Nutrigenomics of Natural Antioxidants in Broilers
Abstract
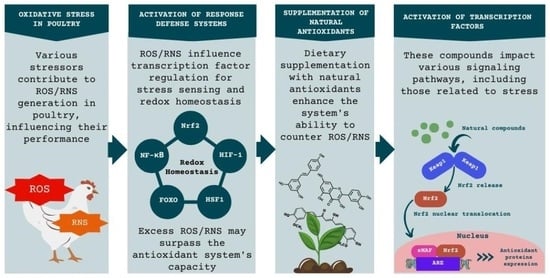
:1. Introduction
2. Data Sourcing and Inclusion Criteria
Range of Interest, Data Extraction, and the Dataset
3. Oxidative Stress
3.1. The Endogenous Antioxidant System
3.2. Oxidative Stress and Gene Expression
3.3. Transcription Factor Nrf2
3.4. Transcription Factor NF-κB
4. Effects of Natural Antioxidants on Gene Expression
5. Supplementation of Natural Antioxidants in the Diet of Broilers and Their Effects on Gene Expression
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Korver, D.R. Review: Current challenges in poultry nutrition, health, and welfare. Animal 2023, 17 (Suppl. 2), 100755. [Google Scholar] [CrossRef]
- Poultry Global Market Report. 2023. Available online: https://www.researchandmarkets.com/report/poultry (accessed on 28 January 2024).
- Surai, F.P. Vitagenes in Avian Biology and Poultry Health; Wageningen Academic: Leiden, The Netherlands, 2020; pp. 25–544. [Google Scholar]
- Surai, P.F. Integrated antioxidant defence network in animals. EC Nutr. 2023, 18, 18–20. [Google Scholar]
- Surai, P.; Kochish, I.I.; Fisinin, I.V.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- George, S.; Abrahamse, H. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols inhibiting MAPK signaling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Knasmüller, S.; Nersesyan, A.; Mišík, M.; Gerner, C.; Mikulits, W.; Ehrlich, V.; Hoelzl, C.; Szakmary, A.; Wagner, K.-H. Use of conventional and -omics based methods for health claims of dietary antioxidants: A critical overview. Br. J. Nutr. 2008, 99, ES3–ES52. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-dependent antioxidant enzymes: Actions and properties of selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Fisinin, V.I. Vitagenes in poultry production: Part 3. Vitagene concept development. World’s Poult. Sci. J. 2016, 72, 793–804. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 activation by natural phytochemicals: An overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Ishii, T.; Wakabayashi, N.; Yamamoto, M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic. Res. 1999, 31, 319–324. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [PubMed]
- Hur, W.; Sun, Z.; Jiang, T.; Mason, D.E.; Peters, E.C.; Zhang, D.D.; Luesch, H.; Schultz, P.G.; Gray, N.S. A small-molecule inducer of the antioxidant response element. Chem. Biol. 2010, 17, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Mimura, J.; Yamamoto, M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxid. Redox Signal. 2010, 13, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Zenkov, N.K.; Kozhin, P.M.; Chechushkov, A.V.; Martinovich, G.G.; Kandalintseva, N.V.; Menshchikova, E.B. Mazes of Nrf2 regulation. Biochemistry 2017, 82, 556–564. [Google Scholar] [CrossRef]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating nrf2–keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.-H.; Chiang, S.; Kalinowski, D.S.; Bae, D.-H.; Sahni, S.; Richardson, D.R. The role of the antioxidant response in mitochondrial dysfunction in degenerative diseases: Crosstalk between antioxidant defense, autophagy, and apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Lyakhovich, V.V.; Vavilin, V.A.; Zenkov, N.K.; Menshchikova, E.B. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry 2006, 71, 962–974. [Google Scholar] [CrossRef]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The dual role of Nrf2 in nonalcoholic fatty liver disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. BioMed Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef] [PubMed]
- Sagin, F.; Sozmen, E. Anti-inflammatory effects of dietary antioxidants. Antiinflamm. Antiallergy Agents Med. Chem. 2004, 3, 19–30. [Google Scholar] [CrossRef]
- Shen, G.; Jeong, W.-S.; Hu, R.; Kong, A.-N.T. Regulation of Nrf2, NF-κB, and AP-1 signaling pathways by chemopreventive agents. Antioxid. Redox Signal. 2005, 7, 1648–1663. [Google Scholar] [CrossRef]
- O’Dea, E.; Hoffmann, A. NF-κB signaling. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 107–115. [Google Scholar] [CrossRef]
- Hayden, S.M.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Kidd, M.T. Redox homeostasis in poultry: Regulatory roles of NF-κB. Antioxidants 2021, 10, 186. [Google Scholar] [CrossRef]
- Moynagh, P.N. The NF-B pathway. J. Cell Sci. 2005, 118, 4589–4592. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Gado, F.; Ferrario, G.; Della Vedova, L.; Zoanni, B.; Altomare, A.; Carini, M.; Aldini, G.; D’Amato, A.; Baron, G. Targeting Nrf2 and NF-κB signaling pathways in cancer prevention: The role of apple phytochemicals. Molecules 2023, 28, 1356. [Google Scholar] [CrossRef]
