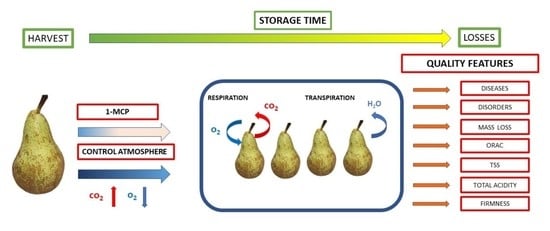
Effect of Storage Conditions on Storability and Antioxidant Potential of Pears cv. ‘Conference’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Storage Conditions
- Normal atmosphere (NA), temp. −1 °C.
- Normal atmosphere (NA), + 1-MCP, temp. −1 °C.
- Controlled atmosphere (CA) 2% O2 + 1% CO2, temp. −1 °C.
- Controlled atmosphere (CA) 2% O2 + 1% CO2, + 1-MCP, temp. −1 °C.
2.3. Quality Measurements
- Loss of fruit mass was measured in each stored box. Ten pears were numbered and weighed with an accuracy of 0.1 g before and after each month of storage. The mass loss is shown as a percentage of the initial mass.
- Firmness was measured using a Fruit Tester 327 EFFEGI FT327 penetrometer (Facchini srl, Alfonsine, Italy), mounted on a stand. The maximum penetration force of a probe of 8 mm in length and 11 mm in diameter, applied to a small area with skin removed, on two opposite sides of the fruit, was recorded.
- Total soluble solids (TSS) were determined using an ATAGO PAL-1 digital refractometer with automatic temperature compensation (Atago, Tokyo, Japan). The results were shown as an average of nine repetitions per sample and expressed as percentage values.
- Titratable acidity (TA): titration with 1n NaOH to 8.1 pH, mval 100 mL−1; the results were expressed as mmol of malic acid per kg of fresh mass.
- Starch pattern during harvest was determined with Lugol’s iodine (measured according to a 10-point scale where 10 means no starch on the pear cross section) [36].
- The Streif index is a combination of firmness (F), soluble solids content (R) and starch index (S) according the formula:Index = F/RS
2.4. Measurement of Antioxidant Capacity
2.5. Incidence of Diseases and Disorders
2.6. Economic Viability
2.7. Statistical Analysis
3. Results and Discussion
3.1. Rootstock Effect
3.2. Mass Loss
3.3. Changes in Quality Parameters during Storage
3.3.1. Total Soluble Solids, Total Acidity and TSS/TA Ratio
3.3.2. Oxygen Radical Absorbance Capacity (ORAC)
3.4. Revenue Differences Related to Differences in Storage Technology
3.5. Incidence of Diseases and Disorders
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. 2020. Available online: www.fao.org (accessed on 20 December 2020).
- European Commission Directorate-General For Agriculture And Rural Development 2019. Brussels, DDG3.G2/JG/Rr (2021) 3000924. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/key_policies/documents/cdg-horticulture-olives-spirits-2019-03-18-minutes_en.pdf (accessed on 5 May 2021).
- Jackson, J.E. The Biology of Apples and Pears; The Biology of Horticultural Crops; Cambridge University Press: Cambridge, UK, 2009; ISBN 978-0-521-38018-8. [Google Scholar]
- Saquet, A.A. Storage of Pears. Sci. Hortic. 2019, 246, 1009–1016. [Google Scholar] [CrossRef]
- Verlinden, B.E.; de Jager, A.; Lammertyn, J.; Schotsmans, W.; Nicolai, B.M. PH—Postharvest Technology: Effect of Harvest and Delaying Controlled Atmosphere Storage Conditions on Core Breakdown Incidence in ‘Conference’ Pears. Biosyst. Eng. 2002, 83, 339–347. [Google Scholar] [CrossRef]
- Chiriboga, M.-A.; Saladié, M.; Giné Bordonaba, J.; Recasens, I.; Garcia-Mas, J.; Larrigaudière, C. Effect of Cold Storage and 1-MCP Treatment on Ethylene Perception, Signalling and Synthesis: Influence on the Development of the Evergreen Behaviour in ‘Conference’ Pears. Postharvest Biol. Technol. 2013, 86, 212–220. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Antkowiak, W. Quality Features of Parthenocarpic Pears Collected from Trees Grown on Different Rootstocks. Acta Sci. Pol.-Hortorum Cultus 2015, 14, 69–82. [Google Scholar]
- Bertolini, P.; Bottardi, S.; Folchi, A.; Dalla Rosa, M. Effect of Controlled Atmosphere Storage on the Physiological Disorders and Quality of Conference Pears. Ital. J. Food Sci. Italy 1997, 9, 303–312. [Google Scholar]
- Wenneker, M.; Thomma, B.P.H.J. Latent Postharvest Pathogens of Pome Fruit and Their Management: From Single Measures to a Systems Intervention Approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef] [Green Version]
- North, M.S.; de Kock, K.; Booyse, M. Effect of Rootstock, Harvest Date and Storage Time on ‘Forelle’ Pear Fruit Quality after Cold Storage. Acta Hortic. 2012, 491–497. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010; ISBN 1-4398-0267-X. [Google Scholar]
- Kader, A.A. Postharvest Technology of Horticultural Crops; University of California: Berkeley, CA, USA, 1992. [Google Scholar]
