Combined Effects of Drought and Soil Fertility on the Synthesis of Vitamins in Green Leafy Vegetables
Abstract
:1. Introduction
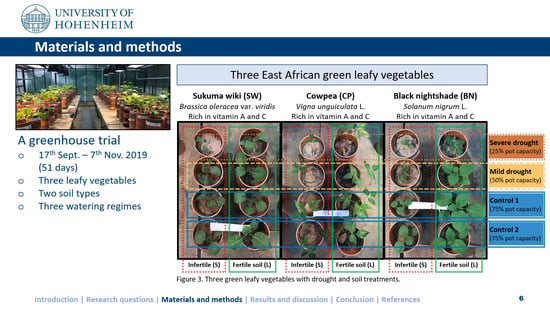
2. Materials and Methods
3. Results
3.1. Plant Yield Analysis
3.2. Δ13C Measurement as an Indicator for Water Stress
3.3. Effects of Drought and Soil Fertility on Vitamin Concentrations
3.3.1. Thiamine
3.3.2. Beta-Carotene (Pro-Vitamin A)
3.3.3. Ascorbic Acid
3.4. Fulfilment of Recommended Nutrient Intake (RNI) by Green Leafy Vegetables under Different Growth Conditions
3.5. Effects of Drought under Two Soil Fertilities on Fresh Yield and Vitamin Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ugandan Bureau of Statistics (UBOS); ICF. Ugandan Demographic and Health Survey 2016; UBOS: Kampala, Uganda; ICF: Rockville, MD, USA, 2018.
- Yang, R.Y.; Fischer, S.; Hanson, P.M.; Keatinge, J.D.H. Increasing micronutrient availability from food in Sub-saharan Africa with indigenous vegetables. ACS Symp. Ser. 2013, 1127, 231–254. [Google Scholar] [CrossRef]
- Whitfield, K.C.; Bourassa, M.W.; Adamolekun, B.; Bergeron, G.; Bettendorff, L.; Brown, K.H.; Cox, L.; Fattal-Valevski, A.; Fischer, P.R.; Frank, E.L.; et al. Thiamine deficiency disorders: Diagnosis, prevalence, and a roadmap for global control programs. Ann. N. Y. Acad. Sci. 2018, 1430, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.; Carr, A.C. Global vitamin c status and prevalence of deficiency: A cause for concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.R.; Richardson, D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption! Free Radic. Biol. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef]
- Ojagbemi, A.; Okekunle, A.; Olowoyo, P.; Akpa, O.; Akinyemi, R.; Ovbiagele, B.; Owolabi, M. Dietary intakes of green leafy vegetables and incidence of cardiovascular diseases. Cardiovasc. J. Afr. 2021, 32, 4. [Google Scholar] [CrossRef]
- Stuetz, W.; Gowele, V.; Kinabo, J.; Bundala, N.; Mbwana, H.; Rybak, C.; Eleraky, L.; Lambert, C.; Biesalski, K. Consumption of Dark Green Leafy Vegetables Predicts Vitamin A and Iron Intake and Status among Female Small-Scale Farmers in Tanzania. Nutrients 2019, 11, 1025. [Google Scholar] [CrossRef]
- Yang, R.Y.; Keding, G.B. Nutritional contributions of important African indigenous vegetables. In African Indigenous Vegetables in Urban Agriculture, 1st ed.; Shackleton, C.M., Pasquini, M.W., Drescher, A.W., Eds.; Earthscan: London, UK, 2009; pp. 105–143. [Google Scholar]
- Moyo, S.M.; Serem, J.C.; Bester, M.J.; Mavumengwana, V.; Kayitesi, E. (2020) African green leafy vegetables health benefits beyond nutrition. Food Rev. Int. 2020, 37, 601–618. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef]
- Mattos, L.M.; Moretti, C.L. Oxidative Stress in Plants Under Drought Conditions and the Role of Different Enzymes. Enzym. Eng. 2016, 5, 136. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Chapman, L.M. The importance of thiamine (vitamin B1) in plant health: From crop yield to biofortification. J. Biol. Chem. 2020, 295, 12002–12013. [Google Scholar] [CrossRef]
