NADPH Oxidase 3: Beyond the Inner Ear
Abstract
:1. NADPH Oxidase 3
1.1. The Family of NADPH Oxidases
Structural Components of Nox Enzymes
1.2. Nox3: Structure and Subunits
1.2.1. Adaptor Subunits of Nox3
1.2.2. The Nox3-p22phox Complex
1.2.3. Nox3 and Rac
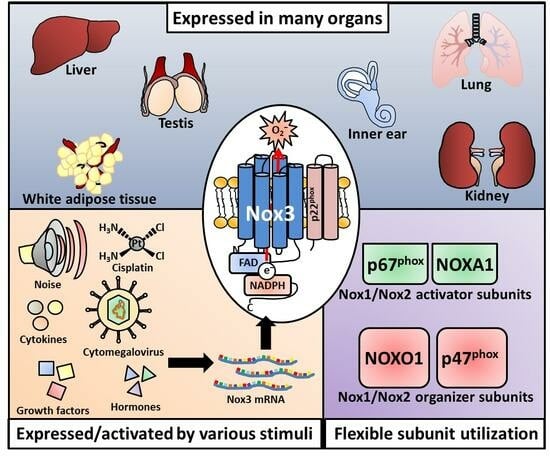
2. Location of Nox3
2.1. Location of Nox3 in Organs and Tissues
2.1.1. Nox3 in the Inner Ear
Nox3 in the Vestibular System
Nox3 in the Cochlea
2.1.2. Nox3 in Other Organs
2.2. Expression of Nox3 in Cell Types
2.2.1. Nox3 in Cells of the Inner Ear
2.2.2. Nox3 in Lung Cells
2.2.3. Nox3 in Liver Cells
2.2.4. Nox3 in Fibroblasts, Endothelial and Epithelial Cells in General
2.2.5. Nox3 in Cells of the Eye
2.2.6. Nox3 in Cells of the Nervous System
2.2.7. Nox3 in Cancer Cells
2.2.8. Nox3 in Immune Cells
2.2.9. Nox3 in Other Cell Types
2.3. Subcellular Locations of Nox3
3. Activation and Regulation of Nox3
3.1. Activation of Nox3
3.2. Regulation of Nox3
3.2.1. Nox3 Regulation on the Expression Level
Up-Regulation Nox3 on the Expression Level
Down-Regulation or No Effect on Nox3 Expression
Nox3 Regulation via Other Factors
4. Functions of Nox3
4.1. Signaling Functions of Nox3
4.2. Functions of Nox3 in Cell Differentiation
4.3. Functions of Nox3 in the Inner Ear
4.3.1. Functions of Nox3 in the Vestibular System
4.3.2. Functions of Nox3 in the Cochlea
5. Roles of Nox3 in Diseases
5.1. Role of Nox3 in Hearing Loss
5.1.1. Noise-Induced Ototoxicity
5.1.2. Cisplatin-Induced Ototoxicity
5.1.3. Cytomegalovirus-Induced Hearing Loss
5.1.4. Age-Induced Hearing Loss
5.2. Role of Nox3 during Vertigo
5.3. Role of Nox3 during Lung Diseases
5.4. Role of Nox3 during Cardiovasclar Diseases
5.4.1. Nox3 and Type 2 Diabetes
5.4.2. Nox3 and Adipositas
5.4.3. Nox3 and Stroke
5.4.4. Nox3 and Heart Failure
5.5. Role of Nox3 during Renal Diseases
5.6. Role of Nox3 during Gastrointetinal Disaeses
5.7. Role of Nox3 in Other Diseases
6. Nox3 as Therapeutic Target
6.1. Therapeutic Nox3 Targeting in the Inner Ear
6.1.1. Therapeutic Treatment of Cisplatin-Induced Hearing Loss
6.1.2. Therapeutic Treatment of Noise-Induced Hearing Loss
6.2. Therapeutic Nox3 Targeting as Diabetic Treatment
6.3. Therapeutic Nox3 Targeting during Cancer
6.4. Therapeutic Nox3 Targeting during Multiple Sclerosis
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inupakutika, M.A.; Sengupta, S.; Devireddy, A.R.; Azad, R.K.; Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 2016, 67, 5933–5943. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best practice & research. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Lambeth, J.D.; Neish, A.S. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Annu. Rev. Pathol. 2014, 9, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. Beobachtungen über die oxydationsprozesse im seeigelei. Biol. Chem. 1908, 57, 1–16. [Google Scholar] [CrossRef]
- Shapiro, B.M. The control of oxidant stress at fertilization. Science 1991, 252, 533–536. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.M.; Johnson, M.H. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development 1991, 113, 551–560. [Google Scholar] [CrossRef]
- Sirokmany, G.; Donko, A.; Geiszt, M. Nox/duox family of NADPH oxidases: Lessons from knockout mouse models. Trends Pharmacol. Sci. 2016, 37, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schroder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The Nox family of Ros-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277. [Google Scholar] [CrossRef]
- Kawahara, T.; Quinn, M.T.; Lambeth, J.D. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol. Biol. 2007, 7, 109. [Google Scholar] [CrossRef]
- Karplus, P.A.; Daniels, M.J.; Herriott, J.R. Atomic structure of ferredoxin-NADP+ reductase: Prototype for a structurally novel flavoenzyme family. Science 1991, 251, 60–66. [Google Scholar] [CrossRef]
- Carrillo, N.; Ceccarelli, E.A. Open questions in ferredoxin-NADP+ reductase catalytic mechanism. Eur. J. Biochem. 2003, 270, 1900–1915. [Google Scholar] [CrossRef]
- Wang, M.; Roberts, D.L.; Paschke, R.; Shea, T.M.; Masters, B.S.; Kim, J.J. Three-dimensional structure of NADPH-cytochrome p450 reductase: Prototype for fmn- and fad-containing enzymes. Proc. Natl. Acad. Sci. USA 1997, 94, 8411–8416. [Google Scholar] [CrossRef]
- Leclerc, D.; Wilson, A.; Dumas, R.; Gafuik, C.; Song, D.; Watkins, D.; Heng, H.H.; Rommens, J.M.; Scherer, S.W.; Rosenblatt, D.S.; et al. Cloning and mapping of a cdna for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc. Natl. Acad. Sci. USA 1998, 95, 3059–3064. [Google Scholar] [CrossRef]
