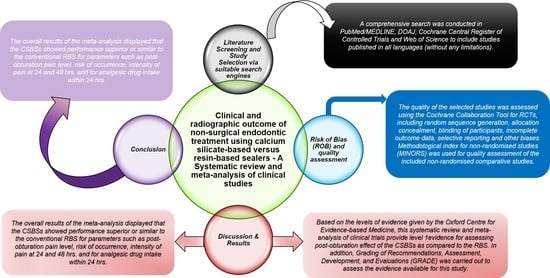
Clinical and Radiographic Outcome of Non-Surgical Endodontic Treatment Using Calcium Silicate-Based Versus Resin-Based Sealers—A Systematic Review and Meta-Analysis of Clinical Studies
Abstract
:1. Introduction
2. Protocol Development
2.1. Literature Screening and Study Selection
2.2. Inclusion and Exclusion Criteria Outline According to the PICOs Strategy
2.3. Screening Process
2.4. Data Extraction
2.5. Quality Assessment and Risk of Bias Analysis (ROB)
2.6. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Risk of Bias (ROB) and Quality Assessment
3.3. Quantitative Analysis
3.4. Mean Pain Levels
3.5. Risk of Occurrence of Pain
3.6. Intensity of Pain
3.7. Analgesic Medicament Intake within 24 h
3.8. Extrusion of the Sealer
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asawaworarit, W.; Pinyosopon, T.; Kijsamanmith, K. Comparison of apical sealing ability of bioceramic sealer and epoxy resin-based sealer using the fluid filtration technique and scanning electron microscopy. J. Dent. Sci. 2020, 15, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Stoll, R.; Betke, K.; Stachniss, V. The Influence of Different Factors on the Survival of Root Canal Fillings: A 10-Year Retrospective Study. J. Endod. 2005, 31, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Sindi, A.M.; Alogaly, J.A.; Ashour, M.S. Factors affecting the success of endodontic treatment (surgical or nonsurgical): A brief. Int. J. Med. Dev. Ctries. 2019, 3, 730–733. [Google Scholar]
- Tabassum, S.; Khan, F.R. Failure of endodontic treatment: The usual suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Thejeswar, E.; Ranjan, M. Evolution of root canal sealers and its impact on success of endodontics. Int. J. Sci. Dev. Res. 2020, 5, 264–269. [Google Scholar]
- Lee, K.-W.; Williams, M.C.; Camps, J.J.; Pashley, D.H. Adhesion of Endodontic Sealers to Dentin and Gutta-Percha. J. Endod. 2002, 28, 684–688. [Google Scholar] [CrossRef]
- Singh, H.; Markan, S.; Kaur, M.; Gupta, G.; Singh, H.; Kaur, M.S. Endodontic sealers: Current concepts and comparative analysis. Dent. Open J. 2015, 2, 32–37. [Google Scholar] [CrossRef]
- Da Fonseca, T.S.; Da Silva, G.F.; Tanomaru-Filho, M.; Sasso-Cerri, E.; Guerreiro-Tanomaru, J.M.; Cerri, P.S. In vivo evaluation of the inflammatory response and IL-6 immunoexpression promoted by Biodentine and MTA Angelus. Int. Endod. J. 2016, 49, 145–153. [Google Scholar] [CrossRef]
- Silva, G.F.; Guerreiro-Tanomaru, J.M.; da Fonseca, T.S.; Bernardi, M.I.B.; Sasso-Cerri, E.; Tanomaru-Filho, M.; Cerri, P.S. Zirconium oxide and niobium oxide used as radiopacifiers in a calcium silicate-based material stimulate fibroblast proliferation and collagen formation. Int. Endod. J. 2017, 50, e95–e108. [Google Scholar] [CrossRef] [Green Version]
- Atteia, M.H. Apical healing, resorbability, and digital radiodensity after apical extrusion of totalfill versus ah-plus sealers (one-year retrospective study). Egypt. Dent. J. 2017, 63, 3717–3723. [Google Scholar] [CrossRef]