- Shih, P.-H.; Yeh, C.-T.; Yen, G.-C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar] [CrossRef]
- Sahin, K. Modulation of NF-κB and Nrf2 pathways by lycopene supplementation in heat-stressed poultry. World’s Poult. Sci. J. 2015, 71, 271–284. [Google Scholar] [CrossRef]
- Arain, M.A.; Mei, Z.; Hassan, F.U.; Saeed, M.; Alagawany, M.; Shar, A.H.; Rajput, I.R. Lycopene: A natural antioxidant for prevention of heat-induced oxidative stress in poultry. World’s Poult. Sci. J. 2017, 74, 89–100. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.-S.; Wang, H.-D.; Zhang, X.; Yu, Q.; Li, W.; Zhou, M.-L.; Wang, X.-L. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar. Drugs 2014, 12, 6125–6141. [Google Scholar] [CrossRef] [PubMed]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Mancuso, C.; Barone, E.; Calafato, S.; Bates, T.; Rizzarelli, E.; Kostova, A.T. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front. Biosci. 2009, 14, 376–397. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive compounds in oxidative stress-mediated diseases: Targeting the NRF2/ARE signaling pathway and epigenetic regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Zoidis, E.; Pappas, A.C.; Goliomytis, M.; Simitzis, P.E.; Sotirakoglou, K.; Tavrizelou, S.; Danezis, G.P.; Georgiou, C.A. Quercetin and Egg Metallome. Antioxidants 2021, 10, 80. [Google Scholar] [CrossRef]
- Roy, A.; Das, S.; Chatterjee, I.; Roy, S.; Chakraborty, R. Anti-inflammatory Effects of Different Dietary Antioxidants. In Plant Antioxidants and Health; Reference Series in Phytochemistry; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Sohrabi, F.; Dianat, M.; Badavi, M.; Radan, M.; Mard, S.A. Gallic acid suppresses inflammation and oxidative stress through modulating Nrf2-HO-1-NF-κB signaling pathways in elastase-induced emphysema in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 56822–56834. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Sahin, K.; Smith, M.O. Regulation of transcription factors by the epigallocatechin-3-gallate in poultry reared under heat stress. World’s Poult. Sci. J. 2016, 72, 299–306. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Activation of Nrf2 signaling pathway by natural and synthetic chalcones: A therapeutic road map for oxidative stress. Expert. Rev. Clin. Pharmacol. 2021, 14, 465–480. [Google Scholar] [CrossRef]
- Surai, P.F.; Surai, A. Silymarin Puzzle; Wageningen Academic Publishers: Wageningen, The Netherlands, 2023. [Google Scholar]
- Surai, P.F.; Earle-Payne, K.; Kidd, M.T. Taurine as a natural antioxidant: From direct antioxidant effects to protective action in various toxicological models. Antioxidants 2021, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.-L.; Jun, M.; Jeong, W.-S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2017, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A. Anti-inflammatory effects of resveratrol: Possible role in prevention of age-related cardiovascular disease. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Marques, M.; Diniz, C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yu, W.; Li, X.; Zhou, F.; Zhang, W.; Shen, Q.; Li, J.; Zhang, C.; Shen, P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017, 136, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Seremelis, I.; Danezis, G.P.; Pappas, A.C.; Zoidis, E.; Fegeros, K. Avian stress-related transcriptome and selenotranscriptome: Role during exposure to heavy metals and heat stress. Antioxidants 2019, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Giamouri, E.; Tavrizelou, S.; Zacharioudaki, M.; Danezis, G.P.; Simitzis, P.E.; Zoidis, E.; Tsiplakou, E.; Pappas, A.C.; Georgiou, C.A.; et al. Impact of Mycotoxins on Animals’ Oxidative Status. Antioxidants 2021, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Surai, F.P.; Karadas, F.; Sparks, H.N. The importance of antioxidants in poultry. In Proceedings of the Annual, North Carolina Poultry Nutrition Conference, Greensboro, NC, USA, 4–6 October 2003; pp. 6–9. [Google Scholar]
- Surai, F.P.; Kochish, I.I.; Fisinin, I.V. Antioxidant systems in poultry biology: Nutritional modulation of vitagenes. Eur. Poult. Sci. 2017, 81, 214. [Google Scholar] [CrossRef]
- Zoidis, Ε.; Simitzis, P.E.; Kapantais, D.; Katsoulas, P.; Pappas, A.C.; Papadomichelakis, G.; Goliomytis, M. Dietary orange pulp and organic selenium effects on growth performance, meat quality, fatty acid profile, and oxidative stability parameters of broiler chickens. Sustainability 2022, 14, 1534. [Google Scholar] [CrossRef]
