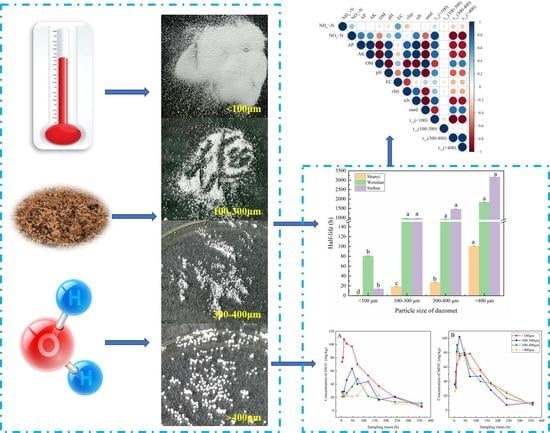
1. Introduction
The expansion of protected agriculture in many countries has increased the continuous production area of many crops. However, continuous production of the same crop on the same soil under protected agriculture increases pathogens and soil-borne disease over time which if left uncontrolled can severely reduce crop yield, farmer profitability and limit further expansion of the crop. Soil fumigation prior to planting seedlings is a well-established procedure that provides effective control of soil-borne disease caused by fungal pathogens, nematodes and weeds [
1,
2]. The research has shown that soil fumigants can increase crop yield and improve food safety [
3]. Fumigants most commonly used are chloropicrin (CP) [
4], dazomet (DZ) [
5], 1,3-dichloropropene (1,3-D) [
6,
7], metam sodium (MS) [
8,
9], and allyl isothiocyanate (AITC) [
10].
DZ is a solid-particle soil fumigant that has been used for decades. Granules of DZ are applied to the soil surface by hand in many countries and then tilled into the soil by machine. Manual application can result in higher levels of DZ granules in some places which can lead to crop phytotoxicity, while lower levels of granules in some areas can lead to poor pathogen control [
11,
12]. DZ is formulated as tetrahydro-3,5-dimethyl-2H-1,3,5-thiadiazine-2-thione, which belongs to the class of chemical called thiadiazines. Methyl isothiocyanate (MITC) is the main active ingredient released from DZ when it comes into contact with soil moisture. DZ in effect acts as a carrier for MITC which has poor mobility in soil [
11]. Soil type and temperature also influence the degradation rate of DZ and the release of MITC [
13,
14]. Zheng et al. [
15] observed that low temperature or dry soil reduce the DZ degradation rate. Fang et al. [
13] showed that the degradation rate of DZ was determined by soil type and water content. While once released from DZ, MITC is toxic to weeds and soil borne pathogens [
16]. However, phytotoxicity caused by DZ often occurred in many countries, and similar phenomena were often encountered in our field experiments. In the field with phytotoxicity, it will be found that there were still DZ particles, so we speculate that DZ particle size is one of the main reasons for phytotoxicity. In addition, Ren et al. [
17] found that the degradation rate of linter particle size was different in the water environment. Therefore, in order to find solutions, it is necessary to study the degradation law of DZ with different particle sizes.
At present, the research on the DZ mostly focuses on the impact of environmental factors on DZ. However, the effect of DZ granule’s own factor, such as DZ particle size range, on DZ degradation has rarely been reported publicly. We cannot deny that this is also one of the important factors affecting the degradation of DZ. Commercially available DZ has wide-ranging granule diameters that vary from 100 to 400 μm [
18]. As a basic physical principle, larger particles degrade more slowly than smaller ones. The wide variation in granular diameter of commercially-available DZ results in a combination of degradation rates and in general unpredictable results for farmers, particularly when DZ is applied to different soil types and temperatures that contain different moisture levels.
Therefore, the research aims to study the degradation rate of DZ with different particle size in soil environment, such as soil type, soil temperature and moisture, so as to formulate more accurate fumigation time. In addition, the research also recorded the concentrations of MITC produced by DZ according to particle size range, soil type and soil conditions. We hypothesized that (1) The smaller the particle size, the faster the degradation rate of DZ; (2) larger particle size would release MITC more slowly over time than those in smaller particle size.