- Lee, B.-N.; Hong, J.-U.; Kim, S.-M.; Jang, J.-H.; Chang, H.-S.; Hwang, Y.-C.; Hwang, I.-N.; Oh, W.-M. Anti-inflammatory and Osteogenic Effects of Calcium Silicate–based Root Canal Sealers. J. Endod. 2019, 45, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Peng, B. Tissue reactions after subcutaneous and intraosseous implantation of iRoot SP, MTA and AH Plus. Dent. Mater. J. 2015, 34, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersahan, S.; Aydin, C. Dislocation Resistance of iRoot SP, a Calcium Silicate–based Sealer, from Radicular Dentine. J. Endod. 2010, 36, 2000–2002. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-G.; Lee, Y.-H.; Lee, N.-H.; Bhattarai, G.; Lee, I.-K.; Yun, B.-S.; Yi, H.-K. The Antioxidant Property of Pachymic Acid Improves Bone Disturbance against AH Plus–induced Inflammation in MC-3T3 E1 Cells. J. Endod. 2013, 39, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.-Y.; Park, Y.-J.; Han, J.-S. Effects of HA released calcium ion on osteoblast differentiation. J. Mater. Sci. Mater. Electron. 2010, 21, 1649–1654. [Google Scholar] [CrossRef]
- Ma, S.; Yang, Y.; Carnes, D.L.; Kim, K.; Park, S.; Oh, S.H.; Ong, J.L. Effects of Dissolved Calcium and Phosphorous on Osteoblast Responses. J. Oral Implant. 2005, 31, 61–67. [Google Scholar] [CrossRef]
- Schröder, U. Effects of Calcium Hydroxide-containing Pulp-capping Agents on Pulp Cell Migration, Proliferation, and Differentiation. J. Dent. Res. 1985, 64, 541–548. [Google Scholar] [CrossRef]
- Lopez-Cazaux, S.; Bluteau, G.; Magne, D.; Lieubeau, B.; Guicheux, J.; Alliot-Licht, B. Culture medium modulates the behaviour of human dental pulp-derived cells: Technical Note. Eur. Cells Mater. 2006, 11, 35–42. [Google Scholar] [CrossRef]
- Nayab, S.N.; Jones, F.H.; Olsen, I. Effects of calcium ion-implantation of titanium on bone cell function in vitro. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 296–302. [Google Scholar]
- Zayzafoon, M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. 2006, 97, 56–70. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Fulzele, K.; McDonald, J.M. Calmodulin and Calmodulin-dependent Kinase IIα Regulate Osteoblast Differentiation by Controlling c-fos Expression. J. Biol. Chem. 2005, 280, 7049–7059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, F.R.; Pashley, D.H. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 2008, 29, 1127–1137. [Google Scholar] [CrossRef]
- Graunaite, I.; Skucaite, N.; Lodiene, G.; Agentiene, I.; Machiulskiene, V. Effect of Resin-based and Bioceramic Root Canal Sealers on Postoperative Pain: A Split-mouth Randomized Controlled Trial. J. Endod. 2018, 44, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Osiri, S.; Banomyong, D.; Sattabanasuk, V.; Yanpiset, K. Root Reinforcement after Obturation with Calcium Silicate–based Sealer and Modified Gutta-percha Cone. J. Endod. 2018, 44, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Nagar, N.; Kumar, N. A Comparative Clinical Evaluation Of A Bioceramic Root Canal Sealer With MTA Based Sealer, Resin Based Sealer And Zinc Oxide Based Sealer-An In Vivo Study. J. Dent. Med. Sci. 2018, 17, 81–85. [Google Scholar]
- Fonseca, B.; Coelho, M.S.; Bueno, C.E.D.S.; Fontana, C.E.; De Martin, A.S.; Rocha, D.G.P. Assessment of Extrusion and Postoperative Pain of a Bioceramic and Resin-Based Root Canal Sealer. Eur. J. Dent. 2019, 13, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, N.D.S.; Gollo, E.K.F.; Boscato, N.; Arias, A.; Silva, E.J.N.L.D. Postoperative pain after root canal filling with different endodontic sealers: A randomized clinical trial. Braz. Oral Res. 2020, 34, 1–8. [Google Scholar] [CrossRef]
- Aslan, T.; Özkan, H.D. The effect of two calcium silicate-based and one epoxy resin-based root canal sealer on postoperative pain: A randomized controlled trial. Int. Endod. J. 2021, 54, 190–197. [Google Scholar] [CrossRef]