- Villalobos-Acuña, M.; Mitcham, E.J. Ripening of European Pears: The Chilling Dilemma. Postharvest Biol. Technol. 2008, 49, 187–200. [Google Scholar] [CrossRef]
- Porritt, S.W. The Effect of Temperature on Postharvest Physiology and Storage Life of Pears. Can. J. Plant Sci. 1964. [Google Scholar] [CrossRef]
- Moya-León, M.A.; Vergara, M.; Bravo, C.; Montes, M.E.; Moggia, C. 1-MCP Treatment Preserves Aroma Quality of ‘Packham’s Triumph’ Pears during Long-Term Storage. Postharvest Biol. Technol. 2006, 42, 185–197. [Google Scholar] [CrossRef]
- Lara, I.; Miró, R.M.; Fuentes, T.; Sayez, G.; Graell, J.; López, M.L. Biosynthesis of Volatile Aroma Compounds in Pear Fruit Stored under Long-Term Controlled-Atmosphere Conditions. Postharvest Biol. Technol. 2003, 29, 29–39. [Google Scholar] [CrossRef]
- Wright, A.H.; Delong, J.M.; Arul, J.; Prange, R.K. The Trend toward Lower Oxygen Levels during Apple (Malus × Domestica Borkh) Storage. J. Hortic. Sci. Biotechnol. 2015, 90, 1–13. [Google Scholar] [CrossRef]
- Weber, A.; Brackmann, A.; Both, V.; Pavanello, E.P.; de Oliveira Anese, R.; Thewes, F.R.; Weber, A.; Brackmann, A.; Both, V.; Pavanello, E.P.; et al. Respiratory Quotient: Innovative Method for Monitoring ‘Royal Gala’ Apple Storage in a Dynamic Controlled Atmosphere. Sci. Agric. 2015, 72, 28–33. [Google Scholar] [CrossRef]
- Veltman, R.H.; Verschoor, J.A.; van Dugteren, J.H.R. Dynamic Control System (DCS) for Apples (Malus Domestica Borkh. Cv ‘Elstar’): Optimal Quality through Storage Based on Product Response. Postharvest Biol. Technol. 2003, 27, 79–86. [Google Scholar] [CrossRef]
- Lum, G.B.; Shelp, B.J.; DeEll, J.R.; Bozzo, G.G. Oxidative Metabolism Is Associated with Physiological Disorders in Fruits Stored under Multiple Environmental Stresses. Plant Sci. 2016, 245, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Streif, J.; Saquet, A.A.; Xuan, H. CA-Related Disorders of Apples and Pears. Acta Hortic. 2003, 223–230. [Google Scholar] [CrossRef]
- Ekman, J.H.; Clayton, M.; Biasi, W.V.; Mitcham, E.J. Interactions between 1-MCP Concentration, Treatment Interval and Storage Time for ‘Bartlett’ Pears. Postharvest Biol. Technol. 2004, 31, 127–136. [Google Scholar] [CrossRef]
- Hendges, M.V.; Neuwald, D.A.; Steffens, C.A.; Vidrih, R.; Zlatić, E.; do Amarante, C.V.T. 1-MCP and Storage Conditions on the Ripening and Production of Aromatic Compounds in Conference and Alexander Lucas Pears Harvested at Different Maturity Stages. Postharvest Biol. Technol. 2018, 146, 18–25. [Google Scholar] [CrossRef]
- Watkins, C.B. The Use of 1-Methylcyclopropene (1-MCP) on Fruits and Vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Spotts, R.A.; Sholberg, P.L.; Randall, P.; Serdani, M.; Chen, P.M. Effects of 1-MCP and Hexanal on Decay of d’Anjou Pear Fruit in Long-Term Cold Storage. Postharvest Biol. Technol. 2007, 44, 101–106. [Google Scholar] [CrossRef]
- Chen, P.M.; Spotts, R.A. Changes in Ripening Behaviors of 1-MCP-Treated ‘d’Anjou’ Pears After Storage. Int. J. Fruit Sci. 2005, 5, 3–18. [Google Scholar] [CrossRef]
- Golding, J.B.; Singh, S.P. Use of 1-MCP in the Storage Life Extension of Fruit. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-08-100596-5. [Google Scholar]
- Silva, F.J.P.; Gomes, M.H.; Fidalgo, F.; Rodrigues, J.A.; Almeida, D.P.F. Antioxidant Properties and Fruit Quality During Long-Term Storage of ‘Rocha’ Pear: Effects of Maturity and Storage Conditions. J. Food Qual. 2010, 33, 1–20. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupińska, A.; Kruczyńska, D.E. Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus Communis L.) Fruits. Molecules 2020, 25, 4444. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durazzo, A. Study Approach of Antioxidant Properties in Foods: Update and Considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ctifl (Centre Technique Interprofessionnel Des Fruits et Légumes) Code Amidon Pomme (Starch Conversion Chart for Apples). Available online: http://www.ctifl.fr/Pages/Kiosque/DetailsOuvrage.aspx?idouvrage=819 (accessed on 20 December 2020).
- Łysiak, G. The Sum of Active Temperatures as a Method of Determining the Optimum Harvest Date of ‘Sampion’and ‘Ligol’Apple Cultivars. Acta Sci. Pol.-Hortorum Cultus 2012, 11, 3–13. [Google Scholar]
- OECD Organisation for Economic Co-Operation and Development. International Standards for Fruits and Vegetables, Pears. Available online: https://www.oecd-ilibrary.org/docserver/87244e04-en-fr.pdf?expires=1616519112&id=id&accname=guest&checksum=32A0D385371504C147657DAE88E6A245 (accessed on 5 May 2021).