2. Materials and Methods
2.1. Chemicals Reagents
Dazomet (DZ, 98% purity) microparticles in four particle size (including <100 μm, 100–300 μm, 300–400 μm, >400 μm) used in this experiment were provided by Nantong Shizhuang Chemical Co., Ltd. (Jiangsu, Chian) (
Figure S1). The specific surface areas of DZ were measured by ASAP 2460 (Version 3.01). Acetonitrile and ethyl acetate (HPLC grade) were purchased from Thermo Fisher Technology Co., Ltd. (Shanghai, China). Anhydrous sodium sulfate and sodium chloride (analytically pure) were obtained from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai China). Methyl isothiocyanate (MITC, 98% purity) was purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China).
2.2. Soil Samples
Soil samples (5–20 cm soil layer) obtained from Shunyi (Beijing Municipality), Wenshan (Yunnan Province) and Suihua (Heilongjiang Province) were used to investigate DZ degradation rate. Soil in those locations had not been fumigated with DZ. After all soil samples were brought back to the laboratory, plant debris and stones in the soil samples were removed with a 2 mm sieve, The sifted soil samples were refrigerated at 4 °C for future use. The physicochemical properties of the three soil samples were summarized in
Table 1 according to Zhang et al. [
19].
2.3. Soil Moisture
The water content of the soil samples was adjusted to 15%, 20% and 30% (w/w) with deionized water, and then incubated in the dark environment at 25 °C for 1 day to homogenize. After the pretreatment of soil samples, 20 grams (dry weight, the same below) of soil samples were transferred into a 50 mL headspace vial into which was added sufficient DZ with different particle sizes to achieve the label dosage rate used commercially in China of 214.3 mg/kg. The vial was immediately sealed with an aluminum cap equipped with Teflon-faced butyl rubber septum. The sealed vial was shaken manually to fully mixed DZ into the soil. The vials were inverted and incubated in the dark at 25 °C. After incubation for 0, 3, 6, 12, 24, 48, 96, 168, 240, 360, 480, 720 and 1080 h, three vials were randomly selected at each sampling time and immediately stored at −80 °C for later analysis of the DZ concentration.
2.4. Soil Temperature
The water content of the soil samples was adjusted to 20%, and then incubated at 4, 15, 25 and 37 °C for 1 day. DZ with different particle sizes was added to each vial sufficient to achieve the label dosage rate used commercially in China of 214.3 mg/kg. Each vial was immediately sealed with an aluminum cap equipped with Teflon-faced butyl rubber septum. The vials were inverted and incubated in the dark at 4, 15, 25 and 37 °C for 0, 3, 6, 12, 24, 48, 96, 168, 240, 360, 480, 720 and 1080 h. Three vials were randomly selected at each sampling time and immediately stored at −80 °C for later analysis of the DZ concentration.
2.5. Soil Type
The research determined the influence of soil type from some of the vials in the soil temperature and moisture experiments above. The research determined the DZ degradation rate in the three soil types exposed to 25 °C and 20% water content for DZ that contained each range of particle size.
2.6. DZ Extraction Procedure and Concentration Determination
Each vial was removed from the −80 °C refrigerator, the DZ extraction procedures followed those previously described [
13].The vials cap was removed, and 20 mL of acetonitrile was immediately added. The vial was re-sealed with new aluminum caps, When the soil sample had thawed, 10 g of sodium chloride were added and the vial which was re-sealed. Each vial was then shaken at 2500 rpm for 10 min and then the DZ was statically extracted at room temperature into the acetonitrile supernatant over a period of 15 min.
Approximately 1.5 mL of the supernatant were filter into a 2 mL vial using a 0.22 μm nylon syringe for high-performance liquid chromatography (HPLC) analysis. The recovery efficiency of DZ obtained by the above extraction method in soil ranged from 88–114%.