- Junior, E.C.S.; Vieira, W.D.A.; Normando, A.G.C.; Pereira, J.V.; Ferraz, C.C.R.; Almeida, J.F.A.; Marciano, M.A.; Gomes, B.P.; De-Jesus-Soares, A. Calcium Silicate-Based Sealers Do Not Reduce the Risk and Intensity of Postoperative Pain after Root Canal Treatment when Compared with Epoxy Resin-Based Sealers: A Systematic Review and Meta-Analysis. Eur. J. Dent. 2021, 15, 347–359. [Google Scholar] [CrossRef]
- Jamali, S.; Darvish, M.; Nasrabadi, N.; Jafarizadeh, S. Evaluation of the Effect of the Intensity and Occurrence of Postoperative Pain of Resin-Based and Bioceramic Root Canal Sealers: A Systematic Review and Meta-Analysis of Randomized Controlled Trial Studies. Pesqui. Bras. Em Odontopediatria E Clínica Integr. 2021, 21, 21. [Google Scholar] [CrossRef]
- Mekhdieva, E.; Del Fabbro, M.; Alovisi, M.; Comba, A.; Scotti, N.; Tumedei, M.; Carossa, M.; Berutti, E.; Pasqualini, D. Postoperative Pain following Root Canal Filling with Bioceramic vs. Traditional Filling Techniques: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 4509. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Ab Aziz, Z.A.C. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valencia Berrospide, J.A.; Farje, J.I.N. Evaluación de la capacidad de sellado de cementos biocerámicos en la obturación de conductos radiculares. Revisión Sist. Estud. Vitr. 2020, Completed Dissertation in Universidad Peruana Caytano Heredia: Facultad DE Esyomatologia. 1–36. [Google Scholar]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, D142. [Google Scholar] [CrossRef] [Green Version]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.S.G.; Lim, K.C.; Lui, J.N.; Lai, W.M.C.; Yu, V.S.H. Postobturation Pain Associated with Tricalcium Silicate and Resin-based Sealer Techniques: A Randomized Clinical Trial. J. Endod. 2021, 47, 169–177. [Google Scholar] [CrossRef]
- Ved, R.P.; Hegde, V. An evaluation of the efficiency of a novel polyamide polymer bioceramic obturating system in cases with periapical lesions: An in vivo study. Int. J. Oral Care Res. 2020, 8, 39. [Google Scholar] [CrossRef]
- Zavattini, A.; Knight, A.; Foschi, F.; Mannocci, F. Outcome of Root Canal Treatments Using a New Calcium Silicate Root Canal Sealer: A Non-Randomized Clinical Trial. J. Clin. Med. 2020, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Ates, A.A.; Dumani, A.; Yoldas, O.; Unal, I. Post-obturation pain following the use of carrier-based system with AH Plus or iRoot SP sealers: A randomized controlled clinical trial. Clin. Oral Investig. 2018, 23, 3053–3061. [Google Scholar] [CrossRef]
- Paz, A.B.G. Evaluation of Postoperative Pain after Using Bioceramic Materials as Endodontic Sealers: Randomized Control Clinical Trial. Doctoral Dissertation, Universidade de Lisboa, Lisboa, Portugal, 2017. [Google Scholar]
- Paulaian, B.; Thakur, S.; Emil, J. Evaluation of mineral trioxide aggregate as root canal sealer: A clinical study. J. Conserv. Dent. 2013, 16, 494–498. [Google Scholar] [CrossRef] [Green Version]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Z.-H.; Wong, D.W.-C.; Lam, W.K.; Wan, A.H.-P.; Lee, W.C.-C. Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef] [Green Version]
- Estrela, C.; Holland, R.; Estrela, C.R.D.A.; Alencar, A.H.G.; Sousa-Neto, M.D.; Pécora, J.D. Characterization of successful root canal treatment. Braz. Dent. J. 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Nagendrababu, V.; El-Karim, I.A.; Dummer, P.M.H. Outcome measures to assess the effectiveness of endodontic treatment for pulpitis and apical periodontitis for use in the development of European Society of Endodontology (ESE) S3 level clinical practice guidelines: A protocol. Int. Endod. J. 2021, 54, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Sathorn, C.; Parashos, P.; Messer, H. The prevalence of postoperative pain and flare-up in single-and multiple-visit endodontic treatment: A systematic review. Int. Endod. J. 2008, 41, 91–99. [Google Scholar] [CrossRef]