- Brookfield, P.; Murphy, P.; Harker, R.; MacRae, E. Starch Degradation and Starch Pattern Indices; Interpretation and Relationship to Maturity. Postharvest Biol. Technol. 1997, 11, 23–30. [Google Scholar] [CrossRef]
- Westwood, M.N. Temperate-Zone Pomology: Physiology and Culture, 3rd ed.; Timber Press. Inc.: Portland, OR, USA, 1993; ISBN 0-88192-253-6. [Google Scholar]
- Łysiak, G.P.; Kurlus, R. Rootstock Effect on Optimum Harvest Date and Storability of Two Apple Cultivars. In Proceedings of the Proc. Int. Conf. Fruit Production and Fruit Breeding, Tartu, Estonia, 12–13 September 2000; pp. 12–13. [Google Scholar]
- Streif, J. Optimum Picking Date for Cox Orange Apples Grown in Bodensee Region. Workshop Optim. Harvest Date 1992, 1, 2–5. [Google Scholar]
- Łysiak, G. The Determination of Harvest Index of Šampion Apples Intended for Long Storage. Acta Sci. Pol. Hortorum Cultus 2011, 10, 273–282. [Google Scholar]
- Mendes da Silva, T.; Torello Marinoni, D.; Peano, C.; Roberta Giuggioli, N. A New Sensory Approach Combined with a Text-Mining Tool to Create a Sensory Lexicon and Profile of Monovarietal Apple Juices. Foods 2019, 8, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullaj, E. Chapter 8—Rootstocks for Improved Postharvest Quality of Fruits: Recent Advances. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 189–207. ISBN 978-0-12-809807-3. [Google Scholar]
- Stern, R.A.; Doron, I. Performance of ‘Coscia’ Pear (Pyrus Communis) on Nine Rootstocks in the North of Israel. Sci. Hortic. 2009, 119, 252–256. [Google Scholar] [CrossRef]
- Murayama, H.; Takahashi, T.; Honda, R.; Fukushima, T. Cell Wall Changes in Pear Fruit Softening on and off the Tree. Postharvest Biol. Technol. 1998, 14, 143–149. [Google Scholar] [CrossRef]
- Saquet, A.A. Storability of ‘Conference’ Pear Under Various Controlled Atmospheres. Erwerbs-Obstbau 2018, 60, 275–280. [Google Scholar] [CrossRef]
- Torregrosa, L.; Echeverria, G.; Illa, J.; Giné-Bordonaba, J. Ripening Behaviour and Consumer Acceptance of ‘Conference’ Pears during Shelf Life after Long Term DCA-Storage. Postharvest Biol. Technol. 2019, 155, 94–101. [Google Scholar] [CrossRef]
- Wawrzyńczak, A.; Rutkowski, K.P.; Kruczyńska, D.E. Changes in Fruit Quality in Pears during CA Storage. J. Fruit Ornam. Plant Res. 2006, 14, 77–84. [Google Scholar]
- Watkins, C.B.; Nock, J.F.; Whitaker, B.D. Responses of Early, Mid and Late Season Apple Cultivars to Postharvest Application of 1-Methylcyclopropene (1-MCP) under Air and Controlled Atmosphere Storage Conditions. Postharvest Biol. Technol. 2000, 19, 17–32. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Michalska-Ciechanowska, A.; Wojdyło, A. Postharvest Changes in Phenolic Compounds and Antioxidant Capacity of Apples Cv. Jonagold Growing in Different Locations in Europe. Food Chem. 2020, 310, 125912. [Google Scholar] [CrossRef]
- Charles, M.; Aprea, E.; Gasperi, F. Factors Influencing Sweet Taste in Apple. In Sweeteners; Merillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–22. ISBN 978-3-319-26478-3. [Google Scholar]
- Vilaplana, R.; Valentines, M.C.; Toivonen, P.; Larrigaudière, C. Antioxidant Potential and Peroxidative State of ‘Golden Smoothee’ Apples Treated with 1-Methylcyclopropene. J. Am. Soc. Hortic. Sci. 2006, 131, 104–109. [Google Scholar] [CrossRef]
- Larrigaudiere, C.; Pintó, E.; Lentheric, I. Oxidative Behaviour of Conference Pears Stored in Air and Controlled-Atmosphere Storage. Acta Hortic. 2003, 355–360. [Google Scholar] [CrossRef]
- Florkowski, W.; Shewfelt, R.L.; Brueckner, B.; Prussia, S.E. Postharvest Handling. A Systems Approach, 3rd ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-408137-6. [Google Scholar]
- Nótári, M.; Ferencz, Á. The Harvest and Post-Harvest of Traditional Pear Varieties in Hungary. APCBEE Procedia 2014, 8, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Florkowski, W.J.; Łysiak, G. Quality Attribute-Price Relationship: Modernization of the Sweet Cherry Sector in Poland. Sci. J. Wars. Univ. Life Sci. SGGW Probl. World Agric. 2015, 15, 1–15. [Google Scholar] [CrossRef]