- Vignais, P.V. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell. Mol. Life Sci. CMLS 2002, 59, 1428–1459. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. Nox enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Quinn, M.T. Assembly of the phagocyte NADPH oxidase: Molecular interaction of oxidase proteins. J. Leukoc. Biol. 1996, 60, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004, 122, 277–291. [Google Scholar] [CrossRef]
- Geiszt, M.; Leto, T.L. The Nox family of NAD(P)H oxidases: Host defense and beyond. J. Biol. Chem. 2004, 279, 51715–51718. [Google Scholar] [CrossRef]
- Petry, A.; Weitnauer, M.; Gorlach, A. Receptor activation of NADPH oxidases. Antioxid. Redox Signal. 2010, 13, 467–487. [Google Scholar] [CrossRef]
- Stasia, M.J. Cyba encoding p22(phox), the cytochrome b558 alpha polypeptide: Gene structure, expression, role and physiopathology. Gene 2016, 586, 27–35. [Google Scholar] [CrossRef]
- Buvelot, H.; Jaquet, V.; Krause, K.-H. Mammalian NADPH oxidases. In NADPH Oxidases: Methods and Protocols; Knaus, U.G., Leto, T.L., Eds.; Springer: New York, NY, USA, 2019; pp. 17–36. [Google Scholar]
- Davis, A.R.; Mascolo, P.L.; Bunger, P.L.; Sipes, K.M.; Quinn, M.T. Cloning and sequencing of the bovine flavocytochrome b subunit proteins, gp91-phox and p22-phox: Comparison with other known flavocytochrome b sequences. J. Leukoc. Biol. 1998, 64, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Onouchi, H.; Hamada, S.; Machida, C.; Hammond-Kosack, K.E.; Jones, J.D. Six arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. Cell Mol. Biol. 1998, 14, 365–370. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species in non-phagocytic cells. J. Leukoc. Biol. 1999, 65, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Teshima, S.; Rokutan, K.; Nikawa, T.; Kishi, K. Guinea pig gastric mucosal cells produce abundant superoxide anion through an NADPH oxidase-like system. Gastroenterology 1998, 115, 1186–1196. [Google Scholar] [CrossRef]
- Linnerz, T.; Hall, C.J. The diverse roles of phagocytes during bacterial and fungal infections and sterile inflammation: Lessons from zebrafish. Front. Immunol. 2020, 11, 1094. [Google Scholar] [CrossRef]
- Inoue, Y.; Suenaga, Y.; Yoshiura, Y.; Moritomo, T.; Ototake, M.; Nakanishi, T. Molecular cloning and sequencing of Japanese pufferfish (Takifugu rubripes) NADPH oxidase cdnas. Dev. Comp. Immunol. 2004, 28, 911–925. [Google Scholar] [CrossRef]
- Ha, E.M.; Oh, C.T.; Bae, Y.S.; Lee, W.J. A direct role for dual oxidase in drosophila gut immunity. Science 2005, 310, 847–850. [Google Scholar] [CrossRef]
- Carol, R.J.; Dolan, L. The role of reactive oxygen species in cell growth: Lessons from root hairs. J. Exp. Bot. 2006, 57, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Damude, H.G.; Werner, D.; Doerner, P.; Dixon, R.A.; Lamb, C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 1998, 10, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.; Dangl, J.L. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in arabidopsis thaliana. Nat. Genet. 2005, 37, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J. Exploitation of reactive oxygen species by fungi: Roles in host-fungus interaction and fungal development. J. Microbiol. Biotechnol. 2014, 24, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ortiz, T.; Riveros-Rosas, H.; Aguirre, J. Reactive oxygen species generated by microbial NADPH oxidase noxa regulate sexual development in aspergillus nidulans. Mol. Microbiol. 2003, 50, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Lardy, B.; Bof, M.; Aubry, L.; Paclet, M.H.; Morel, F.; Satre, M.; Klein, G. NADPH oxidase homologs are required for normal cell differentiation and morphogenesis in dictyostelium discoideum. Biochim. Biophys. Acta 2005, 1744, 199–212. [Google Scholar] [CrossRef]
- Eichinger, L.; Noegel, A.A. Comparative genomics of dictyostelium discoideum and entamoeba histolytica. Curr. Opin. Microbiol. 2005, 8, 606–611. [Google Scholar] [CrossRef]
- Edens, W.A.; Sharling, L.; Cheng, G.; Shapira, R.; Kinkade, J.M.; Lee, T.; Edens, H.A.; Tang, X.; Sullards, C.; Flaherty, D.B.; et al. Tyrosine cross-linking of extracellular matrix is catalyzed by duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 2001, 154, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Wingler, K.; Hermans, J.J.; Schiffers, P.; Moens, A.; Paul, M.; Schmidt, H.H. Nox1, 2, 4, 5: Counting out oxidative stress. Br. J. Pharmacol. 2011, 164, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef]