- Xie, Q.; Xie, K.; Yi, J.; Song, Z.; Zhang, H.; He, X. The effects of magnolol supplementation on growth performance, meat quality, oxidative capacity, and intestinal microbiota in broilers. Poult. Sci. 2022, 101, 101722. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.; Zhu, Y.; Cui, H.; Yang, J.; Lu, M.; Cheng, H.; Gu, L.; Xu, T.; Xu, L. Effect of lycopene on the growth performance, antioxidant enzyme activity, and expression of gene in the Keap1-Nrf2 signaling pathway of arbor acres broilers. Front. Vet. Sci. 2022, 9, 833346. [Google Scholar] [CrossRef]
- Kikusato, M. Phytobiotics to improve health and production of broiler chickens: Functions beyond the antioxidant activity. Anim. Biosci. 2021, 34, 345–353. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Zhang, J.; Zhou, B.; Zhuang, S.; He, X.; Wang, T.; Wang, C. Effects of different levels of rutin on growth performance, immunity, intestinal barrier, and antioxidant capacity of broilers. Ital. J. Anim. Sci. 2022, 21, 1390–1401. [Google Scholar] [CrossRef]
- Tan, Z.; Halter, B.; Liu, D.; Gilbert, E.R.; Cline, M.A. Dietary flavonoids as modulators of lipid metabolism in poultry. Front. Physiol. 2022, 13, 863860. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, Z.; Zhang, H.; Yao, Y.; Ma, H. Genistein activated adenosine 5′-monophosphate-activated protein kinase-sirtuin1/peroxisome proliferator-activated receptor γ coactivator-1α pathway potentially through adiponectin and estrogen receptor β signaling to suppress fat deposition in broiler chickens. Poult. Sci. 2021, 100, 246–255. [Google Scholar] [PubMed]
- Xie, Z.; Shen, G.; Wang, Y.; Wu, C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019, 98, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Hager-Theodorides, A.L.; Massouras, T.; Simitzis, P.E.; Moschou, K.; Zoidis, E.; Sfakianaki, E.; Politi, K.; Charismiadou, M.; Goliomytis, M.; Deligeorgis, S. Hesperidin and naringin improve broiler meat fatty acid profile and modulate the expression of genes involved in fatty acid β-oxidation and antioxidant defense in a dose dependent manner. Foods 2021, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mei, H.; Liu, Y.; Li, Z.; Qamar, H.; Yu, M.; Ma, X. Dietary supplementation with rutin alters meat quality, fatty acid profile, antioxidant capacity, and expression levels of genes associated with lipid metabolism in breast muscle of qingyuan partridge chickens. Foods 2023, 12, 2302. [Google Scholar] [CrossRef]
- Wang, M.; Wang, B.; Wang, S.; Lu, H.; Wu, H.; Ding, M.; Ying, L.; Mao, Y.; Li, Y. Effect of quercetin on lipids metabolism through modulating the gut microbial and AMPK/PPAR signaling pathway in broilers. Front. Cell Dev. Biol. 2021, 9, 616219. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.A.; Elbestawy, A.R.; El-Far, A.H.; Noreldin, A.E.; Emam, M.; Baty, R.S.; Albadrani, G.M.; Abdel-Daim, M.M.; Abd El-Hamid, H.S. Quercetin dietary supplementation advances growth performance, gut microbiota, and intestinal mRNA expression genes in broiler chickens. Animals 2021, 11, 2302. [Google Scholar] [CrossRef]
- Chen, Y.P.; Gu, Y.F.; Zhao, H.R.; Zhou, Y.M. Dietary squalene supplementation alleviates diquat-induced oxidative stress and liver damage of broiler chickens. Poult. Sci. 2021, 100, 100919. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zhang, H.; Wang, T. Pterostilbene as a protective antioxidant attenuates diquat-induced liver injury and oxidative stress in 21-day-old broiler chickens. Poult. Sci. 2020, 99, 3158–3167. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, J.; Zhang, B. Effects of dietary quercetin on the antioxidative status and cecal microbiota in broiler chickens fed with oxidized oil. Poult. Sci. 2020, 99, 4892–4903. [Google Scholar] [CrossRef] [PubMed]
- Tolba, S.A.; Magnuson, A.D.; Sun, T.; Lei, X.G. Dietary supplemental microalgal astaxanthin modulates molecular profiles of stress, inflammation, and lipid metabolism in broiler chickens and laying hens under high ambient temperatures. Poult. Sci. 2020, 99, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Hosseindoust, A.; Oh, S.M.; Ko, H.S.; Jeon, S.M.; Ha, S.H.; Jang, A.; Son, J.S.; Kim, J.S. Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature. Antioxidants 2020, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.; Wang, X.; Zhao, F.; Wang, C.; Zhang, Q.; Chen, X.; Geng, Z.; Zhang, C. Resveratrol attenuates heat stress-induced impairment of meat quality in broilers by regulating the Nrf2 signaling pathway. Animals 2022, 12, 1889. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, F.; Li, Z.; Jin, X.; Chen, X.; Geng, Z.; Hu, H.; Zhang, C. Effects of Resveratrol on Growth Performance, Intestinal Development, and Antioxidant Status of Broilers under Heat Stress. Animals 2021, 11, 1427. [Google Scholar] [CrossRef]