- Waelti, H.; Bartsch, J.A. Controlled atmosphere storage facilities. In Food Preservation by Modified Atmospheres; CRC Press: Boca Raton, FL, USA, 1990; pp. 373–389. ISBN 978-0-8493-6569-0. [Google Scholar]
- Rizzolo, A.; Grassi, M.; Vanoli, M. Influence of Storage (Time, Temperature, Atmosphere) on Ripening, Ethylene Production and Texture of 1-MCP Treated ‘Abbé Fétel’ Pears. Postharvest Biol. Technol. 2015, 109, 20–29. [Google Scholar] [CrossRef]
- Sardella, D.; Muscat, A.; Brincat, J.-P.; Gatt, R.; Decelis, S.; Valdramidis, V. A Comprehensive Review of the Pear Fungal Diseases. Int. J. Fruit Sci. 2016, 16, 351–377. [Google Scholar] [CrossRef]
- Saquet, A.; Almeida, D. Internal Disorders of ‘Rocha’ Pear Affected by Oxygen Partial Pressure and Inhibition of Ethylene Action. Postharvest Biol. Technol. 2017, 128, 54–62. [Google Scholar] [CrossRef]
- Sutton, T.B.; Aldwinckle, H.S.; Agnello, A.M.; Walgenbach, J.F. (Eds.) Compendium of Apple and Pear Diseases and Pests, 2nd ed.; The American Phytopathological Society: St. Paul, MN, USA, 2014; ISBN 978-0-89054-433-4. [Google Scholar]
- Xie, X.; Song, J.; Wang, Y.; Sugar, D. Ethylene Synthesis, Ripening Capacity, and Superficial Scald Inhibition in 1-MCP Treated ‘d’Anjou’ Pears Are Affected by Storage Temperature. Postharvest Biol. Technol. 2014, 97, 1–10. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Einhorn, T.C. Postharvest Physiology, Storage Quality and Physiological Disorders of ‘Gem’ Pear (Pyrus Communis L.) Treated with 1-Methylcyclopropene. Sci. Hortic. 2018, 240, 631–637. [Google Scholar] [CrossRef]
- Hendges, M.V.; Steffens, C.A.; Espindola, B.P.; Amarante, C.V.T.; Neuwald, D.A.; Kittemann, D. 1-MCP Treatment Increases Internal Browning Disorders in ‘Alexander Lucas’ Pears Stored under Controlled Atmosphere. Acta Hortic. 2015, 511–517. [Google Scholar] [CrossRef]
- Bautista-Baños, S. Postharvest Decay: Control Strategies; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-411568-2. [Google Scholar]
- Domingues, A.R.; Roberto, S.R.; Ahmed, S.; Shahab, M.; José Chaves Junior, O.; Sumida, C.H.; De Souza, R.T. Postharvest Techniques to Prevent the Incidence of Botrytis Mold of ‘BRS Vitoria’ Seedless Grape under Cold Storage. Horticulturae 2018, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Tomala, K.; Grzęda, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. The Effects of Preharvest 1-Methylcyclopropene (1-MCP) Treatment on the Fruit Quality Parameters of Cold-Stored ‘Szampion’ Cultivar Apples. Agriculture 2020, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Saquet, A.A.; Streif, J.; Bangerth, F. Changes in ATP, ADP and Pyridine Nucleotide Levels Related to the Incidence of Physiological Disorders in ‘Conference’ Pears and ‘Jonagold’ Apples during Controlled Atmosphere Storage. J. Hortic. Sci. Biotechnol. 2000, 75, 243–249. [Google Scholar] [CrossRef]
- Veltman, R.H.; Kho, R.M.; van Schaik, A.C.R.; Sanders, M.G.; Oosterhaven, J. Ascorbic Acid and Tissue Browning in Pears (Pyrus Communis L. Cvs Rocha and Conference) under Controlled Atmosphere Conditions. Postharvest Biol. Technol. 2000, 19, 129–137. [Google Scholar] [CrossRef]
- Jung, S.-K.; Watkins, C.B. Involvement of Ethylene in Browning Development of Controlled Atmosphere-Stored ‘Empire’ Apple Fruit. Postharvest Biol. Technol. 2011, 59, 219–226. [Google Scholar] [CrossRef]
- Lurie, S.; Watkins, C.B. Superficial Scald, Its Etiology and Control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]





| Rootstock | Streif Index | Starch Index | Firmness (N) | TSS (%) | TSS/TA | Total Acidity (% Malic Acid) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | ||||||||||||
| Q S1 | 0.09 | a 1 | 6.6 | b | 63.7 | a | 12.9 | a | 62.3 | a | 0.21 | b |
| PC | 0.10 | b | 5.4 | a | 64.7 | a | 12.9 | a | 68.6 | a | 0.19 | a |
| PD | 0.10 | b | 5.8 | ab | 63.7 | a | 13.3 | a | 65.8 | a | 0.20 | b |
| 2012 | ||||||||||||
| Q S1 | 0.08 | a | 6.5 | b | 60.8 | a | 12.0 | a | 57.9 | ab | 0.21 | a |
| PC | 0.09 | ab | 6.3 | ab | 61.8 | a | 12.4 | b | 61.6 | b | 0.20 | a |
| PD | 0.09 | b | 6.0 | a | 62.8 | a | 12.2 | ab | 57.0 | a | 0.21 | b |
| 2013 | ||||||||||||
| Q S1 | 0.06 | a | 9.2 | b | 66.7 | b | 12.4 | b | 69.3 | b | 0.18 | a |
| PC | 0.07 | a | 8.6 | a | 66.7 | b | 11.9 | a | 66.1 | a | 0.18 | a |
