Pulmonary Evaluation in Children with Post-COVID-19 Condition Respiratory Symptoms: A Prospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Microbiology
2.3. Pulmonary Function Testing
2.3.1. Spirometry and Exercise Challenge
2.3.2. Plethysmography and Diffusion Capacity
2.3.3. Exhaled Nitric Oxide Levels (FeNo)
2.3.4. Lung Clearance Index (LCI)
2.3.5. Methacholine Testing
2.4. Statistical Methods
3. Results
3.1. Patient Characteristics
3.1.1. Demographic and Clinical Parameters
3.1.2. Post-COVID Respiratory Symptoms and Consequences
3.2. Cardio-Pulmonary Evaluation
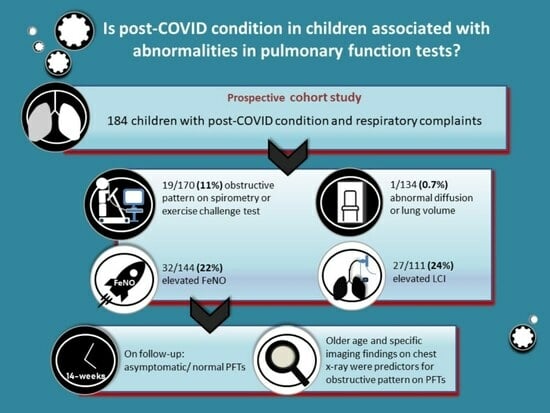
- Among patients who performed PFTs, 170/184 (92%) performed spirometry, with a mean FEV1 of 92.6% (SD 10.6%, Table 3); 19/170 (11%) patients had FEV1 < 80%, of which FEV1/FVC was <80% in three of them, one with a rheumatologic disease and two previously healthy, and one with a personal history of atopy; the latter two also had a positive ECT and all had significant reversibility post bronchodilators.
- ECT was performed by 133 children (Table 3), of whom four had a positive test; all four had complete post bronchodilator reversibility, three were previously healthy, and one had a background gastrointestinal disease; none had a history of asthma, but three had personal atopy. Eleven other children had negative ECT but a rise of ≥10% in FEV1 post bronchodilators; one had asthma and six of the remaining 10 had either a personal or familial history of asthma. All the participants who performed ECT maintained normal O2 saturations during the test.
- Following baseline spirometry with FEV1 < 90% or FEV1/FVC < 90%, 11 children were evaluated for bronchodilator responsiveness, of whom three showed FEV1 improvement of ≥10%. All three had no previous history of asthma or atopy.
- Overall, 19/175 (11%) children had an obstructive pattern, as indicated by FEV1/FVC <80%, and/or positive ECT, and/or post bronchodilator reversibility—of whom only one had a previous asthma diagnosis.
- Altogether, 144 children underwent FeNO testing (Table 3), of whom 32 (22%) had elevated values, and 4/34 had a previous diagnosis of asthma as well as personal atopy. Another four children with elevated FeNO levels also demonstrated an obstructive pattern on spirometry: two with FEV1/FVC < 80% and two more with positive ECT. All four had personal atopy, and 14 others had either a personal or family history of atopy.
- In all of the 147 children who performed plethysmography lung volume testing, TLC was normal (≥80% predicted, Table 3); RV/TLC > 150% was found in 27 (18%), of whom one had a history of asthma.
- In all, 111 children performed MBW test, with a mean LCI of 7.48 (SD 0.9, Table 3); 27/111 (24.3%) had a value of >7.9, of whom two had known asthma, seven more had personal atopy and three had familial atopy; none had a history of prematurity and baseline spirometry was normal in all but one, who also had a significant post bronchodilator reversibility.
- Of the 134 children who performed a lung diffusion test (Table 3) KCO was >75% predicted in all but one patient with a rare genetic syndrome, post kidney transplantation, without a previous known lung disease.
- Overall, abnormal PFTs—including an obstructive pattern, elevated FeNO, air trapping, elevated LCI, or diffusion limitation—were documented in 85 children (46.2%); of those, 20/85 had at least two abnormal tests, and 17/20 demonstrated an obstructive pattern.