- Meng, T.; Deng, J.; Xiao, D.; Arowolo, M.A.; Liu, C.; Chen, L.; Deng, W.; He, S.; He, J. Protective effects and potential mechanisms of dietary resveratrol supplementation on the spleen of broilers under heat stress. Front. Nutr. 2022, 9, 821272. [Google Scholar] [CrossRef]
- Kishawy, A.T.Y.; Ibrahim, D.; Roushdy, E.M.; Moustafa, A.; Eldemery, F.; Hussein, E.M.; Hassan, F.A.M.; Elazab, S.T.; Elabbasy, M.T.; Kanwal, R.; et al. Impact of resveratrol-loaded liposomal nanocarriers on heat-stressed broiler chickens: Effects on performance, sirtuin expression, oxidative stress regulators, and muscle building factors. Front. Vet. Sci. 2023, 10, 1137896. [Google Scholar] [CrossRef]
- Abbas, A.O.; Alaqil, A.A.; Mehaisen, G.M.K.; El Sabry, M.I. Effect of organic selenium-enriched yeast on relieving the deterioration of layer performance, immune function, and physiological indicators induced by heat stress. Front. Vet. Sci. 2022, 9, 880790. [Google Scholar] [CrossRef]
- Li, X.; Hua, J.; Wang, S.; Hu, Z.; Wen, A.; Yang, B. Genes and signaling pathways involved in the regulation of selenium-enriched yeast on liver metabolism and health of broiler (Gallus gallus). Biol. Trace Elem. Res. 2023, 201, 387–402. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhang, Y.; Tong, Y.X.; Guo, P.T.; Zhang, J.; Wang, C.K.; Gao, Y.Y. Effects of lutein on jejunal mucosal barrier function and inflammatory responses in lipopolysaccharide-challenged yellow-feather broilers. Poult. Sci. 2022, 101, 102191. [Google Scholar] [CrossRef]
- He, Z.; Li, Y.; Xiong, T.; Nie, X.; Zhang, H.; Zhu, C. Effect of dietary resveratrol supplementation on growth performance, antioxidant capacity, intestinal immunity and gut microbiota in yellow-feathered broilers challenged with lipopolysaccharide. Front. Microbiol. 2022, 13, 977087. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Chen, Z.L.; Zhang, S.; Ye, J.L.; Wang, Y.B. Protective effects of protocatechuic acid on growth performance, intestinal barrier and antioxidant capacity in broilers challenged with lipopolysaccharide. Animal 2023, 17, 100693. [Google Scholar] [CrossRef]
- Zhang, J.; Han, H.; Zhang, L.; Wang, T. Dietary bisdemethoxycurcumin supplementation attenuates lipopolysaccharide-induced damages on intestinal redox potential and redox status of broilers. Poult. Sci. 2021, 100, 101061. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Gong, J.G.; Li, J.H.; Hao, Y.S.; Xu, H.J.; Liu, Y.C.; Feng, Z.H. Dietary resveratrol supplementation on growth performance, immune function and intestinal barrier function in broilers challenged with lipopolysaccharide. Poult. Sci. 2023, 102, 102968. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, W.; Liu, H.; Wang, Κ.; Gao, Μ.; Guo, L.; Xu, S. SeMet alleviates LPS-induced eggshell gland necroptosis mediated inflammation by regulating the Keap1/Nrf2/HO-1 pathway. Arch. Biochem. Biophys. 2024, 751, 109847. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.L.; Li, N.; Chen, Y.J.; Chen, X.S.; Yang, Z.; Xu, L.; Yang, H.M.; Wang, Z.Y. Protective effects of lycopene on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1-exposed broilers. Poult. Sci. 2021, 100, 101441. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Ge, B.; Li, H.; Zhou, H.; Yan, K.; Wang, W.; Cui, Q.; Zhang, X. Taraxasterol alleviates aflatoxin B1-induced liver damage in broiler chickens via regulation of oxidative stress, apoptosis, and autophagy. Ecotoxicol. Environ. Saf. 2023, 251, 114546. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, L.; Yu, L.; Sun, L.; Li, K.; Tong, C.; Xu, W.; Cui, G.; Long, M.; Li, P. Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes of the diversity of caecal microbiota caused by Ochratoxin-A in broilers. Food Chem. Toxicol. 2020, 137, 111139. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Li, P.; Yu, L.-H.; Li, L.; Li, K.; Chen, Y.; Yang, S.-H.; Long, M. Selenium-rich yeast attenuates ochratoxin A-induced small intestinal injury in broiler chickens by activating the Nrf2 pathway and inhibiting NF-KB activation. J. Funct. Foods 2020, 66, 103784. [Google Scholar] [CrossRef]