- Wong, A.W.-Y.; Zhang, S.; Li, S.K.-Y.; Zhu, X.; Zhang, C.; Chu, C.-H. Incidence of post-obturation pain after single-visit versus multiple-visit non-surgical endodontic treatments. BMC Oral Health 2015, 15, 96. [Google Scholar] [CrossRef] [Green Version]
- Ørstavik, D. Apical periodontitis: Microbial infection and host responses. In Essential Endodontology: Prevention and Treatment of Apical Periodontitis, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1–10. [Google Scholar]
- De Paz, L.E.C.; Dahlén, G.; Molander, A.; Möller, Å.; Bergenholtz, G. Bacteria recovered from teeth with apical periodontitis after antimicrobial endodontic treatment. Int. Endod. J. 2003, 36, 500–508. [Google Scholar] [CrossRef]
- Gomes, B.P.F.A.; Pinheiro, E.; Gade-Neto, C.R.; Sousa, E.L.R.; Ferraz, C.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 2004, 19, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Lodienė, G.; Kopperud, H.M.; Ørstavik, D.; Bruzell, E.M. Detection of leachables and cytotoxicity after exposure to methacrylate-and epoxy-based root canal sealers in vitro. Eur. J. Oral Sci. 2013, 121, 488–496. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Peng, B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int. Endod. J. 2010, 43, 769–774. [Google Scholar] [CrossRef]
- Manfredi, M.; Figini, L.; Gagliani, M.; Lodi, G. Single versus multiple visits for endodontic treatment of permanent teeth. Cochrane Database Syst. Rev. 2016, 2016, CD005296. [Google Scholar] [CrossRef] [PubMed]
- Endodontology, E.S.O. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Boutron, I.; Moher, D. CONSORT and Its Extensions for Reporting Clinical Trials. Princ. Pract. Clin. Trials 2020, 1–15. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Duncan, H.F.; Bjørndal, L.; Kvist, T.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Pigg, M.; Rechenberg, D.K.; Væth, M.; et al. PRIRATE 2020 guidelines for reporting randomized trials in Endodontics: A consensus-based development. Int. Endod. J. 2020, 53, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]







| Search Strategy | |
|---|---|
| Focused Question | Are CSBSs sealers effective in improving the clinical and radiographic outcomes of endodontically treated permanent teeth in comparison to RBSs? |
| Search strategy | |
| Population (#1) | (Permanent Dentition [MeSH] OR Adult Dentition [Text Word] OR Secondary Dentition [Text Word] OR Permanent teeth [Text Word] OR Teeth [Text Word] OR Root Canal Obturation [MeSH] |
| Intervention (#2) | (Bioceramic sealer [Text Word] OR Endosequence BC [Text Word] OR iRoot Plus [Text Word] OR MTA Fillapex [Text Word] OR Totalfill BC [Text Word] OR tricalcium phosphate [Text Word] OR tricalcium phosphate ceramic sealer [Text Word] OR Calcium silicate sealer [Text Word] OR Calcium phosphate sealer [Text Word] OR Endodontic sealer [Text Word] OR Root canal sealer [Text Word]) |
| Comparisons (#3) | (Epoxy resin-based root canal sealer [Text Word] OR AH Plus [Text Word] OR Adseal [Text Word] OR AH 26 [Text Word]) |
| Outcomes (#4) | (Success [Text Word] Pain [Text Word] OR Pain intensity [Text Word] OR medication [Text Word] OR duration [Text Word] OR Visual analogue scale [MeSH] OR Heft Parker Visual Analog Scale [Text Word] OR Apical healing [Text Word]) |