| Test | n | Result, Mean (SD) |
|---|---|---|
| FEV1% predicted | 170 | 92.6% (10.6) |
| FVC% predicted | 170 | 95.9% (11.4) |
| FEV1/FVC% | 170 | 90.38% (6.9) |
| FEV1% post exercise | 133 | 91.7% (10.6) |
| FEV1% post BD * | 96 | 93.9 (9.5) |
| FeNO | ||
| SBT **, ppb (normal < 8 ppb) | 128 | 15.1 (14.6) |
| MBT **, ppb (normal < 20 ppb) | 16 | 13.5 (8.4) |
| TLC% predicted | 147 | 105.2 (12.9) |
| RV/TLC% | 146 | 118.5 (32.7) |
| KCO% predicted | 134 | 104.3 (14.2) |
| LCI | 113 | 7.48 (0.9) |
| MCT ** (n = 7) | ||
| Positive/borderline | 3 | |
| Negative | 4 |
- As part of their assessment, chest X-ray was performed on 157/184, of whom results were normal in 122 (78%), while 13 (8%) exhibited peribronchial cuffing, seven (4%) prominent bronchovascular markings, and 10 had other abnormalities, as detailed in Table 4.
| Result | n (%) |
|---|---|
| Normal | 122 (77.7%) |
| Peribronchial cuffing | 13 (8.3%) |
| Prominent bronchovascular markings | 7 (4.5%) |
| Residual pulmonary infiltrate | 6 (3.8%) |
| Hyperinflation | 2 (1.3%) |
| Enlarged cardiac silhouette | 1 (0.6%) |
| Minimal effusion unilateral | 1 (0.6%) |
- ECG testing was done in 167 children; nine (5.4%) had abnormal results that were previously identified and were not COVID-related.
- Echocardiography was performed in 145 children, showing mild abnormalities in six (4.1%), all detected previous to SARS-CoV-2 infection.
- CPET testing was performed in six children due to continuous severe complaints of exercise intolerance at a median time of 38.8 weeks after acute COVID infection (range 32.8–115.8 weeks); five were previously healthy individuals and one had known asthma. In all cases, CPET demonstrated normal cardio-respiratory function.
3.2.1. Pulmonary Evaluation in Children with Moderate to Severe Acute COVID-19
- Among the 9 children with moderate acute COVID-19 infection according to the NIH criteria [29], one demonstrated an obstructive pattern on PFTs without a previous asthma diagnosis; three had elevated FeNO values, of whom two had either a personal or a familial history of atopy; two had elevated LCI values, one with known asthma.
- Among the 5 children with severe acute COVID-19 infection, none had abnormal spirometry, two had elevated FeNO values, of whom one had a personal history of atopy, and two had elevated LCI values. One child, post kidney transplantation, had diffusion limitation.
3.2.2. Pulmonary Evaluation in Children Previously Vaccinated against SARS-CoV-2
3.2.3. Multivariate Logistic Regression Analysis for Factors Associated with an Obstructive Pattern on Pulmonary Function Testing
3.3. Interventions and Drug Therapy
3.4. Pulmonary Long-Term Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID—Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Stephenson, T.; Allin, B.; Nugawela, M.D.; Rojas, N.; Dalrymple, E.; Pereira, S.P.; Soni, M.; Knight, M.; Cheung, E.Y.; Heyman, I.; et al. Long COVID (post-COVID-19 condition) in children: A modified Delphi process. Arch. Dis. Child. 2022, 107, 674–680. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Excellence (NICE): London, UK, 2020.
- Shachar-Lavie, I.; Shorer, M.; Segal, H.; Fennig, S.; Ashkenazi-Hoffnung, L. Mental health among children with long COVID during the COVID-19 pandemic. Eur. J. Pediatr. 2023, 182, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chen, S.; Zhang, Y.; Dong, F.; Zhang, Z.; Hu, B.; Zhu, Z.; Li, F.; Wang, X.; Wang, Y.; et al. Diffusion Capacity Abnormalities for Carbon Monoxide in Patients with COVID-19 At Three-Month Follow-up. Eur. Respir. J. 2021, 58, 2003677. [Google Scholar] [CrossRef]
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 2020, 27, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yim, J.-J.; Park, J. Pulmonary function and chest computed tomography abnormalities 6–12 months after recovery from COVID-19: A systematic review and meta-analysis. Respir. Res. 2022, 23, 233. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.M.; Prata-Barbosa, A.; da Cunha, A.J.L.A. Update on SARS-CoV-2 infection in children. Paediatr. Int. Child Health 2021, 41, 56–64. [Google Scholar] [CrossRef]