The concentration of DZ in acetonitrile was determined following procedures previously described [
20,
21]. An HPLC was coupled to a diode array detector (DAD) (Agilent Technologies, Santa Clara, CA, USA) and equipped with a Venusil XBP-C8 column (4.6 × 150 mm, 5 μm; Agela Technologies Inc., Torrance, CA, USA). The DZ quantification conditions were: Mobile phase consisted of 45% deionized water and 55% chromatographic grade acetonitrile, in which deionized water contained 0.2% glacial acetic acid. Flow rate of the mobile phase 1 mL/min; the injection volume of the sample 20 μL; column temperature 30 °C; and detection wavelength 282 nm. Under the above conditions, the retention time of DZ was 3.1 min. The limit of detection (LOD) and limit of quantification (LOQ) for DZ in the soil samples were 0.01 and 0.05 mg/kg, respectively.
2.7. Concentration of MITC Produced by DZ Degradation in Soil
The water content of Shunyi soil samples was adjusted to 20% or 30%. Exactly 20.0 g of soil was added into a 50 mL headspace vial. DZ with different particle sizes was added to each vial sufficient to achieve the label dosage rate used commercially in China of 214.3 mg/kg. The vials were incubated at 25 °C for 4, 8, 12, 24, 48, 72, 120, 168, 240, 360 and 480 h. Three soil samples were randomly selected at each sampling time and immediately stored in −80 °C refrigerator for subsequent MITC extraction and concentration determination.
20 mL of ethyl acetate and 10 g of anhydrous sodium sulfate were immediately added to each vial and then re-sealed. The vials were shaken at 2500 rpm for 1 h and then left to stand for 30 min ambient temperature.
Approximately 1.5 mL of supernatant was added to a 2 mL GC vial using a syringe and 0.22 μm nylon syringe filter. MITC in ethyl acetate was analyzed using gas chromatography-mass spectrometry (GC-MS, Agilent Technologies Inc., USA) equipped with 7693A Automatic Liquid Sampler (Agilent Technologies Inc., USA) and an HP-5MS column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies Inc., USA). Helium (99.9999% purity) was used as carrier gas at a flow rate 1.5 mL/min. The inlet temperature, ion source, and transfer line temperatures were set at 250, 230, and 180 °C, respectively. One μL of sample was injected in a 20:1 split mode. The temperature of the oven was 50 °C and the operation time was 5 min. The electron ionization mode was set at 70 eV. MITC was detected at both m/z 45 and m/z 73 but quantified at m/z 73. Under the above conditions, the retention time of MITC was 3.2 min.
2.8. Data Analysis and Statistical Reporting
The degradation of DZ with different particle sizes was fitted to the first-order kinetic equation:[
22]
where
Ct (mg/kg) is the concentration of DZ at different sampling times (h);
C0 (mg/kg) is the initial concentration of DZ;
k is the first-order kinetic constant (h
−1);
t was the sampling time (h).
The degradation half-life (
t1/2) is the time required for a pesticide to degrade to half of its initial concentration:
DZ degradation data were fitted using Origin 2018 software (version 8.0, OriginLab Corporation, 2018). Statistical differences between relevant data sets were analyzed using SPSS 26.0 software (IBM Corporation, 2019).
3. Results
3.1. Effects of Soil Moisture on DZ Degradation with Different Particle Sizes
The degradation rates of DZ with different particle sizes with various water content were shown in
Figure 1. Half-life values of DZ with different particle sizes in soil conformed to the first-order degradation kinetics with water content of 15%, 20% and 30% and temperature of 25 °C (Shunyi: r
2 = 0.79 to 0.99; Wenshan: r
2 = 0.69 to 1.00; Suihua: r
2 = 0.61 to 1.00) (
Table 2,
Table 3 and
Table 4). The DZ degradation rate increased as the soil water content increased (30% > 20% > 15%), which was independent of the particle size of DZ and soil type. For example, 100–300 μm of DZ in Shunyi soil: 30% (5.96 h) >20% (24.70 h) >15% (160.05 h).
At the same soil moisture content, the larger the DZ particle size, the longer the DZ degradation half-life. In all soils, the half-life of >400 μm DZ was the longest, while the half-life of <100 μm DZ was the shortest. In Shunyi soil, when the water content was 30%, the degradation rate of DZ with <100 μm, 100–300 μm, 300–400 μm, and >400 μm were increased by 2.5, 26.9, 24.5 and 23.7 times, respectively, compared with 15% water content.