| Study design (#5) | (Clinical trials [MeSH] OR randomized controlled studies [Text Word] OR randomized control trials [MeSH] OR randomized control clinical trial MeSH OR non-randomized control trials [Text Word] OR Quasi experimental studies [Text Word] OR before and after study design [Text Word] OR cohort studies [Text Word] OR in vivo study [Text Word]) |
| Search Combination | #1 AND #2 AND #3 AND #4 AND #5 |
| Database search | |
| Language | No restriction |
| Electronic Databases | PubMed/MEDLINE, DOAJ, Cochrane Central Register of Controlled Trials, Web of Science |
| Journals | Journal of Endodontics, International Endodontic Journal, Australian Endodontic Journal, Clinical Oral Investigations, Journal of Conservative Dentistry, Journal of American Dental Association |
| Period of Publication | 1 January 2011 to 31 January 2021 |
| Inclusion Criteria |
|---|
Secondary outcome: Studies assessing frequency of analgesics drug intake by individual’s post-treatment clinical success rate (asymptomatic tooth, sinus tract, tenderness on percussion, swelling, tooth mobility) and periapical status (apical healing, resolution of lesion, sealer resorption, sealer extrusion) post-obturation at a minimum of one month of follow-up using radiovisiography. Study design (S): Clinical trials, RCTs, quasi-experimental studies, non-randomised trials (NRS) and in- vivo studies. |
| Exclusion criteria |
|
| Study Id | Place of Study | Age of Participants | Sample Size I1/I2/C | Type of Tooth | Type of Pulpal Disease | Pulp Sensibility Test | Method of Root Canal Preparation | Final Irrigant Used | Obturation Technique I/C | Sealer Used I1/I2/C | Medicament Prescribed | Visit for RCT | Outcome Assessed | Method of Outcome Assessment | Time of Evaluation | Authors’ Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tan, H.S., et al. 2021 [36] | Singapore | 21 and above | 80/-/83 | Maxillary and mandibular anterior and posterior teeth | Vital, non-vital and previously root-filled teeth | - | Nickel– titanium rotary files in crown-down approach | 1.25% NaOCl 17% EDTA | Totalfill® BC point/non-standardised GP cones | Totalfill BC/-/AH plus | Ibuprofen if necessary | Single and Multiple | Post-obturation pain | Likert scale | 1, 3 and 7 days | There was no significant difference in pain experience between teeth filled using AH Plus or Totalfill BC sealer 1, 3 and 7 days after obturation. |
| Aslan., T, et al. 2020 [28] | Turkey | 18–60 | 28/30/26 | Mandibular first and second molar | Asymptomatic irreversible pulpitis | Thermal and electric pulp test | Nickel–titanium file system Reciproc with a VDW | 3 mL of 17% EDTA, 3 mL of 5% NaOCl, 2 mL of distilled water | Single tapered gutta-percha cone | Endoseal MTA/Endosequence BC/AH Plus | Ibuprofen 400 mg only when they encountered severe pain | Single | Pain, frequency of analgesic drug intake | VAS | 6, 12, 24 and 48 h and on 3rd, 4th, 5th, 6th and 7th day | Endoseal MTA, Endosequence BC Sealer and AH Plus were not significantly different in terms of the severity of postoperative pain after single-visit root canal treatment. |
| Ferreira, N., 2020 [27] | Brazil | 18 and above | 20/20/20 | Single rooted anterior teeth and premolars | Pulp necrosis | Cold test Absence of bleeding on access opening | - | 5 mL 2.5% NaOCl 5 mL 17% EDTA | Single-cone and vertical compaction technique | EndoFill/MTA Fillapex/ AH Plus | - | Min. 2 visits | Postoperative pain intensity | Level of pain | 24 h, 48 h and 7 days | Root canal filling using AH Plus, MTA Fillapex and EndoFill resulted in the same postoperative pain occurrence and intensity and need for analgesic intake. |
