Diallyl Trisulfide Protects Rat Brain Tissue against the Damage Induced by Ischemia-Reperfusion through the Nrf2 Pathway
Abstract
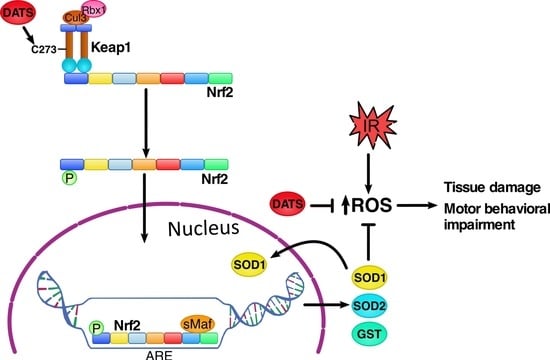
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Experimental Design
2.4. Middle Cerebral Artery Occlusion (MCAO) Model
2.5. Histology
2.6. Nissl Staining
2.7. Behavioral Testing
2.8. Nuclear Extract
2.9. Nrf2 Activation
2.10. Immunohistochemistry
2.11. Preparation of Total Homogenates
2.12. CAT Activity
2.13. GPx Activity
2.14. Statistical Analysis
3. Results
3.1. DATS Decreased the Infarct Area and Protected against Brain Damage and Motor Behavioral Impairment Induced by IR in Both Striatum and Cortex
3.2. DATS Prevented the Increase in the MMP-9 Levels in the Striatum and Cortex
3.3. DATS Decreased Oxidative Stress in the Striatum and Cortex
3.4. DATS Increased Nrf2 Activation in Cerebral Cortex
3.5. DATS Increased the Levels of SOD1 and SOD2 in Striatum and Cortex
3.6. DATS Increased the GST Enzyme Levels in Both Striatum and Cortex
3.7. DATS Did not Increase the Activity of CAT and GPx
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fani, L.; Bos, D.; Mutlu, U.; Portegies, M.L.P.; Zonneveld, H.I.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A.; Ikram, M.K. Global brain perfusion and the risk of transient ischemic attack and ischemic stroke: The Rotterdam study. J. Am. Heart Assoc. 2019, 8, e011565. [Google Scholar] [CrossRef] [PubMed]
- Díez-Tejedor, E. Guía para el diagnóstico y tratamiento del ictus. In Guías Oficiales de la Sociedad Española de Neurología, 3rd ed.; Prous Science: Barcelona, España, 2004; pp. 1–260. [Google Scholar]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; de Ferranti, S.D.; et al. Heart disease and stroke statistics-2018 update: A report from the American heart association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J.; Fernández, A.P.; Serrano, J.; Peinado, M.A.; Martínez, A. The role of free radicals in cerebral hypoxia and ischemia. Free Radic. Biol. Med. 2005, 39, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.; Back, T.; Lindauer, U.; Busch, C.; Megow, D.; Dreier, J.; Dirnagl, U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1998, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.L.; Guo, Z.N.; Yang, Y. Free radical damage in ischemia-reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell. Logev. 2018, 2018, 3804979. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defense pathway: Keap1-dependent and —Independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.D.; Lo, S.C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef]
- Katoh, Y.; Iida, K.; Kang, M.I.; Kobayashi, A.; Mizukami, M.; Tong, K.I.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch. Biochem. Biophys. 2005, 433, 342–350. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc and alkenals. Proc. Nat. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Small Maf proteins serve as transcriptional cofactor for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc. Nat. Sci. Acad. USA 2004, 101, 6379–6384. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, P.; Chánez-Cardenas, M.E.; Ortíz-Plata, A.; León-Aparicio, D.; Barrera, D.; Espinoza-Rojo, M.; Villeda-Hernández, J.; Sánchez-García, A.; Maldonado, P.D. Aged garlic extract delays the appearance of infarct area in cerebral ischemia model, an effect likely conditioned by the cellular antioxidant systems. Phytomedicine 2010, 17, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Al-Qattan, K.K.; Al-Enezi, F.; Khanafer, R.M.; Mustafa, T. Effects of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet. Prostaglandins Leukot. Essent. Fatty Acids 2000, 62, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Colín-González, A.L.; Ortíz-Plata, A.; Villeda-Hernández, J.; Barrera, D.; Molina-Jijón, E.; Padraza-Chaverrí, J.; Maldonado, P.D. Aged garlic extract attenuates cerebral damage and cyclooxygenase-2 induction after ischemia and reperfusion in rats. Plant Foods Hum. Nutr. 2011, 66, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.D.; Agustine, L.M.; Maher, J.M.; Nelson, D.M.; Slitt, A.L.; Klaassen, C.D.; Lehman-McKeeman, L.D.; Cherrington, N.J. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab. Dispos. 2007, 35, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Hosono, T.; Misawa, S.; Seki, T.; Ariga, T. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem. Toxicol. 2004, 42, 743–749. [Google Scholar] [CrossRef]