- Fischer, S.; Hilger, T.; Piepho, H.-P.; Jordan, I.; Karungi, J.; Towett, E.; Shepherd, K.; Cadisch, G. Soil and farm management effects on yields and nutrient concentrations of food crops in East Africa. Sci. Total Environ. 2020, 716, 137078. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid pigments in kale are influenced by nitrogen concentration and form. J. Sci Food. Agric. 2007, 87, 900–907. [Google Scholar] [CrossRef]
- Onyango, C.M.; Harbinson, J.; Imungi, J.K.; Van Kooten, O. Influence of maturity at harvest, N fertiliser and postharvest storage on dry matter, ascorbic acid and ß-carotene contents of vegetable amaranth (Amaranthus hypochondriacus). Int. J. Postharvest. Technol. Innov. 2011, 2, 180–196. [Google Scholar] [CrossRef]
- Cai, W.; Borlace, S.; Lengaigne, M.; van Rensch, P.; Collins, M.; Vecchi, G.; Timmermann, A.; Santoso, A.; McPhaden, M.J.; Wu, L.; et al. Increasing frequency of extreme El Niño events due to greenhouse warming. Nat. Clim. Change 2014, 4, 111–116. [Google Scholar] [CrossRef]
- Fischer, S.; Hilger, T.; Piepho, H.-P.; Jordan, I.; Cadisch, G. Do we need more drought for better nutrition? The effect of precipitation on nutrient concentration in East African food crops. Sci. Total Environ. 2019, 658, 405–415. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- D’Oria, A.; Courbet, G.; Billiot, B.; Jing, L.; Pluchon, S.; Arkoun, M.; Maillard, A.; Roux, C.P.; Trouverie, J.; Etienne, P.; et al. Drought specifically downregulates mineral nutrition: Plant ionomic content and associated gene expression. Plant Direct 2022, 6, e402. [Google Scholar] [CrossRef]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef]
- Fischer, S.; Hilger, T.; Piepho, H.-P.; Jordan, I.; Cadisch, G. Missing association between nutrient concentrations in leaves and edible parts of food crops—A neglected food security issue. J. Food Chem. 2021, 345, 128723. [Google Scholar] [CrossRef]
- Turner, N.C. Imposing and maintaining soil water deficits in drought studies in pots. Plant Soil 2019, 439, 45–55. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Fenollosa, E.; Salguero-Gómez, R.; Munné-Bosch, S. What is the minimal optimal sample size for plant ecophysiological studies? Plant Physiol. 2018, 178, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Bramley, H.; Turner, N.C.; Siddique, K.H.M. Water use efficiency. In Genomics and Breeding for Climate-Resilient Crops, 1st ed.; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 2, pp. 225–268. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.K.; Westergaard, J.C.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 Increases Water Use Efficiency via Growth Stimulation in Both Normal and Drought Conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- BSI Standards: 2014: BS EN 14122:2014; Foodstuffs—Determination of Vitamin B1 by High Performance Liquid Chromatography. BSI: London, UK, 2014.
- Wald, J.P.; Nohr, D.; Biesalski, H.K. Rapid and easy carotenoid quantification in Ghanaian starchy staples using RP-HPLC-PDA. J. Food Comp. Anal. 2018, 67, 119–127. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agricultural Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geeneve, Switzerlnd, 2004; 341p. [Google Scholar]