3.2. Effects of Soil Temperature on DZ Degradation with Different Particle Sizes
Figure 2 showed the effect of soil temperature on the degradation of DZ with different particle sizes. From the figures, it can see that the degradation of DZ was greatly affected by soil temperature, and the degradation of DZ with different particle sizes all conformed to the first-order kinetic equation (Shunyi: r
2 = 0.87 to 1.00; Wenshan: r
2 = 0.55 to 1.00; Suihua: r
2 = 0.53 to 1.00) (
Table 5,
Table 6 and
Table 7). The DZ degradation rate in each DZ particle size increased as the soil temperature increased, and without exception, the degradation half-life increased as the DZ particle size increased.
When the soil temperature was 4 °C, the degradation rate of DZ was very slow in all DZ particle size and soil types. In addition, DZ was not degraded in Wenshan soil at 4 °C in all DZ particle size (
Figure 2B). When the soil temperature increased from 4 °C to 37 °C, the DZ degradation rate with all particle sizes in Shunyi, Wenshan and Suihua soil increased by 3.2 to 23.6 times, 7.9 to 26.1 times and 15.1 to 26.7 times, respectively (
Figure S2).
3.3. Effects of Soil Type on DZ Degradation with Different Particle Sizes
The degradation half-life of DZ with different particle size in Shunyi, Wenshan, and Suihua soil type held at 25 °C and 20% soil moisture content has shown in
Figure 3. The first-order kinetic equation was used to fit the degradation half-life of DZ from soil sources, Shunyi: r
2 = 0.97 to 0.98; Wenshan: r
2 = 0.91 to 0.99; Suihua: r
2 = 0.80 to 0.96. On the whole, regardless of soil type, the degradation rate of DZ with large particle size in soil was slow and the degradation half-life was longer. The degradation rate of DZ with different particle sizes in Shunyi soil was significantly higher (
t1/2 = 2 h (<100 μm); 18.54 h (100–300 μm); 27.09 h (300–400 μm); 101.07 h (>400 μm)) than that in Wenshan and Suihua soil type. Notably, when the particle size of DZ was <100 μm, the degradation half-life of DZ in Wenshan soil (80.83 h) was significantly higher than that in Suihua soil (13.69 h). However, in the other diameter ranges, the DZ degradation rate in Wenshan soil was equivalent to (100–300 μm: Wenshan (964.97 h) ≈ Suihua (970.52 h)) or significantly greater (300–400 μm: Wenshan (930.83 h) < Suihua (1466.7 h); >400 μm: Wenshan (1835.76 h) < Suihua (3168 h)) than Suihua soil.
3.4. Correlation between Soil Physicochemical Properties and the Degradation Half-Life of DZ
Interestingly, there was no significant correlation between any of the soil physicochemical properties and
t1/2 (100–300 μm), and there was also no significant correlation of NH
4+-N concentration with DZ degradation half-life values in any of the DZ diameter ranges tested (r = −0.114–0.235,
p = 0.508–0.771) (
Figure 4,
Table S1). The concentration of NO
3−-N, AP and AK had significant positive correlations with
t1/2 (<100 μm) (r = 0.798–0.926,
p = 0.00306–0.0003) and significant negative correlations with
t1/2 (300–400 μm) (r = −(0.788–0.949),
p = 0.0116–9.20 × 10
−5) and
t1/2 (>400 μm) (r = −(0.796–0.955),
p = 0.0103–6.08 × 10
−5).
Soil organic matter had a significant negative correlation with t1/2 (<100 μm) (r = −0.715, p = 0.0304), but a significant positive correlation with t1/2 (300-400 μm) (r = 0.700, p = 0.0357) and t1/2 (>400 μm) (r = 0.708, p = 0.0329).