- Fukushima, S.; Takada, N.; Hori, T.; Wanibuchi, H. Cancer prevention by organosulfur compounds from garlic and onion. J. Cell. Biochem. Suppl. 1997, 27, 100–105. [Google Scholar] [CrossRef]
- Chan, K.C.; Yin, M.C.; Chao, W.J. Effect of dialliyl trisulfide-rich garlic oil on blood coagulation and plasma activity of anticoagulation factors in rats. Food Chem. Toxicol. 2007, 45, 502–507. [Google Scholar] [CrossRef]
- Kim, J.M.; Chang, H.J.; Kim, W.K.; Chang, N.; Chun, H.S. Structure-activity relationship of neuroprotective and reactive oxygen species scavenging activities for allium organosulfur compounds. J. Agric. Food Chem. 2006, 54, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Sheen, L.Y.; Chen, H.W.; Kuo, W.W.; Tsai, S.J.; Lii, C.K. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J. Agric. Food Chem. 2002, 50, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lii, C.K.; Tsai, S.J.; Sheen, L.Y. Diallyl trisulfide modulates cell viability and the antioxidants and detoxification systems of rat primary hepatocytes. J. Nutr. 2004, 134, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, K.; Wang, Q.; Yin, Y.; Xu, Q.; Duan, W.; Li, C. Neuroprotective effects of diallyl trisulfide in SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. Brain Res. 2011, 1374, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, S.; Tnag, X.; Li, Z.; Zhang, J.; Xue, X.; Han, J.; Liu, Y.; Zhang, Y.; Zhang, Y.; et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: role of SIRT1 activation. Apoptosis 2017, 22, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.G.; Park, S.A.; Kundu, J.K.; Keum, Y.S.; Cha, Y.M.; Na, H.K.; Surh, Y.J. Keap1 Cysteine 288 as a potential target of diallyl trisulfide-induced Nrf2 activation. PLoS ONE 2014, 9, e85984. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pung, D.; Leong, V.; Hebbar, V.; Shen, G.; Nair, S.; Li, W.; Kong, A.N. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signaling. Free Radic. Biol. Med. 2004, 37, 1578–1590. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef]
- Lee, J.M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef]
- Papp, D.; Lenti, K.; Módos, D.; Fazekas, D.; Dúl, Z.; Türei, D.; Földvári-Nagy, L.; Nussinov, R.; Csermely, P.; Korcsmáros, T. The Nrf2-related interactome and regulome contain multifunctional proteins and fine-tuned autoregulatory loops. FEBS Lett. 2012, 586, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Knecht, T.; Borlongan, C.; Dela Peña, I. Combination therapy for ischemic stroke: Novel approaches to lengthen therapeutic window of tissue plasminogen activator. Brain Circ. 2018, 4, 99–108. [Google Scholar] [PubMed]
- Siket, M.S. Treatment of acute ischemic stroke. Emerg. Med. Clin. N. Am. 2016, 34, 861–882. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.W.; Wang, W.J.; Tsai, C.Y.; Way, C.L.; Hsu, H.H.; Chen, L.M. Diallyl trisulfide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFkB signaling via attenuating ROS generation. Int. J. Cardiol. 2013, 168, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Li, G.L.; Wang, B.A.; Qin, Y.; Bai, S.R.; Rong, J.; Deng, T.; Li, Q. Diallyl trisulfide protects against oxygen glucose deprivation-induced apoptosis by scavenging free radicals via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway in B35 neural cells. Brain Res. 2015, 1614, 38–50. [Google Scholar] [CrossRef]
- Franklin, S. The peripheral and central nervous system. In Conn’s Translational Neuroscience, 1st ed.; Conn, M., Ed.; Elsevier Inc.: London, UK, 2016; pp. 113–129. [Google Scholar]
- Shih, A.Y.; Li, P.; Murphy, T.H. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Singh, M.; Sharma, A. Neuroprotective effect of antioxidants in ischaemia and reperfusion-induced cerebral injury. Pharmacol. Res. 2003, 48, 209–215. [Google Scholar] [CrossRef]
- Silva-Islas, C.; Santana, R.A.; Colín-González, A.L.; Maldonado, P.D. Nrf2 activation, an innovative therapeutic alternative in cerebral ischemia. In Advances in the Preclinical Study of Ischemic Stroke, 1st ed.; Balestrino, M., Ed.; InthechOpen: Rijeka, Croatia, 2012; pp. 347–378. [Google Scholar]
- Liu, C.; Leng, B.; Li, Y.; Jiang, H.; Duan, W.; Guo, Y.; Li, C.; Hong, K. Diallyl trisulfide protects motor neurons from the neurotoxic protein TDP-43 via activating lysosomal degradation and the antioxidant response. Neurochem. Res. 2018, 43, 2304–2312. [Google Scholar] [CrossRef]
- Sun, M.M.; Bu, H.; Li, B.; Yu, J.X.; Gou, Y.S.; Li, C.Y. Neuroprotective potential of phase II enzyme inducer diallyl trisulfide. Neurol. Res. 2009, 31, 23–27. [Google Scholar] [CrossRef]