- Hill-Mündel, K.; Schlegl, J.; Biesalski, H.K.; Ehnert, S.; Schröter, S.; Bahrs, C.; Nohr, D.; Nüssler, A.K.; Ihle, C. Preoperative Ascorbic Acid Levels in Proximal Femur Fracture Patients Have No Postoperative Clinical Impact, While Ascorbic Acid Levels upon Discharge Have a Major Effect on Postoperative Outcome. J. Clin. Med. 2019, 9, 66. [Google Scholar] [CrossRef]
- Clay, D.E.; Engel, R.E.; Long, D.S.; Liu, Z. Nitrogen and water stress interact to influence carbon-13 discrimination in wheat. Soil Sci. Soc. Am. J. 2001, 65, 1823–1828. [Google Scholar] [CrossRef]
- Schmitter, P.; Dercon, G.; Hilger, T.; Hertel, M.; Treffner, J.; Lam, N.; Duc Vien, T.; Cadisch, G. Linking spatio-temporal variation of crop response with sediment deposition along paddy rice terraces. Agric. Ecosyst. Environ. 2011, 140, 34–45. [Google Scholar] [CrossRef]
- Hanson, P.; Yang, R.Y.; Chang, L.C.; Ledesma, L.; Ledesma, D. Carotenoids, ascorbic acid, minerals, and total glucosinolates in choysum (Brassica rapa cvg. parachinensis) and kailaan (B. oleraceae Alboglabra group) as affected by variety and wet and dry season production. J. Food Compos. Anal. 2011, 24, 950–962. [Google Scholar] [CrossRef]
- Pathirana, I.; Thavarajah, P.; Siva, N.; Wickramasingh, A.N.K.; Smith, P.; Thavarajah, D. Moisture deficit effects on kale (Brassica oleracea L. var. acephala) biomass, mineral, and low molecular weight carbohydrate concentrations. Sci. Hortic. 2017, 226, 216–222. [Google Scholar] [CrossRef]
- van Averbeke, W.; Netshithuthuni, C. Effect of irrigation scheduling on leaf yield of non-heading chinese cabbage (Brassica rapa L. subsp. chinensis). South Afr. J. Plant Soil 2010, 27, 322–327. [Google Scholar] [CrossRef]
- Maseko, I.; Ncube, B.; Tesfay, S.; Fessehazion, M.; Modi, A.T.; Mabhaudhi, T. Productivity of selected african leafy vegetables under varying water regimes. Agronomy 2020, 10, 916. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Kowalska, E.; Ostrowska, K. Modulation of thiamine metabolism in Zea mays seedlings under conditions of abiotic stress. J. Exp. Bot. 2008, 59, 4133–4143. [Google Scholar] [CrossRef]
- Borhannuddin Bhuyan MH, M.; Hasanuzzaman, M.; Nahar, K.; Mahmud, J.A.; Parvin, K.; Bhuiyan, T.F.; Fujita, M. Plants behavior under soil acidity stress: Insight into morphophysiological, biochemical, and molecular responses. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer Nature: Basel, Switzerland, 2019; pp. 35–82. [Google Scholar]
- Hanson, A.D.; Beaudoin, G.A.; McCarty, D.R.; Gregory, J.F. Does abiotic stress cause functional B vitamin deficiency in plants? Plant Physiol. 2016, 172, 2082–2097. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Soil pH buffering capacity and nitrogen availability following compost application in a tropical acid soil. Compost. Sci. Util. 2018, 26, 1–15. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol. 2003, 131, 1816–1825. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Masih, I.; Maskey, S.; Mussá, F.E.F.; Trambauer, P. A review of droughts on the African continent: A geospatial and long-term perspective. Hydrol. Earth Syst. Sci. 2014, 18, 3635–3649. [Google Scholar] [CrossRef]