On the contrary, pH was positively correlated with t1/2 (<100 μm) and negatively correlated with t1/2 (300–400 μm) and t1/2 (>400 μm), although the correlation in the latter two particle size was not significant. Silt content had a significant positive correlation with t1/2 (<100 μm) (r = 0.856, p = 3.19 × 10−3), a significant negative correlation with t1/2 (300–400 μm) (r = −0.851, p = 3.61 × 10−3) and t1/2 (>400 μm) (r = −0.859, p = 3.61 × 10−3), while sandy soil was the opposite.
3.5. Concentration of MITC Produced by DZ with Different Particle Sizes in Shunyi Soil
Shunyi soil at 25 °C produced different MITC emission profiles when held at moisture content values of 20 or 30% and fumigated with DZ containing different granule diameter ranges (
Figure 5). At 20% soil moisture content, DZ with <100 μm degraded more rapidly than larger diameter granules. The MITC concentration at 4 h was 73.5 mg/kg, peaked at 12 h at 108.0 mg/kg and then declined gradually. The MITC peaks from DZ containing 100–300 μm, 300–400 μm and >400 μm diameter granules occurred much later at 48 h (63.7 mg/kg), 72 h (48.5 mg/kg) and 120 h (45.5 mg/kg), respectively.
At 30% soil moisture content and except for >400 μm, the MITC concentration produced by DZ with <100μm, 100–300 μm, 300–400 μm at 4 h were similar being 36.4 mg/kg, 35.1 mg/kg, 37.2 mg/kg, respectively. DZ with 100–300 μm produced the highest concentration of MITC at 24 h (102.2 mg/kg), while DZ with 300–400 μm and >400 μm reached the peak of MITC at 12 h (91.1 mg/kg) and 48 h (85.1 mg/kg), respectively. However, DZ with <100 μm peaked at 78.6 mg/kg at 72 h, which was significantly lower than the other three particle sizes. After 480 h, the concentration of MITC produced by DZ with different particle sizes in Shunyi soil tended to be slightly higher at 30 than 20% water content.
4. Discussion
The distribution of fumigant in gas and liquid phase, adsorption on soil particles, chemical and biological degradation are affected by soil moisture, soil type, organic matter and temperature [
9,
23,
24]. Dazomet (DZ) is a solid particle, in order to ensure that DZ can be fully and effectively used, it is very important to find the appropriate DZ particle size for different soil conditions according to local conditions.
Soil moisture content is critical to the control effect of fumigant, because fumigants must be present in the soil solution around the pest organisms to be effective, and soil water content also has an effect on soil microbial degradation of fumigants [
25]. Fang et al. [
13] showed that the degradation rate of DZ in aqueous solution was 5.5–22 times faster than in soil. Therefore, hydrolysis plays a leading role in the degradation of DZ, the degradation products mainly include methyl isothiocyanate (MITC), formaldehyde and other compounds (
Figure S3) [
26,
27]. Therefore, the influence of soil water content on the fumigation effect of DZ is significant. Our results showed that DZ degradation rate was positively correlated with increased soil water content (
Figure 1). which was consistent with Fang et al. [
13] who reported that when the soil water content in Beijing and Zhejiang soils increased from 10% to 30% moisture content, the DZ degradation rate increased by 16 and 36 times, respectively. They also reported that the degradation rate of DZ in aqueous solution was 5.5–22 times faster than in soil. Guo et al. [
28] also reported that the degradation of 1,3-D was dominated by hydrolysis, when the soil water content was increased, the soil adsorption capacity of 1,3-D was inhibited and the dissolution of 1,3-D in water was increased. Liu et al. [
10] increased the soil water content of Suihua soil from 3% to 30% and reported that AITC degradation rate increased significantly, even at low temperature of only 4 °C.
Research on MITC emissions reported that an increase of soil water content increased soil porosity, reduced the ability of soil to adsorb fumigant, increased the concentration of fumigant in the liquid phase, expanded the contact area between fumigant and soil and microorganisms, and accelerated the degradation of the fumigant [
29,
30]. Their results also helped to explain our findings that the maximum concentration of MITC produced by DZ with different particle sizes (except <100μm) in soil with 30% water content than 20%, and that MITC peaked earlier at 30% than at 20% soil moisture content. Gan et al. [
25] also found that elevated soil moisture content was critical for fumigant efficacy as the water promotes contact of the fumigant with pests.