- Rajput, S.A.; Zhang, C.; Feng, Y.; Wei, X.T.; Khalil, M.M.; Rajput, I.R.; Baloch, D.M.; Shaukat, A.; Rajput, N.; Qamar, H.; et al. Proanthocyanidins Alleviates AflatoxinB₁-Induced Oxidative Stress and Apoptosis through Mitochondrial Pathway in the Bursa of Fabricius of Broilers. Toxins 2019, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- El-Ghareeb, W.R.; Kishawy, A.T.Y.; Anter, R.G.A.; Aboelabbas Gouda, A.; Abdelaziz, W.S.; Alhawas, B.; Meligy, A.M.A.; Abdel-Raheem, S.M.; Ismail, H.; Ibrahim, D. Novel antioxidant insights of myricetin on the performance of broiler chickens and alleviating experimental infection with Eimeria spp.: Crosstalk between oxidative stress and inflammation. Antioxidants 2023, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashidi, H.E. Gut microbiota and immunity relevance in eubiosis and dysbiosis. Saudi J. Biol. Sci. 2022, 29, 1628–1643. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.C.; Yuan, S.; Hou, X.T.; Meng, H.; Liu, B.H.; Cheng, W.W.; Zhao, M.; Li, H.B.; Guo, X.F.; Di, C.; et al. Natural products modulate NLRP3 in ulcerative colitis. Front. Pharmacol. 2023, 14, 1265825. [Google Scholar] [CrossRef]



| Natural Antioxidant/Dosage/Type of Stress Factor | Oxidative Stress Response | Inflammatory Response/Apoptosis | Metabolism/Intestinal Barrier | Conclusions | Reference |
|---|---|---|---|---|---|
| Rutin (0, 200, or 400 mg/kg) | ↑Nrf2 (400 mg/kg) and ↑CAT (200 mg/kg) | - | ↓AMPKα, ↑FADS1, ACC (200 and 400 mg/kg), ↑PPARγ, FAS, and ELOVL7 (400 mg/kg) | Dietary rutin supplementation appears to alter the fatty acid profile and metabolism by regulating the expression levels of the genes related to lipid metabolism | [73] |
| Lycopene (LYC: 10, 20, or 30 mg/kg) | ↑Nrf2, NQO1, HO-1, and SOD2 (higher expression levels in the 30 mg/kg, 21st day); ↑Nrf2, SOD 2, and NQO1 (higher expression levels in the 30 mg/kg, 42nd day) | - | - | The supplementation with 30 mg/kg of LYC effectively improved the gene expression in the Keap1-Nrf2 signaling pathway. The researchers suggested an increase in the amount of LYC in the early stages of development | [66] |
| Rutin (0, 250, 500, or 1000 mg/kg) | ↑Nrf2 and NQO1 (500 and 1000 mg/kg); ↑HO-1 (500 mg/kg); and ↑SOD with ↑ levels of rutin | ↓BAX in jejunal mucosa (250 and 500 mg/kg); ↓NF-κB (500 mg/kg); ↓TNF-α (500 and 1000 mg/kg); and linear and quadratic ↓IL-2 (dose-dependent) | ↑ZO-1 (500 and 1000 mg/kg), and ↑CLN and CLDN2 (500 mg/kg) | Dietary rutin supplementation improved jejunal morphology and enhanced intestinal barrier function through the inhibition of NF-κB and the activation of the Nrf2/HO-1 pathway. Optimum dose: 500 mg/kg | [68] |
| Magnolol (MAG: 100, 200, 300, or 400 mg/kg) | ↑Nrf2, NQO1, HO-1, GCLC, and SOD (200 and 400 mg/kg, 28th day); ↑GCLC (significant upregulation with 100 and 400 mg/kg); ↑GST and SOD (100 mg/kg and 200 mg/kg, 51st day); and ↑Nrf2, NQO1, HO-1, GST, GCLM, and SOD (300 mg/kg) | - | - | Dietary MAG supplementation enhanced the oxidative stability by activating the Nrf2 pathway, which led to an increase in the expression of related genes such as NQO1, HO-1, and GSH | [65] |
| Genistein (0, 50, 100, or 150 mg/kg) | - | - | ↓LXRα, SREBP-1c, FAS, and ACC (100 and 150 mg/kg); ↑PPARα, ATGL, and CPT-I (100 and 150 mg/kg); ↑FOXO1, ERβ, ↑SIRT1, AMPKα, and PGC-1a (100 and 150 mg/kg) | Dietary genistein supplementation has led to a reduction in abdominal fat deposition by downregulating the expression levels of genes associated with lipogenesis (LXRα, SREBP-1c, FAS, and ACC) and upregulating the expression levels of those associated with lipolysis (PPARα, ATGL, and CPT-I). Researchers have possibly attributed these results to the activation of the AMPK-SIRT1/PGC-1a pathway | [70] |
| Hesperidin (E1: 0.75 or Ε2: 1.5 g/kg feed) + Naringin (Ν1: 0.75 or Ν2: 1.5 g/kg feed) | Ν(+): ↑GSR (positive linear dose–response increase between the N1 and N2 groups) | - | Ν(+): ↑PPARα (positive linear dose–response increase between the two groups with a significant increase in the expression in N2 compared to N1) and ACOX1 (significantly increased in Ν1); Ν(+) ή Ε(+): ↑FASN (significant linear dose–response); and Ε1: ↑ADIPOQ | Supplementation with naringin significantly increased the expression levels of PPARα and ACOX1, i.e., the genes involved in the β-oxidation of fatty acids in the liver. Both hesperidin and naringin supplementation led to an increase in the FASN gene expression in breast muscles, which was perhaps due to the lack of fatty acids in the diet and the need to meet these requirements in the muscles. Meanwhile, both hesperidin and naringin led to the oxidative stability of meat through the regulation of the GSR gene with a significant increase, mainly in naringin supplementation. | [72] |