- Jesaitis, A.J. Structure of human phagocyte cytochrome b and its relationship to microbicidal superoxide production. J. Immunol. 1995, 155, 3286–3288. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A. Activation of the neutrophil respiratory burst oxidase. J. Infect. Dis. 1999, 179 (Suppl. 2), S309–S317. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.H. Tissue distribution and putative physiological function of nox family NADPH oxidases. Jpn. J. Infect. Dis. 2004, 57, S28–S29. [Google Scholar] [PubMed]
- Green, T.R.; Shangguan, X. Stoichiometry of O2 metabolism and NADPH oxidation of the cell-free latent oxidase reconstituted from cytosol and solubilized membrane from resting human neutrophils. J. Biol. Chem. 1993, 268, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Parkos, C.A.; Allen, R.A.; Cochrane, C.G.; Jesaitis, A.J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J. Clin. Investig. 1987, 80, 732–742. [Google Scholar] [CrossRef]
- Parkos, C.A.; Dinauer, M.C.; Walker, L.E.; Allen, R.A.; Jesaitis, A.J.; Orkin, S.H. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc. Natl. Acad. Sci. USA 1988, 85, 3319–3323. [Google Scholar] [CrossRef]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.M.; Dunne, M.J.; Segal, A.W. Purification of cytochrome b-245 from human neutrophils. Biochem. J. 1984, 219, 519–527. [Google Scholar] [CrossRef]
- Dinauer, M.C.; Orkin, S.H.; Brown, R.; Jesaitis, A.J.; Parkos, C.A. The glycoprotein encoded by the x-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 1987, 327, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Lambeth, J.D.; Nauseef, W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002, 397, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Baehner, R.L.; Nathan, D.G. Leukocyte oxidase: Defective activity in chronic granulomatous disease. Science 1967, 155, 835–836. [Google Scholar] [CrossRef] [PubMed]
- Baehner, R.L.; Nathan, D.G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N. Engl. J. Med. 1968, 278, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.Y.; Islam, M.F.; Quastel, J.H. Biochemical aspects of phagocytosis. Nature 1961, 192, 535–541. [Google Scholar] [CrossRef]
- Baldridge, C.W.; Gerard, R.W. The extra respiration of phagocytosis. Am. J. Physiol.-Leg. Content 1932, 103, 235–236. [Google Scholar] [CrossRef]
- Rossi, F.; Zatti, M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. Nadh and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia 1964, 20, 21–23. [Google Scholar] [CrossRef]
- Morel, F.; Doussiere, J.; Vignais, P.V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur. J. Biochem. 1991, 201, 523–546. [Google Scholar] [CrossRef]
- Babior, B.M. NADPH oxidase: An update. Blood 1999, 93, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. The phagocyte nox2 NADPH oxidase in microbial killing and cell signaling. Curr. Opin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Sbarra, A.J.; Karnovsky, M.L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 1959, 234, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Mandell, G.L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect. Immun. 1974, 9, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.T.; Gauss, K.A. Structure and regulation of the neutrophil respiratory burst oxidase: Comparison with nonphagocyte oxidases. J. Leukoc. Biol. 2004, 76, 760–781. [Google Scholar] [CrossRef]
- Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef]
- Cross, A.R.; Segal, A.W. The NADPH oxidase of professional phagocytes—Prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta 2004, 1657, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Nauseef, W.M. How human neutrophils kill and degrade microbes: An integrated view. Immunol. Rev. 2007, 219, 88–102. [Google Scholar] [CrossRef]
- Lam, G.Y.; Huang, J.; Brumell, J.H. The many roles of Nox2 NADPH oxidase-derived ROS in immunity. Semin. Immunopathol. 2010, 32, 415–430. [Google Scholar] [CrossRef]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef]
- Badwey, J.A.; Curnutte, J.T.; Robinson, J.M.; Lazdins, J.K.; Briggs, R.T.; Karnovsky, M.J.; Karnovsky, M.L. Comparative aspects of oxidative metabolism of neutrophils from human blood and guinea pig peritonea: Magnitude of the respiratory burst, dependence upon stimulating agents, and localization of the oxidases. J. Cell. Physiol. 1980, 105, 541–545. [Google Scholar] [CrossRef]