It can be seen that the DZ degradation rate in each DZ particle size range increased as the soil temperature increased. Our results are consistent with previous research that showed temperature is important for determining the rate of many chemical and biological reactions. The degradation of fumigants was reported to be governed by soil temperature [
25,
31]. As early as 1896, Van’t Hoff showed that a temperate increase of 10 °C increases a chemical reaction rate by 2–3 times, which was called Van’t Hoff rule [
32]. Gan et al. [
25] demonstrated that when the soil temperature increased to 30 °C, the degradation rate of a fumigant increased by 7 times compared to 4 °C. Our research showed that the degradation rate of DZ with different particle sizes in the three soil types increased as soil temperature increased, which was consistent with Fang et al. [
13] who showed that when the temperature increased from 5 °C to 35 °C, the degradation rate of DZ in Beijing soil increased by 4.6 times. Han et al. [
33] reported when the temperature increased from 18 °C to 25 °C, the degradation rate of DMDS increased by 2.6 times. However, Ren et al. [
17] showed that the water environment temperature increased from 4 °C to 35 °C, and the half-life of DZ decreased by 4.7–6.6 times, which may be due to the temperature sensitivity of DZ degradation.
Increasing the temperature, when combined with 20 or 30% soil moisture content in our research, would have promoted DZ’s movement from the liquid to the gas phase. Residues of DZ in the soil decreased as the degradation rate increased. Increasing the temperature increases Henry’s law constant (
KH) which makes it more likely for a fumigant to move from an aqueous to the vapor phase. Tang et al. [
34] showed that the
KH of DMDS changed from 0.0332 to 0.0827 when the temperature increased from 15 to 35 °C, indicating that the DMDS had entered the vapor phase and could more easily diffuse through soil.
Many studies have shown that soil type influences the fate of pesticides in soil [
35,
36]. Our research showed that the DZ degradation half-life was different in Shunyi, Wenshan and Suihua soils fumigated different DZ particle sizes (
Figure 4). Our results were consistent with Han et al. [
33] who reported that the degradation half-life of DMDS in 10 soil types varied from 0.75 to 7.88 d. Chellemi et al. [
37] reported that the degradation half-life of 20 mg/kg DMDS in Tifton loamy sand and Dothan sandy loam was varied from 1to1.6 d at 25 °C, respectively. Our research showed that although NH
4+-N had no significant correlation with the degradation of DZ, NO
3−-N had a significant negative correlation with the degradation of
t1/2 (300–400 μm) and
t1/2 (>400 μm) DZ particle size, which was consist with Borek et al. [
38] who reported that there was negative correlated between the rate of AITC degradation and available nitrogen.
Many studies have shown that soil pH also plays an important role in the degradation of fumigants. The results had shown that the degradation half-life of DZ decreased with the increase of pH [
16,
33,
39]. Although DZ can be hydrolyzed in both acidic and alkaline environments, Dungan et al. [
40] showed that the degradation of DZ mainly occurred in alkaline environments. In our results, the pH of Shunyi soil was 8.25, which was higher than Wenshan and Suihua soil, but the degradation half-life was the lowest among the three soils. Qin et al. [
41] showed that DZ contained an isolated amine nitrogen only combined with alkyl, when the pH of soil or water environment was <9, DZ may be more likely to be protonated and hydrolyzed. Organic compounds degrade faster when protonated because the electron density deviates from the central carbon atom when protons bind. Consolazio et al. [
24] also showed that the hydrolysis of DZ was caused by the attack of hydroxide (OH
−) on amine group.