| PD | 0.06 | a | 8.8 | a | 64.7 | a | 12.1 | a | 67.1 | a | 0.18 | a |
| Mean | ||||||||||||
| Q S1 | 0.07 | a | 7.4 | b | 63.7 | a | 12.1 | a | 62.8 | a | 0.20 | b |
| PC | 0.08 | a | 6.8 | a | 64.7 | a | 12.4 | a | 65.4 | a | 0.19 | a |
| PD | 0.08 | a | 6.5 | a | 63.7 | a | 12.5 | a | 63.3 | a | 0.20 | b |
| Rootstock | Storage Atmosphere | 1-MCP Dose | Days of Storage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | 180 | |||||||||
| Q S1 | NA | Control | 1.82 1 | (0.31) | 3.72 | (0.32) | 5.13 | (0.21) | 6.14 | (0.23) | 7.10 | (0.54) | 7.50 | (0.42) |
| 1-MCP | 2.15 | (0.29) | 2.65 | (0.44) | 3.23 | (0.59) | 4.39 | (0.97) | 5.56 | (1.32) | 6.06 | (1.47) | ||
| CA | Control | 1.49 | (0.51) | 1.97 | (0.51) | 2.52 | (0.55) | 3.00 | (0.61) | 3.48 | (0.70) | 4.11 | (0.85) | |
| 1-MCP | 0.92 | (0.19) | 1.37 | (0.29) | 1.88 | (0.42) | 2.02 | (0.37) | 2.42 | (0.38) | 3.32 | (0.61) | ||
| PC | NA | Control | 1.52 | (0.47) | 2.69 | (0.28) | 4.74 | (0.34) | 5.60 | (0.59) | 6.24 | (0.56) | 6.77 | (0.59) |
| 1-MCP | 2.31 | (0.79) | 3.01 | (1.00) | 3.89 | (1.43) | 5.38 | (1.80) | 5.51 | (1.58) | 6.22 | (1.86) | ||
| CA | Control | 1.01 | (0.06) | 2.02 | (0.30) | 3.05 | (0.44) | 3.46 | (0.60) | 4.32 | (0.62) | 5.18 | (0.64) | |
| 1-MCP | 0.93 | (0.13) | 1.78 | (0.15) | 2.26 | (0.46) | 2.77 | (0.41) | 3.43 | (0.44) | 3.93 | (0.47) | ||
| PD | NA | Control | 1.41 | (0.23) | 2.82 | (0.31) | 4.52 | (0.42) | 5.41 | (0.55) | 6.30 | (0.55) | 6.64 | (0.58) |
| 1-MCP | 1.83 | (0.25) | 2.34 | (0.28) | 3.41 | (0.51) | 4.59 | (1.01) | 5.23 | (1.12) | 5.74 | (1.29) | ||
| CA | Control | 1.07 | (0.17) | 1.98 | (0.49) | 2.56 | (0.50) | 3.08 | (0.60) | 3.59 | (0.74) | 4.44 | (0.75) | |
| 1-MCP | 0.94 | (0.19) | 1.42 | (0.28) | 1.94 | (0.39) | 2.26 | (0.45) | 2.79 | (0.56) | 3.31 | (0.68) | ||
| Main effects 2 | ||||||||||||||
| Rootstock (A) | ** | ** | * | * | ns | ns | ||||||||
| Storage atmosphere (B) | *** | *** | *** | *** | *** | *** | ||||||||
| 1-MCP dose (C) | ns | *** | *** | *** | *** | *** | ||||||||
| Interaction | ||||||||||||||
| A × B | ns | ** | ns | ns | ** | * | ||||||||
| A × C | * | *** | ns | * | ns | ns | ||||||||
| B × C | *** | ns | ** | ns | ns | ns | ||||||||
| A × B × C | ns | * | ns | ns | ns | ns | ||||||||
| Rootstock | Storage Atmosphere | 1-MCP Dose | Days of Storage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | 180 | |||||||||
| Q S1 | NA | Control | 1.96 1 | (0.60) | 4.00 | (0.90) | 5.47 | (0.78) | 6.32 | (0.66) | 7.17 | (0.53) | 7.96 | (0.50) |
| 1-MCP | 1.94 | (0.28) | 2.78 | (0.56) | 3.78 | (0.57) | 4.42 | (0.53) | 5.13 | (0.69) | 5.98 | (0.95) | ||
| CA | Control | 1.21 | (0.29) | 1.92 | (0.27) | 2.59 | (0.31) | 2.93 | (0.25) | 3.90 | (0.43) | 4.70 | (0.44) | |
| 1-MCP | 1.23 | (0.30) | 1.68 | (0.30) | 1.99 | (0.32) | 2.75 | (0.57) | 3.26 | (0.76) | 4.23 | (0.93) | ||
| PC | NA | Control | 2.54 | (0.62) | 3.90 | (0.70) | 5.15 | (0.62) | 5.92 | (0.49) | 6.71 | (0.56) | 7.88 | (0.65) |
| 1-MCP | 1.96 | (0.43) | 2.90 | (0.76) | 4.06 | (0.62) | 4.74 | (0.58) | 5.50 | (0.84) | 6.36 | (0.89) | ||
| CA | Control | 1.15 | (0.23) | 1.89 | (0.33) | 2.49 | (0.37) | 3.01 | (0.38) | 3.79 | (0.44) | 4.62 | (0.48) | |
| 1-MCP | 1.19 | (0.13) | 1.55 | (0.20) | 1.86 | (0.24) | 2.37 | (0.33) | 2.96 | (0.45) | 3.92 | (0.59) | ||
| PD | NA | Control | 2.18 | (0.25) | 4.02 | (0.37) | 5.39 | (0.65) | 6.05 | (0.59) | 6.70 | (0.53) | 7.53 | (0.47) |
| 1-MCP | 1.84 | (0.57) | 2.55 | (0.51) | 3.63 | (0.59) | 4.38 | (0.59) | 5.10 | (0.51) | 6.06 | (0.47) | ||
| CA | Control | 1.01 | (0.14) | 1.88 | (0.34) | 2.67 | (0.53) | 3.03 | (0.66) | 3.78 | (0.77) | 4.73 | (0.81) | |
| 1-MCP | 1.17 | (0.07) | 1.55 | (0.14) | 1.85 | (0.20) | 2.36 | (0.37) | 3.09 | (0.70) | 4.10 | (0.93) | ||
| Main effects 2 | ||||||||||||||
| Rootstock (A) | ns | ns | ns | ns | ns | ns | ||||||||
| Storage atmosphere (B) | *** | *** | *** | *** | *** | *** | ||||||||
| 1-MCP dose (C) | ns | *** | *** | *** | *** | *** | ||||||||
| Interaction | ||||||||||||||
| A × B | ns | ns | ns | ns | ns | ns | ||||||||
| A × C | ns | ns | ns | ns | ns | ns | ||||||||
| B × C | ** | *** | *** | *** | *** | *** | ||||||||