| Quercetin (0, 0.2, 0.4, or 0.6 g/kg) | - | - | ↑AMPKγ (0.6 g/kg), ↑AMPKα1, AMPKα2, AMPKβ2 (0.2 g/kg), ↑PI3K (0.2 g/kg), ↑LKB1 (0.2, 0.4 and 0.6 g/kg), ↓ACC (0.4 and 0.6 g/kg), ↑CPT1 (0.6 g/kg), ↑PPARα (0.6 g/kg), ↓PPARγ, SREBP1 (0.2, 0.4 and 0.6 g/kg), and ↓HMGR (0.4 and 0.6 g/kg) | The supplementation with quercetin succeeded through increasing the expression levels of PI3K, PKB/ATK, and LKB1, as well as in activating the AMPK pathway and thus limiting adipogenesis and lipid synthesis by reducing the expression levels of SREBP1, PPARγ, and HMGR genes in the liver. The activation of the AMPK signaling pathway also prevented fatty acid intake, thus inducing lipolysis and oxidation by decreasing the expression levels of ACC and increasing that of CPT1 and PPARα, thereby contributing to the overall reduction in fat deposition in the livers of the broilers | [74] |
| Quercetin (200, 400, or 800 ppm) | ↑SOD1 and GSH-Px (400 and 800 ppm) | - | ↑GLUT2, PEPT1 (significantly increased in 800 ppm), and ↑FAS (significantly increased in 400 ppm) | In addition to increasing the expression levels of the antioxidant enzymes SOD1 and GSH-Px, quercetin supplementation upregulated the expression levels of nutrient transporter genes such as GLUT2 (sensor for glucose and its homeostasis), PEPT1 (key role in the absorption of small peptides), and FAS (contributes to the palmitoylation of the intestinal mucus barrier to protect the intestine from pathogens). Recommended doses are 200 and 400 ppm as 800 ppm did not affect the gut morphology as effectively as the other doses | [75] |
| Curcumin (0, 500, 1000, or 2000 mg/kg) | - | - | ↓FAS, SREBP-1c, ↓ACLY, and ACC (2000 mg/kg); and ↑CPT-1 and PPARα (1000 mg/kg and 2000 mg/kg) | Curcumin supplementation contributed to the reduction in abdominal fat by decreasing the expression levels of the genes associated with lipogenesis (ACC, FAS, and SREBP-1c) and increasing the expression levels of the genes related to lipolysis (PPARα and CPT-I) | [71] |
| Resveratrol-loaded liposomal nanocarriers (Resv-Lipo NPs: 0, 50, 100, or 150 mg/kg) + Heat Stress (HS) | HS: ↑HSP70 and HSP90; and ↓SIRT1, SIRT3, SIRT7; Resv-Lipo NPs: ↑SOD, CAT, GSH-Px, Nrf2, and HO-1; ↑SIRT3, ↑SIRT1, and SIRT7 (100 mg/kg and 150 mg/kg); and ↓HSP70 and HSP90 (dose-dependent) | HS: ↑TNF-α and IL-6 Resv-Lipo NPs: ↑IL-10 (in a dose-dependent manner), ↓TNF-α, and IL-6 | HS: ↓MyoD and mTOR; Resv-Lipo NPs: ↓myostatin, as well as ↑MyoD and mTOR (highest expression levels with 150 mg/kg) | Heat stress resulted in the upregulation of HSP70 and HSP90, which were restored to normal levels with the administration of 150 mg/kg of Resv-Lipo NP. The dietary supplementation with Resv-Lipo NPs could also alleviate the oxidative damage and modulate the expression levels of some myogenic regulatory factors, thus instigating muscle growth | [84] |
| Resveratrol (Resv: 400 mg/kg) + Heat Stress (HS) | HS: ↓Nrf2, HO-1, NQO1, GSH Px, and ↑Keap1; HS + Resv: ↑Nrf2, HO-1, NQO1 and ↓Keap1 | - | - | Dietary Resv supplementation relieved the deterioration of meat quality by improving the muscle antioxidant capacity through the activation of Nrf2, thereby promoting its translocation within the nucleus and inducing the expression of genes related to antioxidant activity | [81] |
| Resveratrol (Resv: 500 mg/kg) + Heat Stress (HS) | HS + Resv: ↓HSP70 | HS: ↑BCL-2, MDM2, and ↑ERK, IKB-α; HS + Resv: ↓BCL-2, Apaf-1, MDM2, and ↓NF-κB; and ERK, IKB-a, and p38 MAPK | - | Resv supplementation could reduce the inflammatory response by inhibiting the heat stress-induced activation of NF-κB, MAPK, and HSP70, as well as even prevent the activation of mitochondrial apoptotic pathways | [83] |
| Resveratrol (Resv: 400 mg/kg) + Heat Stress (HS) | HS: ↓Nrf2, SOD1, GPX, GST, and ↑Keap1; HS + Resv: ↑Nrf2, SOD1, GPX, GST, and ↓Keap1 | - | - | Resv supplementation has proven to be an effective way through which to prevent the effects of heat stress by enhancing the intestinal antioxidant capacity through the activation of the Nrf2 signaling pathway | [82] |
| Astaxanthin (0, 10, 20, 40, or 80 mg/kg) + Heat Stress | ↓HSP70, HSTF1, and ↑GST (quadratic changes); ↓GPX1, GR, and SOD1 (linear decrease) | ↓JNK1 and TNF-α (linear decrease); ↑AKT1 and P38MAKP (quadratic changes) | ↓SREBP1 (linear decrease); ↑DGAT2 (linear increase) | Dietary supplementation with astaxanthin affected the expression levels of the genes related to redox status (GST), heat stress (HSP70 and HSF1), inflammation (TNF-α), and lipid metabolism (SREBP1 and DGAT2) | [79] |