Our results showed that soil organic matter had a significant negative correlation with
t1/2 (<100 μm), but a significant positive correlation with
t1/2 (300–400 μm) and
t1/2 (>400 μm). Previous research reported that increased soil organic matter content accelerated the degradation rate of a fumigant [
38], While Fang et al. [
13] showed that the addition of organic manure amendments inhibited the degradation rate of DZ. The degradation of DZ is mainly dominated by abiotic degradation process [
13] and organic matter can adsorb fumigant, which also affects the degradation of fumigant in soil. Gimsing et al. [
42] considered that isothiocyanate was readily absorbed by soil organic matter. Tang et al. [
34] reported that the higher the content of soil organic matter, the stronger the adsorption of fumigant, which prevented the rapid transition of the fumigant to liquid phase. Therefore, we speculated that <100 μm DZ rapidly dissolved in water almost immediately after coming into contact with the soil, thereby reducing the opportunity for its adsorption by organic matter, while 300–400 μm and >400 μm DZ took longer time to dissolve in water, resulting in greater opportunity for adsorption by organic matter. This might also provide an explanation for the longer degradation time in the larger DZ granule diameter ranges in Wenshan than in Suihua soil.
From our results, it can see that the particle size of DZ also had a great influence on the degradation rate. Generally speaking, DZ particle in the larger size degraded more slowly than those in the smaller size, and this phenomenon did not change with soil type, soil temperature and soil moisture content. The specific surface area (SSA) of DZ with different particle size was listed in
Table S2. The larger the diameter of DZ particles, the smaller their SSA. <100 μm DZ had a large SSA (3.21 m
2/g), therefore, we speculated that enabling granules in that range to be rapidly saturated by water which maximized the DZ degradation rate. However, compared with <100 μm, the SSA of DZ with >100 μm was smaller (100–300 μm: 0.79 m
2/g, 300–400 μm: 0.0059 m
2/g, >400 μm: −0.071 m
2/g), in the process of being wetted by water, it was also affected by soil adsorption, so the degradation rate slowed down.
Although individual physicochemical component in the soil has greater or lesser impact on the rate of degradation of a fumigant, we acknowledge that the soil environment is complex, resulting in a fumigant degradation rate being determined by many factors acting together, rather than individual factors acting alone. The distribution of fumigant in gas and liquid phase, adsorption on soil particles, chemical and biological degradation are affected by soil moisture, soil type, organic matter and temperature [
9,
23,
24].
5. Conclusions
Our research investigated DZ’s degradation rate when DZ produced in four particle size was used to fumigate three different soil types at three different moisture content levels and four temperatures. The elevated temperatures and water content increased the DZ degradation rate in all particle size. Low temperatures and soil water content had the greatest impact on the rate of DZ degradation in the largest particle size. MITC produced from DZ in all particle size peaked higher and earlier at 30 than at 20% water content in Shunyi soil. In addition, 100–300 μm DZ produced the highest concentration of MITC in soil with 30% water content.
We found that there was no significant correlation between any of the soil physicochemical properties and t1/2 (100–300 μm). NO3−-N, available phosphorus (AP), available potassium (AK), pH and silt content in the soil all had significant positive correlations with the DZ degradation rate when fumigated with DZ < 100 μm, but the DZ degradation rate was significantly negatively correlated when fumigated with DZ with 300–400 μm and >400 μm. organic matter (OM) and sand content were significant positive correlations when fumigated with DZ with 300–400 μm and >400 μm. The soil environment is complex. It should comprehensively evaluate its impact on the degradation of DZ according to the actual situation.
DZ’s control of soil-borne disease mainly depends on the release of MITC from the DZ particle when they come into contact with moisture. DZ is a solid particle, and its degradation is affected by many interconnected factors in the soil environment. From our results, we believe the most important factors affecting MITC emissions from DZ are soil water content, soil temperature, DZ granule diameter range and soil type. These factors should be taken into consideration prior to applying DZ to soil in order to maximize its efficacy and minimize the risk of phytotoxicity. In Shunyi soil, for example, we advise selecting a warm day of about 25 °C, using DZ produced with a granule diameter range of 100–300 μm, using a dazomet applicator for even distribution and spread of granules, and adding sufficient water after fumigation to bring the soil to about 30% water content. These conditions will ensure that DZ does not degrade too fast and that sufficient MITC is generated to control pests in the soil and reduce phytotoxicity. To sum up, our research was mainly carried out in the laboratory at present, and we will verify our results in the field in future research and produce and transport appropriate DZ particle size for different regions.