| A × B × C | ns | ns | ns | * | ns | ns | ||||||||
| Rootstock | Storage Atmosphere | 1-MCP Dose | Days of Storage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | 180 | |||||||||
| Q S1 | NA | Control | 1.86 1 | (0.31) | 3.41 | (0.40) | 4.48 | (0.43) | 5.09 | (0.52) | 5.93 | (0.50) | 6.63 | (0.45) |
| 1-MCP | 1.79 | (0.52) | 2.60 | (0.51) | 3.31 | (0.50) | 4.02 | (0.44) | 4.71 | (0.43) | 5.36 | (0.45) | ||
| CA | Control | 1.10 | (0.15) | 1.61 | (0.22) | 2.12 | (0.37) | 3.02 | (0.28) | 3.69 | (0.25) | 4.09 | (0.33) | |
| 1-MCP | 0.92 | (0.17) | 1.63 | (0.60) | 1.98 | (0.57) | 2.48 | (0.71) | 2.98 | (0.86) | 3.16 | (0.60) | ||
| PC | NA | Control | 2.15 | (0.27) | 3.66 | (0.68) | 5.01 | (0.90) | 5.86 | (0.95) | 6.28 | (0.62) | 7.13 | (0.68) |
| 1-MCP | 1.93 | (0.28) | 3.29 | (0.38) | 3.67 | (0.38) | 4.51 | (0.37) | 5.26 | (0.39) | 6.33 | (0.48) | ||
| CA | Control | 1.02 | (0.13) | 1.88 | (0.18) | 2.73 | (0.23) | 3.21 | (0.30) | 4.07 | (0.28) | 4.83 | (0.38) | |
| 1-MCP | 0.92 | (0.08) | 1.82 | (0.19) | 2.16 | (0.41) | 2.53 | (0.65) | 3.02 | (0.49) | 3.88 | (0.50) | ||
| PD | NA | Control | 2.27 | (0.27) | 3.79 | (0.43) | 5.27 | (0.77) | 5.83 | (0.85) | 6.13 | (0.74) | 6.96 | (0.44) |
| 1-MCP | 1.68 | (0.61) | 2.54 | (0.77) | 3.57 | (0.45) | 4.08 | (0.56) | 4.82 | (0.58) | 5.47 | (0.64) | ||
| CA | Control | 0.98 | (0.06) | 1.67 | (0.29) | 2.37 | (0.50) | 3.07 | (0.60) | 3.62 | (0.59) | 4.03 | (0.49) | |
| 1-MCP | 0.98 | (0.06) | 1.75 | (0.54) | 1.99 | (0.81) | 2.26 | (0.72) | 2.77 | (0.85) | 3.27 | (0.99) | ||
| Main effects 2 | ||||||||||||||
| Rootstock (A) | ns | ** | ** | * | * | *** | ||||||||
| Storage atmosphere (B) | *** | *** | *** | *** | *** | *** | ||||||||
| 1-MCP dose (C) | *** | *** | *** | *** | *** | *** | ||||||||
| Interaction | ||||||||||||||
| A × B | ns | ns | ns | ns | ns | ns | ||||||||
| A × C | ns | ns | ns | ns | ns | ns | ||||||||
| B × C | ns | ns | *** | ** | ns | ns | ||||||||
| A × B × C | * | ns | ns | ns | ns | ns | ||||||||
| Storage Duration | Firmness | TSS (%) | TA (% Malic Acid) | TSS/TA | ORAC µmol TE/100 g (d.m.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N) | Monthly Loss (%) | Total Loss (%) | |||||||||||
| NA | |||||||||||||
| 0 | 64.2 1 | n | 0 | 12.6 | a | 0.21 | l | 60.0 | a | 2858.8 | d | ||
| 30 | 62.2 | mn | 3.0 | a | 3.0 | 12.9 | a–c | 0.18 | i | 73.2 | b | ||
| 60 | 55.9 | g–i | 10.1 | c | 12.8 | 13.3 | b–d | 0.15 | ef | 89.9 | c–e | ||
| 90 | 52.2 | f | 6.8 | b | 18.7 | 14.6 | g–i | 0.11 | c | 134.5 | g | ||
| 120 | 50.9 | ef | 2.4 | a | 20.7 | 14.9 | i | 0.11 | c | 135.5 | g | ||
| 150 | 43.0 | ab | 15.5 | d | 32.9 | 14.6 | g-i | 0.10 | b | 154.0 | h | ||
| 180 | 40.6 | a | 5.7 | b | 36.8 | 13.9 | ef | 0.06 | a | 231.7 | i | 2562.5 | a |
| NA + 1-MCP | |||||||||||||
| 0 | 64.2 | n | 0 | 12.6 | a | 0.21 | l | 60.0 | a | 2858.8 | d | ||
| 30 | 63.4 | n | 1.1 | a | 1.1 | 12.8 | ab | 0.19 | i-l | 67.8 | ab | ||
| 60 | 58.1 | i–k | 8.4 | c | 9.5 | 12.9 | a–c | 0.19 | i-k | 69.3 | ab | ||
| 90 | 53.5 | fg | 7.9 | b | 16.6 | 13.4 | c–e | 0.16 | f-h | 84.8 | cd | ||
| 120 | 47.6 | cd | 11.0 | e | 25.8 | 14.3 | f–i | 0.16 | e-h | 92.1 | c–e | ||
| 150 | 43.4 | b | 8.9 | d | 32.4 | 14.5 | f–i | 0.15 | e | 99.5 | e | ||
| 180 | 42.9 | ab | 1.1 | a | 33.2 | 14.7 | hi | 0.10 | bc | 147.8 | h | 2782.5 | b |
| CA | |||||||||||||
| 0 | 64.2 | n | 0 | 12.6 | a | 0.21 | l | 60.0 | a | 2858.8 | d | ||
| 30 | 64.6 | n | −0.7 | a | −0.7 | 12.5 | a | 0.18 | ij | 69.0 | ab | ||
| 60 | 62.3 | mn | 3.5 | b | 2.9 | 12.7 | a | 0.19 | i-l | 66.7 | ab | ||
| 90 | 57.8 | h–j | 7.3 | e | 10.0 | 13.1 | a–d | 0.18 | i | 74.5 | b | ||
| 120 | 54.8 | g | 5.1 | c | 14.6 | 14.1 | fg | 0.15 | e-g | 93.9 | c-e | ||
| 150 | 48.6 | de | 11.3 | f | 24.2 | 14.5 | g-i | 0.15 | e-h | 95.1 | de | ||
| 180 | 45.3 | bc | 6.7 | d | 29.3 | 14.8 | i | 0.12 | d | 123.7 | f | 2817.5 | c |
| CA + 1-MCP | |||||||||||||
| 0 | 64.2 | n | 0 | 12.6 | a | 0.21 | l | 60.0 | a | 2858.8 | d | ||
| 30 | 63.6 | n | 0.8 | b | 0.8 | 12.8 | ab | 0.21 | m | 60.6 | a | ||
| 60 | 59.4 | kl | 6.7 | f | 7.4 | 12.6 | a | 0.20 | kl | 64.1 | ab | ||
| 90 | 60.7 | lm | −2.2 | a | 5.4 | 13.1 | a–d | 0.19 | ij | 71.0 | ab | ||