- Berg, S.K.; Palm, P.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Rosenkilde, S.; Thorsted, A.B.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D.; et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc. Health 2022, 6, 614–623. [Google Scholar] [CrossRef]
- Borch, L.; Holm, M.; Knudsen, M.; Ellermann-Eriksen, S.; Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children—A nationwide cohort study. Eur. J. Pediatr. 2022, 181, 1597–1607. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, Z.; Zhang, T.; Guo, W.; Guo, W.; Zheng, J.; Zhang, J.; Dong, C.; Na, R.; Zheng, L.; et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J. Med. Virol. 2020, 93, 1057–1069. [Google Scholar] [CrossRef]
- Palacios, S.; Krivchenia, K.; Eisner, M.; Young, B.; Ramilo, O.; Mejias, A.; Lee, S.; Kopp, B.T. Long-term pulmonary sequelae in adolescents post-SARS-CoV-2 infection. Pediatr. Pulmonol. 2022, 57, 2455–2463. [Google Scholar] [CrossRef]
- Dobkin, S.C.L.; Collaco, J.M.; McGrath-Morrow, S.A. Protracted respiratory findings in children post-SARS-CoV-2 infection. Pediatr. Pulmonol. 2021, 56, 3682–3687. [Google Scholar] [CrossRef]
- Knoke, L.; Schlegtendal, A.; Maier, C.; Eitner, L.; Lücke, T.; Brinkmann, F. Pulmonary Function and Long-Term Respiratory Symptoms in Children and Adolescents After COVID-19. Front. Pediatr. 2022, 10, 851008. [Google Scholar] [CrossRef] [PubMed]
- Doležalová, K.; Tuková, J.; Pohunek, P. The respiratory consequences of COVID-19 lasted for a median of 4 months in a cohort of children aged 2–18 years of age. Acta Paediatr. 2022, 111, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Martino, L.; Morello, R.; De Rose, C.; Buonsenso, D. Persistent respiratory symptoms associated with post-covid condition (Long covid) in children: A systematic review and analysis of current gaps and future perspectives. Expert Rev. Respir. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi-Hoffnung, L.; Shmueli, E.; Ehrlich, S.; Ziv, A.; Bar-On, O.; Birk, E.; Lowenthal, A.; Prais, D. Long COVID in Children. Pediatr. Infect. Dis. J. 2021, 40, e509–e511. [Google Scholar] [CrossRef]
- Testing—Corona Traffic Light Model (Ramzor) Website. Available online: https://corona.health.gov.il/en/testing-lobby/ (accessed on 1 December 2022).
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- American Thoracic Society Guidelines for Methacholine and Exercise Challenge. Available online: www.atsjournals.org (accessed on 4 August 2023).
- Coates, A.L.; Wanger, J.; Cockcroft, D.W.; Culver, B.H.; Carlsen, K.-H.; Diamant, Z.; Gauvreau, G.; Hall, G.L.; Hallstrand, T.S.; Horvath, I.; et al. ERS technical standard on bronchial challenge testing: General considerations and performance of methacholine challenge tests. Eur. Respir. J. 2017, 49, 1601526. [Google Scholar] [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef]
- Polgar, G.; Varuni, P. Pulmonary Function Testing in Children: Techniques and Standards; Saunders Limited: Collingwood, ON, Canada, 1971. [Google Scholar]
- Weng, T.R.; Levison, H. Standards of pulmonary function in children. Am. Rev. Respir. Dis. 1969, 99, 879–894. [Google Scholar]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Robinson, P.D.; Latzin, P.; Verbanck, S.; Hall, G.L.; Horsley, A.; Gappa, M.; Thamrin, C.; Arets, H.G.; Aurora, P.; Fuchs, S.I.; et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur. Respir. J. 2013, 41, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulou, P.; Latzin, P.; Jensen, R.; Stahl, M.; Harper, A.; Yammine, S.; Usemann, J.; Foong, R.E.; Spycher, B.; Hall, G.L.; et al. Normative data for multiple breath washout outcomes in school-aged Caucasian children. Eur. Respir. J. 2019, 55, 1901302. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, G.; Bar-Yishay, E. Methacholine inhalation challenge: A shorter, cheaper and safe approach. Eur. Respir. J. 2001, 17, 46–51. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines 2. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 4 August 2023).