| Astaxanthin (0, 20, 40, or 80 mg/kg) + Heat Stress | ↓HSP27 and HSP70 (40 or 80 mg/kg) | ↓TNF-α (80 mg/kg); ↓IL-6 (40 or 80 mg/kg) | - | The decrease in HSP expression levels that came with astaxanthin supplementation was due to its antioxidant activity, which resulted in a reduction in the negative effects of heat stress and led to a reduction in heat stress-induced inflammation (TNF-α and IL-6) | [80] |
| Quercetin (Q: 200, 400, or 800 ppm) + Oxidized oil (Ox) | Ox(+): ↓TXN and HO-1; Ox(+)Q(+): ↑Nrf2, GCLM, CAT, SOD1, GPX2, GLRDX, TXN, and HO-1 (800 ppm); d ↓NOX2 (800 ppm) | Ox(+): ↓IL-8; Ox(+)Q(+): ↑IL-8 | Ox(+): ↓MUC2 and ZO-1; Ox(+)Q(+): ↑MUC2, claudin-2 (800 ppm), and ↑ZO-1 (400 ppm) | Supplementation with 800 ppm of quercetin ameliorated the oxidative stress caused by oxidized oil, restored the redox balance, and strengthened the intestinal barrier | [78] |
| Squalene (SQ: 1.0 g/kg) + Diquat (DQ: 20 mg/mL) | DQ(+): ↑Nrf2, GPX1, and ↓NQO1; DQ(+)SQ(+): ↓GPX1 and ↑NQO1 | DQ(+): ↑Bax and CASP3; DQ(+)SQ(+): ↓Bax and CASP3 | - | SQ supplementation improved the oxidative status and alleviated liver injury by regulating apoptosis, thus indicating its hepatoprotective effect | [76] |
| Pterostilbene (PT: 400 mg/kg) + Diquat (DQ) | DQ(+): ↓SOD1, ↓SIRT1, PGC1a, and NRF1; PT(+): ↑NrF2, HO-1, SOD1, GSTA2, γ-GCLc, ↑SIRT1, and TFAM | PT(-)DQ(+): ↓BCL2 and ↑CASP3; PT(+)DQ(+): ↓CASP3 | - | PT supplementation through its effect on the expression levels of the genes related to oxidative stress and apoptosis proved its protective potential against hepatic damage | [77] |
| Protocatechuic Acid (PCA: 300 or 600 mg/kg) + Lipopolysaccharide (LPS) | LPS(+): ↓CAT, SOD1, and GPx-1; PCA(+): ↑SOD1, CAT, and GPx-1 (600 mg/kg) | - | LPS(+): ↓OCLN, ZO-1, JAM2, and MUC2; PCA(+): ↑OCLN, JAM2, and MUC2 (300 or 600 mg/kg) | Dietary supplementation with PCA was able to protect the intestinal health of broilers by alleviating mucosal damage and preserving the morphological structure of the intestine through the regulation of OCLN, JAM2, and MUC2 genes, while 600 mg/kg of PCA supplementation restored the activity of antioxidant enzymes by regulating the expressions of CAT, SOD1, and GPx-1 | [89] |
| Resveratrol (Resv: 400 mg/kg) + Lipopolysaccharide (LPS: 0.5 mg/kg BW) | - | Resv(-)LPS(+): ↑TLR4, MyD88, NF-kB, IL-1β, IL-6, TNF-α, and ↓IL-10; Resv(+)LPS(-): ↓TLR4, NF-kB, IL-1β, IL-6, and TNF-α; and Resv(+)LPS(+): ↓TLR4, NF-kB, and TNF-α | Resv(-)LPS(+): ↓MUC2, ZO-1, occludin, and claudin-1; Resv(+)LPS(-): ↑MUC2, ZO-1, occludin, claudin-1; and Resv(+)LPS(+): ↑occludin | Supplementation with Resv significantly helped in alleviating the LPS-induced intestinal barrier damage and inflammation by upregulating the expression levels of MUC2, ZO-1, occludin, and claudin-1, as well as by reducing the expression of the TLR4/NF-kB pathway and inflammatory factors | [91] |
| Lutein (LU: 0, 20, or 40 mg/kg) + Lipopolysaccharide (LPS: 1 mg/kg BW) | - | LPS(+)LU(+): ↓TLR4, MyD88, NF-κΒ, IL-1β, IL-6, ↑IL-4 (20 and 40 mg/kg), and ↑IL-10 (higher expression in 40 mg/kg) | LPS(+)LU(+): ↑occludin, vlaudin-1, and ZO-1 (20 and 40 mg/kg) | Supplementation with mainly 40 mg/kg of LU restored the LPS-induced intestinal barrier function by regulating the expression levels of occludin, claudin-1, and ZO-1, as well as by taming inflammation through the inhibition of the TLR4/MyD88 pathway, which reduces the expression levels of pro-inflammatory cytokines (IL-1β and IL-6) and increases that of anti-inflammatory cytokines (IL-4 and IL-10) | [87] |
| Resveratrol (Resv: 400 mg/kg) + Lipopolysaccharide (LPS: 1 mg/kg BW) | - | Resv(-)LPS(+): ↑IL-1β, IL-8, IL-17, TNF-α, and ↓TGF-β; Resv(+)LPS(+): ↓IL-8, IL-6, IL-17, and TNF-α | Resv(-)LPS(+): ↓claudin-1, claudin-5, occludin, and ZO-1 Resv(+)LPS(+): ↑claudin-5, occludin, and ZO-1 | Supplementation with Resv could improve the intestinal barrier function by increasing the expression levels of the genes associated with it, and it could also effectively alleviate the intestinal inflammation caused by LPS | [88] |
| Bisdemethoxycurcumin (BDC: 0 or 150 mg/kg) + Lipopolysaccharide (LPS) | BDC(-)LPS(+): ↓CAT, CuZnSOD, γ-GCLc, γ-GCLm, GSH-Px, GR, Nrf2, HO-1, and NQO1 (in jejunum and ileum); BDC(+)LPS(-): ↑CuZnSOD, γ-GCLc, Nrf2, HO-1, and NQO1 (in jejunum and ileum); ↑CAT and GR (in jejunum); BDC(+)LPS(+): significant ↑CAT, γ-GCLc, Nrf2, HO-1, NQO1 (in jejunum); and ↑γ-GCLc, Nrf2, and CuZn-SOD (in ileum) | - | - | Supplementation with BDC, a related compound of curcumin, demonstrated favorable protection against the oxidative damage that was caused by LPS in the small intestine by activating Nrf2 and upregulating the expression levels of the genes related to the antioxidant system | [90] |