| Ved, R.P., 2020 [37] | India | 20–40 | 10/-/10 | Upper central or lateral incisor | Asymptomatic apical periodontitis | - | Rotary Protaper (F3) files | 3% NaOCl (2 mL) 17% aqueous EDTA | Syringe method/ cold lateral condensation | Smart seal/-/AH plus sealer | - | Min. 2 visits | Resolution of the lesion | Change in area of the periapical lesion using radiographs | 3, 6 and 12 months | Smart seal group showed better healing of the lesion as compared to gutta percha and AH Plus group at both 6 and 12 months following root canal treatment. |
| Zavattini, A., 2020 [38] | Russia | NR | 53/-/51 | - | Irreversible pulpitis Necrotic pulp | - | Protaper rotary instruments in a crown-down approach | 2% sodium hypochlorite 15% EDTA | Single-cone technique/warm vertical condensation | BioRootTM/-/AH plus | - | Two | Success rate | CBCT images, periapical radiographs | 12 months | BioRootTM RCS in combination with single cone resulted in a comparable success rate of cases compared to that of warm vertical condensation and AH plus. |
| Fonseca, B., 2019 [26] | Brazil | 25–55 | 32/-/32 | Single- rooted anterior maxillary teeth | Necrotic pulps | Cold and electric pulp test | VDW Silver motor | 17% EDTA 2.5% NaOCl | Single-cone technique | Sealer Plus BC/-/AH Plus | 600 mg Ibuprofen every 6 h if they experienced any pain | Single | Postoperative pain intensity | VAS | 24, 48, 72 h and 1 week | BG sealer presented significantly more extrusion than RG sealer, which was not associated with pain. |
| Ates, A.A., 2018 [39] | Turkey | 18–65 | 39/-/39 | Mandibular premolar or molar | Devitalised teeth | Electric pulp tester | One Shape system and VDW Silver motor | 5 mL 2.5% NaOCl, 5 mL 17% EDTA, and 5 mL sterile saline | Carrier-based obturation system- Hero fill™ Soft-Core obturators | iRoot SP/-/AH Plus | 200 mg ibuprofen | Single | Preoperative and postoperative pain rating, frequency of analgesic drug intake | Huskisson 10 cm VAS | 6, 12, 24 and 72 h. | iRoot SP sealer was associated with lower analgesic intake than AH Plus sealer. |
| Graunaite, I., 2018 [23] | Lithuania | 35–65 | 61/-/61 | Single-rooted teeth | Asymptomatic apical periodontitis | - | Protaper Gold system driven by an X-Smart endodontic motor | Ultrasonic activation for 30 s with 2.0 mL NaOCl, 2.0 mL 17% EDTA | Warm vertical condensation technique using the Calamus Dual System | Total Fill/-/AH Plus | - | Single | Postoperative pain | VAS | 24, 48, 72 h and 7 days | AH Plus and Total Fill perform similarly in terms of the occurrence and intensity of postoperative pain in teeth with AAP with no material extrusion beyond the apex. |
| Nagar, N., 2018 [25] | India | 15–47 | 16/16/16 | Maxillary anterior teeth | Apical periodontitis, small periapical lesion, Root resorption | - | - | 2 mL of 2.5% NaOCl and 2 mL of sterile saline followed by 10 mL 17% EDTA | - | Bioceramic sealer/MTA-based sealer/AH Plus | - | - | Pain, tenderness on percussion, sinus tract, swelling and mobility | VAS, radiovisiography measurement scale | 1, 3 and 6 months | Bioceramic Sealer was found to be of greatest efficiency followed by MTA, AH PLUS and Zinc Oxide Eugenol for all the evaluated parameters. |