- Ohno, Y.; Hirai, K.; Kanoh, T.; Uchino, H.; Ogawa, K. Subcellular localization of H2O2 production in human neutrophils stimulated with particles and an effect of cytochalasin-b on the cells. Blood 1982, 60, 253–260. [Google Scholar] [CrossRef]
- Heymes, C.; Bendall, J.K.; Ratajczak, P.; Cave, A.C.; Samuel, J.L.; Hasenfuss, G.; Shah, A.M. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 2003, 41, 2164–2171. [Google Scholar] [CrossRef]
- Javeshghani, D.; Magder, S.A.; Barreiro, E.; Quinn, M.T.; Hussain, S.N. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 2002, 165, 412–418. [Google Scholar] [CrossRef]
- Reinehr, R.; Becker, S.; Eberle, A.; Grether-Beck, S.; Haussinger, D. Involvement of NADPH oxidase isoforms and src family kinases in cd95-dependent hepatocyte apoptosis. J. Biol. Chem. 2005, 280, 27179–27194. [Google Scholar] [CrossRef]
- Piccoli, C.; Ria, R.; Scrima, R.; Cela, O.; D’Aprile, A.; Boffoli, D.; Falzetti, F.; Tabilio, A.; Capitanio, N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J. Biol. Chem. 2005, 280, 26467–26476. [Google Scholar] [CrossRef]
- Altenhofer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, Z.M.; Henneke, P.; Kolter, J. From flies to men: Ros and the NADPH oxidase in phagocytes. Front. Cell Dev. Biol. 2021, 9, 628991. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Banfi, B.; Maturana, A.; Jaconi, S.; Arnaudeau, S.; Laforge, T.; Sinha, B.; Ligeti, E.; Demaurex, N.; Krause, K.H. A mammalian h+ channel generated through alternative splicing of the NADPH oxidase homolog noh-1. Science 2000, 287, 138–142. [Google Scholar] [CrossRef]
- Bokoch, G.M.; Diebold, B.A. Current molecular models for NADPH oxidase regulation by Rac gtpase. Blood 2002, 100, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Kopp, J.B.; Varnai, P.; Leto, T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, X. The human Nox4: Gene, structure, physiological function and pathological significance. J. Drug Target. 2015, 23, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Madyastha, P.; Bingel, S.; Ries, W.; Key, L. A new superoxide-generating oxidase in murine osteoclasts. J. Biol. Chem. 2001, 276, 5452–5458. [Google Scholar] [CrossRef]
- Banfi, B.; Molnar, G.; Maturana, A.; Steger, K.; Hegedus, B.; Demaurex, N.; Krause, K.H. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001, 276, 37594–37601. [Google Scholar] [CrossRef] [PubMed]
- De Deken, X.; Wang, D.; Many, M.C.; Costagliola, S.; Libert, F.; Vassart, G.; Dumont, J.E.; Miot, F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000, 275, 23227–23233. [Google Scholar] [CrossRef]
- Bokoch, G.M.; Knaus, U.G. NADPH oxidases: Not just for leukocytes anymore! Trends Biochem. Sci. 2003, 28, 502–508. [Google Scholar] [CrossRef]
- Sumimoto, H.; Miyano, K.; Takeya, R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 2005, 338, 677–686. [Google Scholar] [CrossRef]
- Donko, A.; Peterfi, Z.; Sum, A.; Leto, T.; Geiszt, M. Dual oxidases. Philosophical transactions of the Royal Society of London. Ser. B Biol. Sci. 2005, 360, 2301–2308. [Google Scholar]
- Geiszt, M.; Witta, J.; Baffi, J.; Lekstrom, K.; Leto, T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003, 17, 1502–1504. [Google Scholar] [CrossRef]
- Belikov, A.V.; Schraven, B.; Simeoni, L. T cells and reactive oxygen species. J. Biomed. Sci. 2015, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, R.; Sareila, O.; Pizzolla, A.; Winter, S.; Hagert, C.; Jaakkola, N.; Kelkka, T.; Olsson, L.M.; Wing, K.; Backdahl, L. Hydrogen peroxide as an immunological transmitter regulating autoreactive t cells. Antioxid. Redox Signal. 2013, 18, 1463–1474. [Google Scholar] [CrossRef]
- Meier, B.; Cross, A.R.; Hancock, J.T.; Kaup, F.J.; Jones, O.T. Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochem. J. 1991, 275 Pt 1, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Takeya, R.; Sumimoto, H. Molecular mechanism for activation of superoxide-producing NADPH oxidases. Mol. Cells 2003, 16, 271–277. [Google Scholar] [CrossRef]
- Schroder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef]
- Nayernia, Z.; Jaquet, V.; Krause, K.H. New insights on Nox enzymes in the central nervous system. Antioxid. Redox Signal. 2014, 20, 2815–2837. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Zalba, G.; San Jose, G.; Moreno, M.U.; Fortuno, M.A.; Fortuno, A.; Beaumont, F.J.; Diez, J. Oxidative stress in arterial hypertension: Role of NAD(P)H oxidase. Hypertension 2001, 38, 1395–1399. [Google Scholar] [CrossRef]