- Miltonprabu, S.; Sumedha, N.C.; Senthilraja, P. Diallyl trisulfide, a garlic polysulfide protects against As-induced renal oxidative neprotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int. Immunopharmacol. 2017, 50, 107–120. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suzuki, T.; Kobayashi, A.; Wakabayashi, J.; Maher, J.; Motohashi, H.; Yamamoto, M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell. Biol. 2008, 28, 2758–2770. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastavas, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W.; et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acid Res. 2010, 38, 5718–5734. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Wang, C.C.; Lai, T.Y.; Tsu, H.N.; Wang, C.H.; Liang, H.Y.; Kuo, W.W. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int. J. Cardiol. 2013, 168, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Škiljić, D.; Petersen, A.; Karlsson, J.O.; Nehndig, A.; Nilsson, S.; Zetterberg, M. Effects of 17 β—Estradiol on activity, gene and protein expression of superoxide dismutase in primary cultured human lens epithelial cells. Curr. Eye Res. 2018, 43, 639–646. [Google Scholar] [CrossRef]
- Bordoni, M.; Pansarasa, O.; Dell’Orco, M.; Crippa, V.; Gagliardi, S.; Sproviero, D.; Bernuzzi, S.; Diamanto, L.; Ceroni, M.; Tedeschi, G.; et al. Nuclear phosphor-SOD1 protects DNA from oxidative stress damage in amyotrophic lateral sclerosis. J. Clin. Med. 2019, 8, 729. [Google Scholar] [CrossRef]
- Guo, Z.; Kozlov, S.; Lavin, M.F.; Person, M.D.; Paull, T.T. ATM activation by oxidative stress. Science 2010, 330, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Song, J.H.; Hwang, B.; Park, S.L.; Kim, W.T.; Park, S.S.; Kim, W.J.; Moon, S.K. Angiopoietin-like protein 4 potentiates DATS-induced inhibition of proliferation, migration, and invasion of bladder cancer EJ cells; involvement of G2/M-phase cell cycle arrest, signaling pathways, and transcription factors-mediated MPP-9 expression. Food Nutr. Res. 2017, 61, 1338918. [Google Scholar] [CrossRef]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Cummun. 2014, 5, 3446. [Google Scholar] [CrossRef] [PubMed]
- Lii, C.K.; Liu, K.L.; Cheng, Y.P.; Lin, A.H.; Chen, H.W.; Tsai, C.W. Sulforophane and alpha-lipoic acid upregulate the expression of the pi class of glutathione S-transferase through c-jun and Nrf2 activation. J. Nutr. 2010, 140, 885–892. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Sheen, L.Y.; Chen, H.W.; Tsai, S.J.; Lii, C.K. Effects of organosulfur compounds from garlic oil on the antioxidant system in rat liver and red blood cells. Food Chem. Toxicol. 2001, 39, 563–569. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, C.L.; Zhu, Z.P.; Yu, L.H.; Zhao, X.L.; Xie, K.Q. Diallyl trisulfide (DATS) effectively attenuated oxidative stress-mediated liver injury and hepatic mitochondrial dysfunction in acute ethanol-exposed mice. Toxycology 2008, 252, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell. Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef] [PubMed]










| Group | Striatum CAT Activity (k/mg Protein) | Cortex CAT Activity (k/mg Protein) | Cortex GPx Activity (U/mg Protein) |
|---|---|---|---|
| CT | 0.003193 ± 0.000392 | 0.003133 ± 0.000306 | 0.004643 ± 0.001362 |
| DATS | 0.003388 ± 0.000236 | 0.001608 ± 0.000055 | 0.006772 ± 0.001077 |
| IR | 0.005246 ± 0.000299 *** | 0.004442 ± 0.000337 | 0.009219 ± 0.001125 * |
| IR + DATS | 0.005583 ± 0.000359 *** | 0.004105 ± 0.000898 | 0.010490 ± 0.001646 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Barrera-Oviedo, D.; Ortiz-Plata, A.; Pedraza-Chaverri, J.; Maldonado, P.D. Diallyl Trisulfide Protects Rat Brain Tissue against the Damage Induced by Ischemia-Reperfusion through the Nrf2 Pathway. Antioxidants 2019, 8, 410. https://doi.org/10.3390/antiox8090410
Silva-Islas CA, Chánez-Cárdenas ME, Barrera-Oviedo D, Ortiz-Plata A, Pedraza-Chaverri J, Maldonado PD. Diallyl Trisulfide Protects Rat Brain Tissue against the Damage Induced by Ischemia-Reperfusion through the Nrf2 Pathway. Antioxidants. 2019; 8(9):410. https://doi.org/10.3390/antiox8090410
Chicago/Turabian StyleSilva-Islas, Carlos A., María E. Chánez-Cárdenas, Diana Barrera-Oviedo, Alma Ortiz-Plata, José Pedraza-Chaverri, and Perla D. Maldonado. 2019. "Diallyl Trisulfide Protects Rat Brain Tissue against the Damage Induced by Ischemia-Reperfusion through the Nrf2 Pathway" Antioxidants 8, no. 9: 410. https://doi.org/10.3390/antiox8090410