- Morrow, A.K.; Malone, L.A.; Kokorelis, C.; Petracek, L.S.; Eastin, E.F.; Lobner, K.L.; Neuendorff, L.; Rowe, P.C. Long-Term COVID 19 Sequelae in Adolescents: The Overlap with Orthostatic Intolerance and ME/CFS. Curr. Pediatr. Rep. 2022, 10, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Grayson, M.H. Comparing respiratory syncytial virus and rhinovirus in development of post-viral airway disease. J. Asthma 2020, 59, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.L.; Hoang, V.T.; Colson, P.; Million, M.; Gautret, P. Co-infection of SARS-CoV-2 and influenza viruses: A systematic review and meta-analysis. J. Clin. Virol. Plus 2021, 1, 100036. [Google Scholar] [CrossRef] [PubMed]
- Palmon, P.A.; Jackson, D.J.; Denlinger, L.C. COVID-19 Infections and Asthma. J. Allergy Clin. Immunol. Pract. 2021, 10, 658–663. [Google Scholar] [CrossRef]
- Cervia, C.; Zurbuchen, Y.; Taeschler, P.; Ballouz, T.; Menges, D.; Hasler, S.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Warner, J.O.; Warner, J.A.; Munblit, D. Hypotheses to explain the associations between asthma and the consequences of COVID-19 infection. Clin. Exp. Allergy 2022, 52, 7–9. [Google Scholar] [CrossRef]
- Neville, R.D.; Lakes, K.D.; Hopkins, W.G.; Tarantino, G.; Draper, C.E.; Beck, R.; Madigan, S. Global Changes in Child and Adolescent Physical Activity During the COVID-19 Pandemic. JAMA Pediatr. 2022, 176, 886–894. [Google Scholar] [CrossRef]
- Say, D.; Crawford, N.; McNab, S.; Wurzel, D.; Steer, A.; Tosif, S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc. Health 2021, 5, e22–e23. [Google Scholar] [CrossRef] [PubMed]

| Age, mean ± SD, years | 12.64 ± 4.06 |
| Female, n (%) | 111 (60.3%) |
| BMI%, mean ± SD | 60.8% ± 31.7% |
| Underlying disease, n (%) * | 45 (24.5%) |
| Asthma | 11 (5.4%) |
| Depression/anxiety | 8 (4.3%) |
| Gastrointestinal disease | 5 (2.7%) |
| Rheumatologic disease | 4 (2.2%) |
| Immunodeficiency ** | 4 (2.2%) |
| Neurological impairment *** | 4 (2.2%) |
| Nephrological disease | 3 (1.6%) |
| Other **** | 9 (4.9%) |
| Prematurity (<37 weeks) | 4 (2.2%) |
| Atopic background by history, n (%) | |
| Personal | 50 (27.2%) |
| Familial | 32 (17.4%) |
| Attention deficit disorder (ADHD), n (%) | 17 (9.2%) |
| Competitive athletes, n (%) | 24 (13%) |
| Severity of acute COVID-19 illness according to NIH *****, n (%) | |
| Asymptomatic | 9 (4.9%) |
| Mild | 161 (87.5%) |
| Moderate | 9 (4.9%) |
| Severe | 5 (2.7%) |
| Respiratory Complaint, n (%) | |
| Dyspnea | 136 (73.9%) |
| Chest pain | 89 (48.4%) |
| Cough | 19 (10.3%) |
| Functional status, n (%) | |
| Mild | 104 (56.5%) |
| Moderate | 74 (40.2%) |
| Severe | 6 (3.3%) |
| Influence ADL *, n (%) | 116 (63%) |
| Physical activity effected, n (%) | 142 (77.2%) |
| OR | 95%CI | p-Value | |
|---|---|---|---|
| Age | 1.36 | 1.07–1.75 | 0.014 |
| Chest pain | 2.97 | 0.88–10.08 | 0.080 |
| CXR | |||
| Prominent bronchovascular markings | 43.28 | 4.50–416.49 | 0.001 |
| Hyperinflation | 28.42 | 2.18–370.84 | 0.011 |
| Peribronchial cuffing | 4.06 | 0.84–19.77 | 0.082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmueli, E.; Bar-On, O.; Amir, B.; Mei-Zahav, M.; Stafler, P.; Levine, H.; Steuer, G.; Rothschild, B.; Tsviban, L.; Amitai, N.; et al. Pulmonary Evaluation in Children with Post-COVID-19 Condition Respiratory Symptoms: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 6891. https://doi.org/10.3390/jcm12216891
Shmueli E, Bar-On O, Amir B, Mei-Zahav M, Stafler P, Levine H, Steuer G, Rothschild B, Tsviban L, Amitai N, et al. Pulmonary Evaluation in Children with Post-COVID-19 Condition Respiratory Symptoms: A Prospective Cohort Study. Journal of Clinical Medicine. 2023; 12(21):6891. https://doi.org/10.3390/jcm12216891
Chicago/Turabian StyleShmueli, Einat, Ophir Bar-On, Ben Amir, Meir Mei-Zahav, Patrick Stafler, Hagit Levine, Guy Steuer, Benjamin Rothschild, Lior Tsviban, Nofar Amitai, and et al. 2023. "Pulmonary Evaluation in Children with Post-COVID-19 Condition Respiratory Symptoms: A Prospective Cohort Study" Journal of Clinical Medicine 12, no. 21: 6891. https://doi.org/10.3390/jcm12216891