- Jones, S.A.; Hancock, J.T.; Jones, O.T.; Neubauer, A.; Topley, N. The expression of NADPH oxidase components in human glomerular mesangial cells: Detection of protein and mRNA for p47phox, p67phox, and p22phox. J. Am. Soc. Nephrol. JASN 1995, 5, 1483–1491. [Google Scholar] [CrossRef]
- Ahmarani, L.; Avedanian, L.; Al-Khoury, J.; Perreault, C.; Jacques, D.; Bkaily, G. Whole-cell and nuclear NADPH oxidases levels and distribution in human endocardial endothelial, vascular smooth muscle, and vascular endothelial cells. Can. J. Physiol. Pharmacol. 2013, 91, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.; Rajadurai, A.; Carle, A.B.; Kochevar, I.E. 7-dehydrocholesterol enhances ultraviolet a-induced oxidative stress in keratinocytes: Roles of NADPH oxidase, mitochondria, and lipid rafts. Free Radic. Biol. Med. 2006, 41, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Sambo, P.; Baroni, S.S.; Luchetti, M.; Paroncini, P.; Dusi, S.; Orlandini, G.; Gabrielli, A. Oxidative stress in scleroderma: Maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum. 2001, 44, 2653–2664. [Google Scholar] [CrossRef]
- Quinn, M.T.; Ammons, M.C.; Deleo, F.R. The expanding role of NADPH oxidases in health and disease: No longer just agents of death and destruction. Clin. Sci. 2006, 111, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.R.; Kachra, Z.; Spokes, K.C.; Aird, W.C. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000, 486, 252–256. [Google Scholar] [CrossRef]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Lassegue, B.; San Martin, A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef]
- Lambeth, J.D. Regulation of the phagocyte respiratory burst oxidase by protein interactions. J. Biochem. Mol. Biol. 2000, 33, 427–439. [Google Scholar]
- Reeves, E.P.; Lu, H.; Jacobs, H.L.; Messina, C.G.; Bolsover, S.; Gabella, G.; Potma, E.O.; Warley, A.; Roes, J.; Segal, A.W. Killing activity of neutrophils is mediated through activation of proteases by k+ flux. Nature 2002, 416, 291–297. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef]
- Skonieczna, M.; Hejmo, T.; Poterala-Hejmo, A.; Cieslar-Pobuda, A.; Buldak, R.J. NADPH oxidases: Insights into selected functions and mechanisms of action in cancer and stem cells. Oxidative Med. Cell. Longev. 2017, 2017, 9420539. [Google Scholar] [CrossRef]
- Juhasz, A.; Ge, Y.; Markel, S.; Chiu, A.; Matsumoto, L.; van Balgooy, J.; Roy, K.; Doroshow, J.H. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic. Res. 2009, 43, 523–532. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ros levels that regulate self-renewal and neurogenesis in a pi3k/akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, J.; Post, J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 2004, 337, 1–13. [Google Scholar] [CrossRef]
- Arnold, R.S.; Shi, J.; Murad, E.; Whalen, A.M.; Sun, C.Q.; Polavarapu, R.; Parthasarathy, S.; Petros, J.A.; Lambeth, J.D. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA 2001, 98, 5550–5555. [Google Scholar] [CrossRef] [PubMed]
- Teshima, S.; Kutsumi, H.; Kawahara, T.; Kishi, K.; Rokutan, K. Regulation of growth and apoptosis of cultured guinea pig gastric mucosal cells by mitogenic oxidase 1. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1169–G1176. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Bunderson, M.; Wilham, J.; Black, S.M. Important role for Rac1 in regulating reactive oxygen species generation and pulmonary arterial smooth muscle cell growth. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1314–L1322. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y. Aiding and abetting roles of nox oxidases in cellular transformation. Nat. Rev. Cancer 2012, 12, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Weyemi, U.; Redon, C.E.; Parekh, P.R.; Dupuy, C.; Bonner, W.M. NADPH oxidases Noxs and Duoxs as putative targets for cancer therapy. Anti-Cancer Agents Med. Chem. 2013, 13, 502–514. [Google Scholar]
- Brar, S.S.; Kennedy, T.P.; Sturrock, A.B.; Huecksteadt, T.P.; Quinn, M.T.; Whorton, A.R.; Hoidal, J.R. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am. J. Physiol. Cell Physiol. 2002, 282, C1212–C1224. [Google Scholar] [CrossRef] [PubMed]
- Seshiah, P.N.; Weber, D.S.; Rocic, P.; Valppu, L.; Taniyama, Y.; Griendling, K.K. Angiotensin ii stimulation of NAD(P)H oxidase activity: Upstream mediators. Circ. Res. 2002, 91, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc. Res. 2006, 71, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 is involved in angiotensin ii-mediated hypertension: A study in nox1-deficient mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef]