- von Grebmer, K.; Bernstein, J.; Wiemers, M.; Schiffer, T.; Hanano, A.; Towey, O.; Chéilleachair, R.N.; Foley, C.; Gitter, S.; Ekstrom, K.; et al. Global Hunger Index, 16th ed.; Welthungerhilfe: Bonn, Germany, 2021; 60p, Available online: https://www.globalhungerindex.org/ (accessed on 3 August 2022).



| Fresh Yield (g/pot) | Belowground (g/pot FW) | No. of Nodules | Irrigation Water Added (mL/pot) | WUE (g/L) | |||
|---|---|---|---|---|---|---|---|
| Vigna unguiculata | |||||||
| Fertile soil | |||||||
| 75% PC | 25.2 ± 1.3 c | 12.0 ± 1.1 bc | 11.2 ± 3.4 a | 2882 ± 115 a | 8.8 ± 2.0 def | ||
| 50% PC | 18.6 ± 1.9 d | 9.0 ± 1.5 cd | 6.3 ± 2.1 b | 1871 ± 162 bc | 10.1 ± 2.9 cdef | ||
| 25% PC | 12.2 ± 1.9 ef | 5.2 ± 1.5 de | 1.2 ± 0.5 bc | 832 ± 162 de | 15.3 ± 2.9 bcd | ||
| Infertile soil | |||||||
| 75% PC | 13.8 ± 1.3 e | 2.0 ± 1.1 ef | 0.0 c | 2192 ± 165 b | 6.3 ± 2.0 ef | ||
| 50% PC | 7.9 ± 1.9 fg | 1.0 ± 1.5 ef | 0.0 c | 1239 ± 163 d | 6.2 ± 2.9 ef | ||
| 25% PC | 2.5 ± 1.9 h | 0.9 ± 1.5 f | 0.0 c | 405 ± 162 ef | 8.8 ± 2.9 cdef | ||
| Brassica oleraceae | |||||||
| Fertile soil | |||||||
| 75% PC | 41.5 ± 1.7 a | 9.4 ± 1.3 c | - | 2842 ± 142 a | 14.9 ± 2.5 bcd | ||
| 50% PC | 32.8 ± 2.1 b | 10.2 ± 1.6 c | - | 2080 ± 179 bc | 16.4 ± 3.2 bc | ||
| 25% PC | 12.6 ± 2.1 ef | 1.4 ± 1.6 ef | - | 398 ± 179 ef | 43.2 ± 3.2 a | ||
| Infertile soil | |||||||
| 75% PC | 40.3 ± 1.4 a | 3.9 ± 1.1 ef | - | 2928 ± 120 a | 13.9 ± 2.1 bcde | ||
| 50% PC | 15.0 ± 1.9 de | 1.0 ± 1.5 ef | - | 1204 ± 162 d | 11.8 ± 2.9 cdef | ||
| 25% PC | 4.7 ± 1.9 gh | 0.6 ± 1.5 f | - | 342 ± 162 f | 14.1 ± 2.9 bcde | ||
| Solanum scabrum | |||||||
| Fertile soil | |||||||
| 75% PC | 35.0 ± 1.3 b | 21.5 ± 1.1 a | - | 2844 ± 115 a | 12.4 ± 2.0 cde | ||
| 50% PC | 25.9 ± 1.9 c | 15.4 ± 1.5 b | - | 1783 ± 162 c | 14.8 ± 2.9 bcd | ||
| 25% PC | 17.4 ± 1.9 de | 10.4 ± 1.5 c | - | 917 ± 162 d | 20.6 ± 2.9 b | ||
| Infertile soil | |||||||
| 75% PC | 16.5 ± 1.3 de | 3.0 ± 1.1 ef | - | 1804 ± 115 c | 9.0 ± 2.0 cdef | ||
| 50% PC | 4.8 ± 1.9 gh | 0.9 ± 1.5 f | - | 1013 ± 162 d | 4.8 ± 2.9 f | ||
| 25% PC | 1.1 ± 1.9 h | 0.7 ± 1.5 f | - | 209 ± 162 f | 14.4 ± 2.9 bcd | ||
| β-Carotene (mg/100 g FW) | RAE (μg/100 g FW) | β-Carotene (mg/Fresh Yield) | RAE (μg/Fresh Yield) | |||
|---|---|---|---|---|---|---|
| Vigna unguiculata | ||||||
| Fertile soil | ||||||
| 75% PC | 7.37 ± 0.26 bc | 614 ± 21 bc | 1.86 ± 0.09 bc | 155 ± 7 bc | ||
| 50% PC | 7.64 ± 0.36 bc | 636 ± 30 bc | 1.42 ± 0.13 de | 118 ± 10 de | ||
| 25% PC | 8.04 ± 0.36 b | 670 ± 30 b | 0.97 ± 0.13 f | 80 ± 10 f | ||
| Infertile soil | ||||||
| 75% PC | 6.89 ± 0.26 cd | 574 ± 21 cd | 0.97 ± 0.09 f | 81 ± 7 f | ||
| 50% PC | 6.41 ± 0.36 de | 534 ± 30 de | 0.57 ± 0.13 g | 47 ± 10 g | ||
| 25% PC | 4.92 ± 0.36 gh | 410 ± 30 gh | 0.12 ± 0.13 h | 10 ± 10 h | ||
| Brassica oleraceae | ||||||
| Fertile soil | ||||||
| 75% PC | 4.02 ± 0.32 hi | 335 ± 26 hi | 1.66 ± 0.11 cd | 138 ± 9 cd | ||
| 50% PC | 4.11 ± 0.40 ghi | 343 ± 33 ghi | 1.36 ± 0.14 de | 114 ± 12 de | ||
| 25% PC | 3.66 ± 0.40 i | 305 ± 33 i | 0.46 ± 0.14 gh | 38 ± 12 gh | ||
| Infertile soil | ||||||
| 75% PC | 4.03 ± 0.27 hi | 336 ± 22 hi | 1.62 ± 0.10 cd | 135 ± 8 cd | ||
| 50% PC | 3.48 ± 0.36 i | 290 ± 30 i | 0.55 ± 0.13 g | 46 ± 10 g | ||