| 120 | 59.1 | jk | 2.7 | c | 7.9 | 13.5 | de | 0.16 | gh | 84.0 | c | ||
| 150 | 55.3 | gh | 6.4 | e | 13.8 | 13.9 | ef | 0.17 | h | 84.7 | cd | ||
| 180 | 53.3 | fg | 3.5 | d | 16.9 | 14.3 | f–i | 0.16 | e-h | 89.4 | c-e | 2962.5 | e |
| Storage Duration | Firmness | TSS (%) | TA (% Malic Acid) | TSS/TA (N) | ORAC µmol TE/100 g (d.m.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N) | Monthly Loss (%) | Total Loss (%) | |||||||||||
| NA | |||||||||||||
| 0 | 60.6 1 | l | 0 | 12.2 | a | 0.23 | m | 53.0 | a | 2635.0 | c | ||
| 30 | 56.4 | hi | 6.9 | c | 6.9 | 12.6 | a–d | 0.20 | j–l | 64.7 | a–d | ||
| 60 | 53.5 | fg | 5.1 | a | 11.6 | 13.1 | e–h | 0.19 | h–j | 70.4 | c–f | ||
| 90 | 50.5 | de | 5.6 | b | 16.6 | 13.5 | f–i | 0.17 | f | 79.4 | gh | ||
| 120 | 46.9 | c | 7.1 | d | 22.6 | 14.0 | j–l | 0.15 | e | 93.3 | i–k | ||
| 150 | 43.7 | b | 6.9 | c | 27.9 | 14.2 | l | 0.11 | b–e | 137.4 | l | ||
| 180 | 39.8 | a | 8.9 | e | 34.3 | 13.6 | k | 0.07 | a | 194.3 | m | 2435.0 | a |
| NA + 1-MCP | |||||||||||||
| 0 | 60.6 | l | 0 | 12.2 | a | 0.23 | m | 53.0 | a | 2635.0 | c | ||
| 30 | 58.9 | jl | 2.8 | b | 2.8 | 12.4 | ab | 0.19 | i–k | 66.5 | b–e | ||
| 60 | 58.3 | i–l | 1.1 | a | 3.8 | 12.5 | a–c | 0.19 | h–j | 67.4 | b–f | ||
| 90 | 56.3 | hi | 3.4 | c | 7.1 | 13.0 | d–g | 0.18 | g–i | 71.9 | d–g | ||
| 120 | 52.0 | ef | 7.6 | d | 14.2 | 13.4 | f–i | 0.17 | f | 79.4 | gh | ||
| 150 | 47.9 | c | 7.8 | e | 20.9 | 13.9 | j–l | 0.12 | c | 122.8 | k | ||
| 180 | 44.2 | b | 7.8 | e | 27.1 | 14.3 | l | 0.11 | bc | 130.9 | l | 2537.5 | b |
| CA | |||||||||||||
| 0 | 64.2 | m | 0 | 12.2 | a | 0.23 | m | 53.0 | a | 2635.0 | c | ||
| 30 | 60.4 | l | 5.9 | d | 5.9 | 12.2 | a | 0.20 | lm | 60.9 | a–c | ||
| 60 | 58.6 | i–l | 2.9 | b | 8.7 | 12.5 | a–c | 0.19 | j–l | 65.3 | a–d | ||
| 90 | 57.4 | h–k | 2.1 | a | 10.6 | 12.8 | b–e | 0.18 | g–i | 70.7 | c–f | ||
| 120 | 55.7 | h | 2.8 | b | 13.1 | 13.2 | f–i | 0.18 | fg | 75.4 | f–h | ||
| 150 | 53.1 | fg | 4.7 | c | 17.2 | 13.6 | i–k | 0.15 | de | 92.6 | i–k | ||
| 180 | 48.9 | cd | 8.1 | e | 23.9 | 14.2 | l | 0.14 | d–g | 100.4 | j–l | 2627.5 | c |
| CA + 1-MCP | |||||||||||||
| 0 | 60.6 | l | 0 | 12.2 | a | 0.23 | m | 53.0 | a | 2635.0 | c | ||
| 30 | 60.6 | l | 0.0 | b | 0.0 | 12.2 | a | 0.21 | m | 58.8 | ab | ||
| 60 | 59.5 | kl | 1.8 | c | 1.8 | 12.3 | ab | 0.20 | l | 62.5 | a–c | ||
| 90 | 58.3 | i–l | 2.0 | d | 3.8 | 12.7 | b–e | 0.20 | j–l | 65.5 | a–d | ||
| 120 | 58.5 | i–l | −0.4 | a | 3.3 | 12.9 | c–f | 0.18 | f-h | 72.7 | d–g | ||
| 150 | 56.9 | h–j | 2.8 | e | 6.0 | 13.5 | h–j | 0.18 | g-i | 73.9 | e–h | ||
| 180 | 55.2 | gh | 3.0 | f | 8.8 | 14.0 | kl | 0.17 | fg | 81.0 | h–j | 2777.5 | d |
| Storage Duration | Firmness | TSS (%) | TA (% Malic Acid) | TSS/TA (N) | ORAC µmol TE/100 g (d.m.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N) | Monthly Loss (%) | Total Loss (%) | |||||||||||
| NA | |||||||||||||
| 0 | 65.9 1 | k | 0 | 12.1 | a | 0.18 | m | 67.5 | a | 2975.0 | c | ||
| 30 | 64.4 | i–k | 2.2 | a | 2.2 | 12.5 | a–c | 0.18 | m | 70.7 | ab | ||
| 60 | 62.8 | hi | 2.5 | b | 4.7 | 12.7 | b–d | 0.15 | h | 87.6 | cd | ||
| 90 | 56.1 | f | 10.6 | d | 14.8 | 13.6 | f | 0.13 | fg | 101.7 | ef | ||
| 120 | 49.7 | cd | 11.4 | e | 24.6 | 14.3 | gh | 0.11 | c | 130.0 | h | ||
| 150 | 45.4 | b | 8.6 | c | 31.0 | 14.8 | jk | 0.07 | b | 208.3 | i | ||
| 180 | 39.9 | a | 12.3 | f | 39.5 | 13.7 | f | 0.06 | a | 228.3 | j | 2750.0 | a |
| NA + 1-MCP | |||||||||||||
| 0 | 65.9 | k | 0 | 12.1 | a | 0.18 | m | 67.5 | a | 2975.0 | c | ||
| 30 | 64.4 | i–k | 2.2 | b | 2.2 | 12.4 | ab | 0.18 | m | 70.7 | ab | ||
| 60 | 64.1 | i–k | 0.5 | a | 2.7 | 12.4 | a–c | 0.16 | j–l | 76.8 | ab | ||
| 90 | 61.1 | h | 4.6 | c | 7.2 | 12.8 | c–e | 0.15 | h | 88.3 | cd | ||
| 120 | 55.8 | f | 8.8 | f | 15.4 | 13.6 | f | 0.12 | de | 107.7 | f | ||
| 150 | 51.4 | de | 7.9 | e | 22.0 | 14.6 | ij | 0.12 | d | 121.7 | g | ||
| 180 | 47.6 | bc | 7.3 | d | 27.7 | 14.6 | ij | 0.11 | c | 132.7 | h | 2890.8 | b |
| CA | |||||||||||||
| 0 | 65.9 | k | 0 | 12.1 | a | 0.18 | m | 67.5 | a | 2975.0 | c | ||
| 30 | 66.2 | k | −0.5 | a | −0.5 | 12.3 | ab | 0.18 | m | 69.1 | a | ||