- Lassegue, B.; Clempus, R.E. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R277–R297. [Google Scholar] [CrossRef] [PubMed]
- Santillo, M.; Colantuoni, A.; Mondola, P.; Guida, B.; Damiano, S. Nox signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Schramm, A.; Matusik, P.; Osmenda, G.; Guzik, T.J. Targeting NADPH oxidases in vascular pharmacology. Vascul. Pharmacol. 2012, 56, 216–231. [Google Scholar] [CrossRef]
- Corvilain, B.; van Sande, J.; Laurent, E.; Dumont, J.E. The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology 1991, 128, 779–785. [Google Scholar] [CrossRef]
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7A–11A. [Google Scholar] [CrossRef] [PubMed]
- Dworakowski, R.; Anilkumar, N.; Zhang, M.; Shah, A.M. Redox signalling involving NADPH oxidase-derived reactive oxygen species. Biochem. Soc. Trans. 2006, 34, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Irani, K.; Xia, Y.; Zweier, J.L.; Sollott, S.J.; Der, C.J.; Fearon, E.R.; Sundaresan, M.; Finkel, T.; Goldschmidt-Clermont, P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 1997, 275, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, P.; Cirri, P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem. Sci. 2003, 28, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Ziff, M. Increased superoxide anion release from human endothelial cells in response to cytokines. J. Immunol. 1986, 137, 3295–3298. [Google Scholar] [CrossRef] [PubMed]
- Werner, E. Gtpases and reactive oxygen species: Switches for killing and signaling. J. Cell Sci. 2004, 117, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Anilkumar, N.; Weber, R.; Zhang, M.; Brewer, A.; Shah, A.M. Nox4 and Nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1347–1354. [Google Scholar] [CrossRef]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Cooney, S.J.; Bermudez-Sabogal, S.L.; Byrnes, K.R. Cellular and temporal expression of NADPH oxidase (nox) isotypes after brain injury. J. Neuroinflamm. 2013, 10, 155. [Google Scholar] [CrossRef]
- Coyoy, A.; Olguin-Albuerne, M.; Martinez-Briseno, P.; Moran, J. Role of reactive oxygen species and NADPH-oxidase in the development of rat cerebellum. Neurochem. Int. 2013, 62, 998–1011. [Google Scholar] [CrossRef]
- Olguin-Albuerne, M.; Moran, J. Ros produced by nox2 control in vitro development of cerebellar granule neurons development. ASN Neuro 2015, 7, 1759091415578712. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kawada, K.; Gotoh, Y.; Shiba, T.; Ogita, K. Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem. Int. 2010, 56, 740–746. [Google Scholar] [CrossRef]
- Cook-Mills, J.M. Reactive oxygen species regulation of immune function. Mol. Immunol. 2002, 39, 497–498. [Google Scholar] [CrossRef]
- Irani, K. Oxidant signaling in vascular cell growth, death, and survival: A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. Ros-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. Ros function in redox signaling and oxidative stress. Curr. Biol. CB 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Tsukasaki, Y.; Chan, W.C.; Le, J.P.; Kwok, M.L.; Zhou, J.; Natarajan, V.; Mostafazadeh, N.; Maienschein-Cline, M.; Papautsky, I.; et al. Trans-endothelial neutrophil migration activates bactericidal function via piezo1 mechanosensing. Immunity 2024, 57, 52–67.e10. [Google Scholar] [CrossRef]
- Kawahara, T.; Teshima, S.; Oka, A.; Sugiyama, T.; Kishi, K.; Rokutan, K. Type i Helicobacter pylori lipopolysaccharide stimulates toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect. Immun. 2001, 69, 4382–4389. [Google Scholar] [CrossRef]
- Li, J.; Stouffs, M.; Serrander, L.; Banfi, B.; Bettiol, E.; Charnay, Y.; Steger, K.; Krause, K.H.; Jaconi, M.E. The NADPH oxidase nox4 drives cardiac differentiation: Role in regulating cardiac transcription factors and map kinase activation. Mol. Biol. Cell 2006, 17, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- Oakley, F.D.; Smith, R.L.; Engelhardt, J.F. Lipid rafts and caveolin-1 coordinate interleukin-1beta (il-1beta)-dependent activation of nfkappab by controlling endocytosis of Nox2 and il-1beta receptor 1 from the plasma membrane. J. Biol. Chem. 2009, 284, 33255–33264. [Google Scholar] [CrossRef] [PubMed]
