, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Bigham, A.; Rahimkhoei, V.; Abasian, P.; Delfi, M.; Naderi, J.; Ghomi, M.; Dabbagh Moghaddam, F.; Waqar, T.; Nuri Ertas, Y.; Sharifi, S.; et al. Advances in tannic acid-incorporated biomaterials: Infection treatment, regenerative medicine, cancer therapy, and biosensing. Chem. Eng. J. 2022, 432, 134146. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Polkowska, I.; Paździor-Czapula, K.; Nowicka, B.; Gierszewska, M.; Michalska-Sionkowska, M.; Otrocka-Domagała, I. Chitosan/Phenolic Compounds Scaffolds for Connective Tissue Regeneration. J. Funct. Biomater. 2023, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Widsten, P.; Salo, S.; Niemelä, K.; Helin, H.; Salonen, M.; Alakomi, H.-L. Tannin-Based Microbicidal Coatings for Hospital Privacy Curtains. J. Funct. Biomater. 2023, 14, 187. [Google Scholar] [CrossRef]
- Amarowicz, R. Tannins: The new natural antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 549–551. [Google Scholar] [CrossRef]
- Sanguedolce, M.; Saffioti, M.R.; Rotella, G.; Curcio, F.; Cassano, R.; Umbrello, D.; Filice, L. The Effects of Substrate Material on Chitosan Coating Performance for Biomedical Application. Procedia CIRP 2022, 108, 817–820. [Google Scholar] [CrossRef]
- Ganesh, S.S.; Anushikaa, R.; Swetha Victoria, V.S.; Lavanya, K.; Shanmugavadivu, A.; Selvamurugan, N. Recent Advancements in Electrospun Chitin and Chitosan Nanofibers for Bone Tissue Engineering Applications. J. Funct. Biomater. 2023, 14, 288. [Google Scholar] [CrossRef]
- Oe, T.; Dechojarassri, D.; Kakinoki, S.; Kawasaki, H.; Furuike, T.; Tamura, H. Microwave-Assisted Incorporation of AgNP into Chitosan–Alginate Hydrogels for Antimicrobial Applications. J. Funct. Biomater. 2023, 14, 199. [Google Scholar] [PubMed]
- Yu, K.; Song, Y.; Lin, J.; Dixon, R.A. The complexities of proanthocyanidin biosynthesis and its regulation in plants. Plant Commun. 2023, 4, 100498. [Google Scholar] [CrossRef] [PubMed]
- Banavar, S.; Deshpande, A.; Sur, S.; Andreescu, S. Ceria nanoparticle theranostics: Harnessing antioxidant properties in biomedicine and beyond. J. Phys. Mater. 2021, 4, 042003. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hussain, S.; Garantziotis, S.; Demokritou, P.; Castranova, V.; Cassee, F.R. The yin: An adverse health perspective of nanoceria: Uptake, distribution, accumulation, and mechanisms of its toxicity. Environ. Sci. Nano 2014, 1, 406–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [Green Version]




| Technique | Description | Ref. |
|---|---|---|
| Layer-by-layer technique | Bottom-up adsorption technique which involves the development of multi-layered (layers of oppositely charged species) thin films bound together through electrostatic interactions. | [79,80] |
| Immersion/dip coating | Solution-based deposition method which involves the immersion of substrate into a solution of material to be coated which depends on parameters such as dwelling time, substrate-withdrawal speed, number of dip-coating cycles and coating evaporation factor. | [81,82] |
| Spin coating | A technique which uses centrifugal force for deposition, in which a suspension is dropped from top into the rotating substrate, and the resulting centrifugal force will assist in spreading out the coating on the substrate, thereby coating it. The process is dependent on parameters such as dispense volume, spin speed, solution viscosity, solution concentration, spin time, etc. | [83,84,85] |
| Plasma immersion ion implantation (PIII) | Method to improve biocompatibility aspects of material surfaces by immersing in a plasma environment and applying negative-high-voltage pulsed bias. Compared to traditional plasma techniques, PIII can extend to some tens of nanometers beneath sample surface and can treat complex geometries. | [86,87,88] |
| Plasma enhanced chemical vapor deposition | Low temperature chemical vapor deposition in which plasma is used to drive chemical reactions between plasma-generated-reactive species and substrate instead of high temperatures. | [89,90,91] |
| Type of Coating/Surface | Antioxidant Mechanism | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Tannic acid | Ability to chelate metal ions such as Fe(II), thereby interfering with one of the reaction steps in the Fenton reaction and thereby slowing oxidation | Antibacterial, antioxidant, high hemostatic efficiency, anticancer property, regenerative potential | Weak lipid solubility, low bioavailability, and short half-life, release rate should be controlled to exclude potential cytotoxicity, unstable adhesion | [152,153,154,155] |
| Chitosan | Reasonable mechanisms include presence of intra-molecular hydrogen bonding, metal chelation, ability of NH2 amino groups to react with hydroxyl groups | Biological activity, antimicrobial activity, hydrophilicity, and biodegradability | Delamination, unstable adhesion | [156,157,158] |
| Proanthocyanidin | By scavenging free radicals and by modifying signaling pathways, including those involving nuclear factor erythroid 2-related factor 2 (Nrf2), mitogen-activated protein kinase (MAPK), nuclear factor-kappaB (NF-κB), and phosphoinositide 3-kinase (PI3K)/Akt | Antioxidant, anticancer, antidiabetic, neuroprotective, and antimicrobial | High cost, low chemical stability and limited binding sites, difficulties in resolving the chemical labeling pattern of PAs with their proposed biosynthetic pathway, and defining the subcellular sites of biosynthesis | [121,124,159] |
| Ceria | Ability to rapidly switch between multiple valence states. SOD mimic activity is elicited by a shift from Ce3+ to Ce4+ (scavenging of O2−) and catalase mimic activity is induced by a shift from Ce4+ to Ce3+ (deactivating hydrogen peroxide) | Antioxidant, anticancer and anti-inflammatory properties, biosensors | Toxicity associated with small-sized nano-ceria | [160,161] |
| Silica | Hydroxylation degree, By regulation of antioxidants enzyme activity | Accelerated bone fracture healing, biomineral synthesis | Lipid peroxidation induced toxicity | [149,162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnu, J.; Kesavan, P.; Shankar, B.; Dembińska, K.; Swiontek Brzezinska, M.; Kaczmarek-Szczepańska, B. Engineering Antioxidant Surfaces for Titanium-Based Metallic Biomaterials. J. Funct. Biomater. 2023, 14, 344. https://doi.org/10.3390/jfb14070344
Vishnu J, Kesavan P, Shankar B, Dembińska K, Swiontek Brzezinska M, Kaczmarek-Szczepańska B. Engineering Antioxidant Surfaces for Titanium-Based Metallic Biomaterials. Journal of Functional Biomaterials. 2023; 14(7):344. https://doi.org/10.3390/jfb14070344
Chicago/Turabian StyleVishnu, Jithin, Praveenkumar Kesavan, Balakrishnan Shankar, Katarzyna Dembińska, Maria Swiontek Brzezinska, and Beata Kaczmarek-Szczepańska. 2023. "Engineering Antioxidant Surfaces for Titanium-Based Metallic Biomaterials" Journal of Functional Biomaterials 14, no. 7: 344. https://doi.org/10.3390/jfb14070344