| 25% PC | 3.61 ± 0.36 i | 301 ± 30 i | 0.17 ± 0.13 h | 14 ± 10 h | ||
| Solanum scabrum | ||||||
| Fertile soil | ||||||
| 75% PC | 7.59 ± 0.26 bc | 632 ± 21 bc | 2.66 ± 0.09 a | 221 ± 7 a | ||
| 50% PC | 8.09 ± 0.36 b | 674 ± 30 b | 2.11 ± 0.13 b | 176 ± 10 b | ||
| 25% PC | 9.48 ± 0.36 a | 790 ± 30 a | 1.62 ± 0.13 cd | 135 ± 10 cd | ||
| Infertile soil | ||||||
| 75% PC | 7.08 ± 0.26 cd | 590 ± 21 cd | 1.17 ± 0.09 ef | 97 ± 7 ef | ||
| 50% PC | 5.99 ± 0.36 ef | 499 ± 30 ef | 0.31 ± 0.13 gh | 26 ± 10 gh | ||
| 25% PC | 5.13 ± 0.45 fg | 428 ± 37 fg | 0.06 ± 0.16 h | 5 ± 13 h | ||
| % of RNI 1 for Vitamin Content (mg or μg/150 g FW) | % of RNI 1 for Vitamin Yield (mg or μg/Plant) | |||||||
|---|---|---|---|---|---|---|---|---|
| Thiamine | Ascorbic Acid | RAE 2 | Thiamine | Ascorbic Acid | RAE 2 | |||
| Vigna unguiculata | ||||||||
| Fertile soil (L) | ||||||||
| 75% PC | 13.0 | 118 | 184 | 2.4 | 21 | 31 | ||
| 50% PC | 14.7 | 101 | 191 | 1.8 | 11 | 24 | ||
| 25% PC | 20.2 | 105 | 201 | 1.7 | 9 | 16 | ||
| Infertile soil (S) | ||||||||
| 75% PC | 15.5 | 75 | 172 | 1.6 | 8 | 16 | ||
| 50% PC | 19.5 | 92 | 160 | 1.1 | 6 | 9 | ||
| 25% PC | 33.0 | 170 | 123 | 0.5 | 2 | 2 | ||
| Brassica oleraceae | ||||||||
| Fertile soil (L) | ||||||||
| 75% PC | 9.8 | 238 | 101 | 2.7 | 68 | 28 | ||
| 50% PC | 9.7 | 237 | 103 | 2.3 | 53 | 23 | ||
| 25% PC | 10.2 | 132 | 92 | 1.2 | 51 | 8 | ||
| Infertile soil (S) | ||||||||
| 75% PC | 11.3 | 189 | 101 | 3.0 | 15 | 27 | ||
| 50% PC | 12.0 | 117 | 87 | 1.5 | 14 | 9 | ||
| 25% PC | 20.6 | 135 | 90 | 0.6 | 4 | 3 | ||
| Solanum scabrum | ||||||||
| Fertile soil (L) | ||||||||
| 75% PC | 2.9 | 152 | 190 | 0.7 | 37 | 44 | ||
| 50% PC | 3.4 | 127 | 202 | 0.6 | 22 | 35 | ||
| 25% PC | 4.3 | 180 | 237 | 0.4 | 17 | 27 | ||
| Infertile soil (S) | ||||||||
| 75% PC | 4.9 | 122 | 177 | 0.5 | 13 | 19 | ||
| 50% PC | 5.3 | 125 | 150 | 0.2 | 5 | 5 | ||
| 25% PC | 5.5 | 166 | 128 | 0.1 | 1 | 1 | ||
| Brassica oleraceae | Vigna unguiculata | Solanum scabrum | ||
|---|---|---|---|---|
| Fresh yield | Fertile soil (L) |  |  |  |
| Infertile soil (S) |  |  |  | |
| β-carotene | Fertile soil (L) | - | - |  |
| Infertile soil (S) | - |  |  | |
| Ascorbic acid | Fertile soil (L) |  | - |  |
| Infertile soil (S) |  |  |  | |
| Thiamine | Fertile soil (L) | - |  | - |
| Infertile soil (S) |  |  | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, T.; Fischer, S.; Lambert, C.; Hilger, T.; Jordan, I.; Cadisch, G. Combined Effects of Drought and Soil Fertility on the Synthesis of Vitamins in Green Leafy Vegetables. Agriculture 2023, 13, 984. https://doi.org/10.3390/agriculture13050984
Park T, Fischer S, Lambert C, Hilger T, Jordan I, Cadisch G. Combined Effects of Drought and Soil Fertility on the Synthesis of Vitamins in Green Leafy Vegetables. Agriculture. 2023; 13(5):984. https://doi.org/10.3390/agriculture13050984
Chicago/Turabian StylePark, Taewan, Sahrah Fischer, Christine Lambert, Thomas Hilger, Irmgard Jordan, and Georg Cadisch. 2023. "Combined Effects of Drought and Soil Fertility on the Synthesis of Vitamins in Green Leafy Vegetables" Agriculture 13, no. 5: 984. https://doi.org/10.3390/agriculture13050984