- Prosser, B.L.; Ward, C.W.; Lederer, W.J. X-ros signaling: Rapid mechano-chemo transduction in heart. Science 2011, 333, 1440–1445. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Coyoy, A.; Valencia, A.; Guemez-Gamboa, A.; Moran, J. Role of NADPH oxidase in the apoptotic death of cultured cerebellar granule neurons. Free Radic. Biol. Med. 2008, 45, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carbente, M.R.; Castro-Obregon, S.; Covarrubias, L.; Narvaez, V. Motoneuronal death during spinal cord development is mediated by oxidative stress. Cell Death Differ. 2005, 12, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Jezek, P.; Hlavata, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sulik, K.K.; Chen, S.Y. The role of nox enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos. Toxicol. Lett. 2010, 193, 94–100. [Google Scholar] [CrossRef]
- Valencia, A.; Sapp, E.; Kimm, J.S.; McClory, H.; Reeves, P.B.; Alexander, J.; Ansong, K.A.; Masso, N.; Frosch, M.P.; Kegel, K.B.; et al. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in huntington’s disease. Hum. Mol. Genet. 2013, 22, 1112–1131. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The role of oxidative stress in atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Elnakish, M.T.; Hassanain, H.H.; Janssen, P.M.; Angelos, M.G.; Khan, M. Emerging role of oxidative stress in metabolic syndrome and cardiovascular diseases: Important role of rac/NADPH oxidase. J. Pathol. 2013, 231, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef]
- Frederiksen, T.W.; Ramlau-Hansen, C.H.; Stokholm, Z.A.; Grynderup, M.B.; Hansen, A.M.; Kristiansen, J.; Vestergaard, J.M.; Bonde, J.P.; Kolstad, H.A. Noise-induced hearing loss—A preventable disease? Results of a 10-year longitudinal study of workers exposed to occupational noise. Noise Health 2017, 19, 103–111. [Google Scholar]
- Kuroda, J.; Sadoshima, J. NADPH oxidase and cardiac failure. J. Cardiovasc. Transl. Res. 2010, 3, 314–320. [Google Scholar] [CrossRef]
- Hahn, N.E.; Meischl, C.; Kawahara, T.; Musters, R.J.; Verhoef, V.M.; van der Velden, J.; Vonk, A.B.; Paulus, W.J.; van Rossum, A.C.; Niessen, H.W.; et al. Nox5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am. J. Pathol. 2012, 180, 2222–2229. [Google Scholar] [CrossRef]
- Byrne, J.A.; Grieve, D.J.; Bendall, J.K.; Li, J.M.; Gove, C.; Lambeth, J.D.; Cave, A.C.; Shah, A.M. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin ii-induced cardiac hypertrophy. Circ. Res. 2003, 93, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, B.; Sorrentino, A.; Badole, S.; Bogdanova, Y.; Belousov, V.; Michel, T. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 2018, 9, 4044. [Google Scholar] [CrossRef]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Sirker, A.; Zhang, M.; Shah, A.M. NADPH oxidases in cardiovascular disease: Insights from in vivo models and clinical studies. Basic Res. Cardiol. 2011, 106, 735–747. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Lambeth, J.D. Nox enzymes, ros, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, J.H.; Lee, K.H.; Kim, H.Y.; Kim, Y.S.; Choi, W.S.; Lee, J. Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PLoS ONE 2015, 10, e0116814. [Google Scholar] [CrossRef] [PubMed]
- Infanger, D.W.; Sharma, R.V.; Davisson, R.L. NADPH oxidases of the brain: Distribution, regulation, and function. Antioxid. Redox Signal. 2006, 8, 1583–1596. [Google Scholar] [CrossRef]
- Kahles, T.; Luedike, P.; Endres, M.; Galla, H.J.; Steinmetz, H.; Busse, R.; Neumann-Haefelin, T.; Brandes, R.P. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 2007, 38, 3000–3006. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.A.; Guberski, D.L.; Somogyi-Mann, M.; Grant, M.B. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the bbz/wor diabetic rat. Free Radic. Biol. Med. 2000, 28, 91–101. [Google Scholar] [CrossRef]
- Gardner, C.D.; Eguchi, S.; Reynolds, C.M.; Eguchi, K.; Frank, G.D.; Motley, E.D. Hydrogen peroxide inhibits insulin signaling in vascular smooth muscle cells. Exp. Biol. Med. 2003, 228, 836–842. [Google Scholar] [CrossRef]
- Nakabeppu, Y.; Sakumi, K.; Sakamoto, K.; Tsuchimoto, D.; Tsuzuki, T.; Nakatsu, Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol. Chem. 2006, 387, 373–379. [Google Scholar] [CrossRef]
- Roy, K.; Wu, Y.; Meitzler, J.L.; Juhasz, A.; Liu, H.; Jiang, G.; Lu, J.; Antony, S.; Doroshow, J.H. NADPH oxidases and cancer. Clin. Sci. 2015, 128, 863–875. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. A J. Virtual Libr. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Han, M.; Zhang, T.; Yang, L.; Wang, Z.; Ruan, J.; Chang, X. Association between NADPH oxidase (NOX) and lung cancer: A systematic review and meta-analysis. J. Thorac. Dis. 2016, 8, 1704–1711. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar]