| Taraxasterol (TAR: 25, 50, or 100 mg/kg BW) + Aflatoxin B1 (AFB1: 1 mg/kg) | AFB1(+)TAR(-): ↓Nrf2, NQO1, HO-1, and ↑Keap1; AFB1(+)TAR(+): ↑Nrf2 (100 mg/kg), ↑NQO1, and HO-1 (50 and 100 mg/kg); and ↓Keap1 (25, 50 and 100 mg/kg) | AFB1(+)TAR(-): ↓Bcl-2, ↑Bax, and caspase-3; ↑PI3K, AKT, and mTOR; AFB1(+)TAR(+): ↑Bcl-2 (25, 50 and 100 mg/kg), ↓Bax (50 and 100 mg/kg), and ↓caspase-3 (25, 50 and 100 mg/kg); and ↓PI3K, AKT, and mTOR (50 and 100 mg/kg) | AFB1(+)TAR(-): ↑CYP1A1 and CYP2A6; AFB1(+)TAR(+): ↓CYP2A6 (50 mg/kg); and ↓CYP1A1 and CYP2A6 (100 mg/kg) | TAR supplementation could alleviate the liver damage caused by AFB1, which inhibits oxidative stress, through regulation of the Keap1/Nrf2 signaling pathway. This would improve anti-apoptotic ability and restore the autophagy of hepatocytes by regulating the expression levels of Bcl-2, Bax, and caspase-3, as well as by inhibiting the PI3K/AKT/mTOR pathway | [94] |
| Lycopene (LYC: 200 mg/kg) + Aflatoxin B1 (AFB1: 100 μg/kg) | AFB1(+): ↓MnSOD, Trx2, TrxR2, Prx3, and PGC-1a; ↓NRF1 and TFAM; and AFB1(+)LYC(+): ↑MnSOD, TrxR2, PGC-1a, and ↑TFAM | - | - | LYC supplementation ameliorated AFB1-induced liver injury by upregulating the expression levels of the genes responsible for antioxidant defense and by maintaining mitochondrial biogenesis | [93] |
| Selenium-enriched Yeast (SY: 0.4 mg/kg/feed) + Ochratoxin-A (OTA: 50 μg/kg/BW) | - | (OTA): ↑NF-κB, TLR4, and MYD88; (OTA + SY): ↓NF-κB, TLR4, and MYD88 | (OTA): ↓claudin-1, occludin, and ZO-1; (OTA + SY): ↑claudin-1 and occludin | The supplementation with SY alleviated the oxidative injury that was caused by OTA, and this was achieved through regulation of the TLR4/MYD88 pathway and inhibition of the NF-κB signaling pathway while protecting the intestinal barrier | [95] |
| Selenium-enriched Yeast (SY: 0.4 mg/kg/feed) + Ochratoxin-A (OTA: 50 μg/kg/BW) | (OTA): ↓Nrf2 and HO-1 (OTA + SY): ↑Nrf2 and HO-1 | (OTA): ↑NF-κB, IL-1β, and TNF-α (OTA + SY): ↓NF-κB, IL-1β, and TNF-α | - | SY supplementation alleviated the intestinal toxicity that was caused by OTA through the activation of Nrf2 and inhibition of NF-κB | [96] |
| Proanthocyanidins (PC: 250 mg/kg) + Aflatoxin B1 (AFB1: 1 mg/kg) | PC(-)AFB1(+): ↓SOD, GST, CAT, and GPx1; PC(+)AFB1(+): ↑SOD, GST, CAT, and GPx1 | PC(-)AFB1(+): ↑Bax, caspase-3, caspase-9, p53, cytochrome-C, and ↓Bcl-2; PC(+)AFB1(+): ↓Bax, caspase-3, caspase-9, p53, cytochrome-C, and ↑Bcl-2 | - | PC supplementation could reverse the negative effect of AFB1 by reducing oxidative stress, thereby enhancing the antioxidant defense system and simultaneously inhibiting AFB1-induced apoptosis by regulating the expression levels of the genes associated with it | [97] |
| Myricetin (Myc: 200, 400, or 600 mg/kg) + Eimeria spp. (IC) | IC(+)Myc(-): ↓CAT, SOD, GSH-Px, HO-1, NQO1 (intestine and muscles), and ↑COX-2; IC(+)Myc(+): ↑CAT, SOD, GSH-Px, HO-1, NQO1 (↑expression with ↑dose), and ↓COX-2 (↓expression with ↑dose) | IC(+)Myc(-): ↑IL-1β, IL-6, TNF-α, ↓IL-10, AvBD6, and AvBD612; ↑CCL4, CCL20, and CXCL13; IC(+)Myc(+): ↓IL-1β, IL-6, TNF-α (in a dose-dependent manner),↑IL-10, AvBD6, AvBD612; and ↓CCL4, CCL20, and CXCL13 (higher levels of Myc led to more significant downregulation) | - | Supplementation with Myc improved the broiler’s response against Eimeria spp. by regulating the expression levels of the genes related to antioxidant defense (CAT, SOD, GSH-Px, HO-1, and NQO1) and the genes with anti-inflammatory (IL-10) and pro-inflammatory (IL-1β, IL-6, TNF-α) activity, as well as by decreasing the expression of chemotactic cytokines (CCL4, CCL20, and CXCL13) and increasing the expression of peptides that confer direct defense against microbial invasion (AvBD6 and AvBD612) | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouvedaki, I.; Pappas, A.C.; Surai, P.F.; Zoidis, E. Nutrigenomics of Natural Antioxidants in Broilers. Antioxidants 2024, 13, 270. https://doi.org/10.3390/antiox13030270
Kouvedaki I, Pappas AC, Surai PF, Zoidis E. Nutrigenomics of Natural Antioxidants in Broilers. Antioxidants. 2024; 13(3):270. https://doi.org/10.3390/antiox13030270
Chicago/Turabian StyleKouvedaki, Ioanna, Athanasios C. Pappas, Peter F. Surai, and Evangelos Zoidis. 2024. "Nutrigenomics of Natural Antioxidants in Broilers" Antioxidants 13, no. 3: 270. https://doi.org/10.3390/antiox13030270