| 60 | 63.3 | h–j | 4.4 | c | 4.0 | 12.4 | ab | 0.18 | m | 69.0 | a | ||
| 90 | 62.0 | hi | 2.1 | b | 6.0 | 12.6 | a–c | 0.16 | jk | 80.4 | bc | ||
| 120 | 57.7 | fg | 6.9 | e | 12.4 | 13.8 | fg | 0.15 | h | 93.4 | de | ||
| 150 | 52.9 | e | 8.4 | f | 19.8 | 14.7 | ij | 0.14 | g | 107.9 | f | ||
| 180 | 49.9 | cd | 5.6 | d | 24.2 | 15.1 | k | 0.13 | ef | 119.0 | g | 2990.0 | c |
| CA + 1-MCP | |||||||||||||
| 0 | 65.9 | k | 0 | 12.1 | a | 0.18 | m | 67.5 | a | 2975.0 | c | ||
| 30 | 66.3 | k | −0.6 | a | −0.6 | 12.2 | a | 0.18 | m | 68.9 | a | ||
| 60 | 64.3 | i–k | 3.0 | c | 2.4 | 12.4 | ab | 0.17 | l | 73.9 | ab | ||
| 90 | 63.3 | h–k | 1.5 | b | 3.9 | 12.5 | a–c | 0.16 | kl | 75.9 | ab | ||
| 120 | 61.4 | h | 3.1 | c | 6.9 | 13.1 | de | 0.16 | j–l | 80.6 | bc | ||
| 150 | 58.6 | g | 4.5 | d | 11.1 | 13.7 | fg | 0.16 | ij | 88.5 | cd | ||
| 180 | 55.8 | f | 4.7 | e | 15.3 | 14.1 | hi | 0.15 | hi | 96.7 | de | 3107.5 | d |
| Storage Days | NA | NA 1-MCP | CA | CA 1-MCP | Price kg in (EUR) | NA | NA 1-MCP | CA | CA 1-MCP |
| 2011/2012 (Av. Yield = 14.03 t·h−1) 1 | |||||||||
| Transpiration (%) | Value after Storage (EUR) | ||||||||
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.55 2 | 7723 3 | 7723 | 7723 | 7723 |
| 30 | 1.6 | 2.1 | 1.2 | 0.9 | 0.55 | 7600 | 7561 | 7631 | 7651 |
| 60 | 3.1 | 2.7 | 2.0 | 1.5 | 0.55 | 7485 | 7517 | 7569 | 7605 |
| 90 | 4.8 | 3.5 | 2.7 | 2.0 | 0.57 | 7659 | 7762 | 7827 | 7882 |
| 120 | 5.7 | 4.8 | 3.2 | 2.3 | 0.57 | 7585 | 7659 | 7789 | 7856 |
| 150 | 6.5 | 5.4 | 3.8 | 2.9 | 0.62 | 8119 | 8216 | 8358 | 8438 |
| 180 | 7.0 | 6.0 | 4.6 | 3.5 | 0.64 | 8382 | 8469 | 8598 | 8693 |
| Storage diseases (%) | |||||||||
| 16.0 ± 3.9 4 | 11.6 ± 3.5 | 9.1 ± 3.3 | 7.4 ± 3.5 | 6944 | 7424 | 7782 | 8026 | ||
| Value difference after storage | −779 | −299 | 59 | 303 | |||||
| 2012/2013 (Av. Yield = 11.78 t·h−1) | |||||||||
| Transpiration (%) | Value after storage (EUR) | ||||||||
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.58 | 6846 | 6846 | 6846 | 6846 |
| 30 | 2.2 | 1.9 | 1.1 | 1.2 | 0.58 | 6693 | 6714 | 6769 | 6763 |
| 60 | 4.0 | 2.7 | 1.9 | 1.6 | 0.65 | 7395 | 7490 | 7555 | 7579 |
| 90 | 5.3 | 3.8 | 2.6 | 1.9 | 0.73 | 8100 | 8230 | 8336 | 8395 |
| 120 | 6.1 | 4.5 | 3.0 | 2.5 | 0.82 | 9106 | 9260 | 9408 | 9456 |
| 150 | 6.9 | 5.2 | 3.8 | 3.1 | 0.99 | 10,892 | 11,082 | 11,247 | 11,332 |
| 180 | 7.8 | 6.1 | 4.7 | 4.1 | 1.14 | 12,361 | 12,583 | 12,778 | 12,858 |
| Storage diseases (%) | |||||||||
| 25.0 ± 3.7 | 19.9 ± 3.6 | 14.0 ± 2.7 | 9.9 ± 2.6 | 9004 | 9916 | 10,899 | 11,531 | ||
| Value difference after storage | 3070 | 4053 | 4686 | 3070 | |||||
| 2013/2014 (Av. Yield = 18.85 t·h−1) | |||||||||
| Transpiration (%) | Value after storage (EUR) | ||||||||
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.43 | 8122 | 8122 | 8122 | 8122 |
| 30 | 2.1 | 1.8 | 1.0 | 0.9 | 0.53 | 9719 | 9748 | 9824 | 9834 |
| 60 | 3.6 | 2.8 | 1.7 | 1.7 | 0.53 | 9567 | 9648 | 9756 | 9754 |
| 90 | 4.9 | 3.5 | 2.4 | 2.0 | 0.57 | 10,296 | 10,448 | 10,568 | 10,608 |
| 120 | 5.6 | 4.2 | 3.1 | 2.4 | 0.60 | 10,649 | 10,806 | 10,930 | 11,007 |
| 150 | 6.1 | 4.9 | 3.8 | 2.9 | 0.60 | 10,590 | 10,724 | 10,852 | 10,950 |
| 180 | 6.9 | 5.7 | 4.3 | 3.4 | 0.60 | 10,501 | 10,635 | 10,793 | 10,892 |
| Storage diseases (%) | |||||||||
| 29.2 ± 4.4 | 23.3 ± 3.1 | 17.9 ± 3.9 | 11.9 ± 1.8 | 7211 | 8006 | 8776 | 9550 | ||
| Value difference after storage | −911 | −115 | 654 | 1428 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysiak, G.P.; Rutkowski, K.; Walkowiak-Tomczak, D. Effect of Storage Conditions on Storability and Antioxidant Potential of Pears cv. ‘Conference’. Agriculture 2021, 11, 545. https://doi.org/10.3390/agriculture11060545
Łysiak GP, Rutkowski K, Walkowiak-Tomczak D. Effect of Storage Conditions on Storability and Antioxidant Potential of Pears cv. ‘Conference’. Agriculture. 2021; 11(6):545. https://doi.org/10.3390/agriculture11060545
Chicago/Turabian StyleŁysiak, Grzegorz P., Krzysztof Rutkowski, and Dorota Walkowiak-Tomczak. 2021. "Effect of Storage Conditions on Storability and Antioxidant Potential of Pears cv. ‘Conference’" Agriculture 11, no. 6: 545. https://doi.org/10.3390/agriculture11060545