| Paz, A., et al. 2018 [40] | Portugal | NR | 10/10 and 10 | Maxillary and mandibular anterior and posterior teeth | Asymptomatic irreversible pulpitis, pulp necrosis or disease that needed retreatment | - | Protaper Next engine driven rotary nickel-titanium files | 2.5% NaOCl 10% Citric acid | Single-cone technique -/cold lateral condensation and continuous wave of condensation | BioRoot RCS/AH Plus | Ibuprofen 600 mg if needed | Single and Multiple | Postoperative pain | Modified VAS | 24, 48, 72, 96, 120, 144 and 168 h | Single cone + Bioceramic and Continuous wave + resin sealer presented the highest percentage of moderate and the lowest levels of postoperative pain intensity felt, respectively, during the 7 day evaluation period |
| Atteia, M.H., 2017 [10] | Egypt | 20–35 | 15/-/15 | Mandibular first molars | Chronic apical periodontitis | Electronic apex locator | Protaper-NEXT NiTi rotary files | 3% NaOCl 2 mL of 17% EDTA | Lateral compaction technique of gutta-percha | Totalfill sealer/-/AH Plus | - | Single | Apical healing, sealer resorption and extruded sealer | Periapical radiographs, digital radiography | 12 months | Totalfill recorded higher observations of complete apical healing, compared to AH-Plus. |
| Thakur, S., 2013 [41] | India | 18–50 | 15/-/15 | Single rooted tooth | Apical radiolucency and periapical index Score 2 or more Diagnosis | - | Protaper rotary system | 2.5% NaOCl, EDTA and normal saline | Lateral compaction technique | ProRoot MTA/-/AH Plus | - | Multiple | Pain evaluation Periapical status Area measurement | VAS, periapical Index, VixWin Pro digital image analysis software | 1 week and 6 months | MTA could be used as a root canal sealer with equal effectiveness compared with epoxy resin- or zinc oxide eugenol-based sealers. |
| Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Collection of Data | Endpoints Appropriate to the Aim of the Study | Unbiased Assessment of the Study Endpoint | Follow-Up Period Appropriate to the Aim of the Study | Loss to Follow-Up Less than 5% | Prospective Calculation of the Study Size | * An Adequate Control Group | * Contemporary Groups | * Baseline Equivalence of Groups | * Adequate Statistical Analyses | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thakur et al., 2013 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Zavattini et al., 2020 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 22 |
| Pain Intervals | Observations |
|---|---|
| 24 h | Mild (RR: 0.90 95% CI = 0.55–1.47, p = 0.66, I2 = 0%) and |
| Moderate (RR: 1.26 95% CI = 0.65–2.46, p = 0.49, I2 = 0%) | |
| 48 h | Mild (RR: 1.25 95% CI = 0.54–2.89, p = 0.59, I2 = 10%) |
| Moderate (RR: 1.74 95% CI = 0.29–10.25, p = 0.54, I2 = 0%) | |
| Seven days | Mild (RR: 1.73 95% CI = 0.43–7.00, p = 0.44) |
| Moderate (RR: 3.00 95% CI = 0.12–72.56, p = 0.50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, V.; Davis, G.; Baysan, A. Clinical and Radiographic Outcome of Non-Surgical Endodontic Treatment Using Calcium Silicate-Based Versus Resin-Based Sealers—A Systematic Review and Meta-Analysis of Clinical Studies. J. Funct. Biomater. 2022, 13, 38. https://doi.org/10.3390/jfb13020038
Chopra V, Davis G, Baysan A. Clinical and Radiographic Outcome of Non-Surgical Endodontic Treatment Using Calcium Silicate-Based Versus Resin-Based Sealers—A Systematic Review and Meta-Analysis of Clinical Studies. Journal of Functional Biomaterials. 2022; 13(2):38. https://doi.org/10.3390/jfb13020038
Chicago/Turabian StyleChopra, Viresh, Graham Davis, and Aylin Baysan. 2022. "Clinical and Radiographic Outcome of Non-Surgical Endodontic Treatment Using Calcium Silicate-Based Versus Resin-Based Sealers—A Systematic Review and Meta-Analysis of Clinical Studies" Journal of Functional Biomaterials 13, no. 2: 38. https://doi.org/10.3390/jfb13020038