- Fukui, H.; Moraes, C.T. The mitochondrial impairment, oxidative stress and neurodegeneration connection: Reality or just an attractive hypothesis? Trends Neurosci. 2008, 31, 251–256. [Google Scholar] [CrossRef]
- Sompol, P.; Ittarat, W.; Tangpong, J.; Chen, Y.; Doubinskaia, I.; Batinic-Haberle, I.; Abdul, H.M.; Butterfield, D.A.; St Clair, D.K. A neuronal model of alzheimer’s disease: An insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience 2008, 153, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.T.; Sharma, R.; Lim, J.L.; Haider, L.; Frischer, J.M.; Drexhage, J.; Mahad, D.; Bradl, M.; van Horssen, J.; Lassmann, H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain A J. Neurol. 2012, 135, 886–899. [Google Scholar] [CrossRef]
- Tarafdar, A.; Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Yu, L.; Quinn, M.T.; Cross, A.R.; Dinauer, M.C. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. USA 1998, 95, 7993–7998. [Google Scholar] [CrossRef]
- Quinn, M.T.; Mullen, M.L.; Jesaitis, A.J. Human neutrophil cytochrome-b contains multiple hemes—Evidence for heme associated with both subunits. J. Biol. Chem. 1992, 267, 7303–7309. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Otsuka-Murakami, H.; Lambeth, D.J. Reconstitution of flavin-depleted neutrophil flavocytochrome b558 with 8-mercapto-fad and characterization of the flavin-reconstituted enzyme. J. Biol. Chem. 1995, 270, 16428–16434. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.D.; Parkar, M.H.; Verhoeven, A.J.; Levinsky, R.J.; Collins, M.K.; Kinnon, C. P22-phox-deficient chronic granulomatous disease: Reconstitution by retrovirus-mediated expression and identification of a biosynthetic intermediate of gp91-phox. Blood 1994, 84, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; DeLeo, F.R.; Biberstine-Kinkade, K.J.; Renee, J.; Nauseef, W.M.; Dinauer, M.C. Biosynthesis of flavocytochrome b558. Gp91(phox) is synthesized as a 65-kda precursor (p65) in the endoplasmic reticulum. J. Biol. Chem. 1999, 274, 4364–4369. [Google Scholar] [CrossRef]
- Yu, L.; Zhen, L.; Dinauer, M.C. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J. Biol. Chem. 1997, 272, 27288–27294. [Google Scholar] [CrossRef]
- Wallach, T.M.; Segal, A.W. Analysis of glycosylation sites on gp91phox, the flavocytochrome of the NADPH oxidase, by site-directed mutagenesis and translation in vitro. Biochem. J. 1997, 321 Pt 3, 583–585. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Burritt, J.B.; Yu, L.; Jesaitis, A.J.; Dinauer, M.C.; Nauseef, W.M. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J. Biol. Chem. 2000, 275, 13986–13993. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W.; West, I.; Wientjes, F.; Nugent, J.H.; Chavan, A.J.; Haley, B.; Garcia, R.C.; Rosen, H.; Scrace, G. Cytochrome b-245 is a flavocytochrome containing fad and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem. J. 1992, 284 Pt 3, 781–788. [Google Scholar] [CrossRef]
- Rotrosen, D.; Yeung, C.L.; Leto, T.L.; Malech, H.L.; Kwong, C.H. Cytochrome b558: The flavin-binding component of the phagocyte NADPH oxidase. Science 1992, 256, 1459–1462. [Google Scholar] [CrossRef]
- Koshkin, V.; Lotan, O.; Pick, E. Electron transfer in the superoxide-generating NADPH oxidase complex reconstituted in vitro. Biochim. Biophys. Acta 1997, 1319, 139–146. [Google Scholar] [CrossRef]
- Koshkin, V.; Pick, E. Generation of superoxide by purified and relipidated cytochrome b559 in the absence of cytosolic activators. FEBS Lett. 1993, 327, 57–62. [Google Scholar] [CrossRef]
- Segal, A.W.; Jones, O.T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature 1978, 276, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Pember, S.O.; Heyl, B.L.; Kinkade, J.M., Jr.; Lambeth, J.D. Cytochrome b558 from (bovine) granulocytes. Partial purification from triton x-114 extracts and properties of the isolated cytochrome. J. Biol. Chem. 1984, 259, 10590–10595. [Google Scholar] [CrossRef] [PubMed]
- Knoller, S.; Shpungin, S.; Pick, E. The membrane-associated component of the amphiphile-activated, cytosol-dependent superoxide-forming NADPH oxidase of macrophages is identical to cytochrome b559. J. Biol. Chem. 1991, 266, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.R.; Higson, F.K.; Jones, O.T.; Harper, A.M.; Segal, A.W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem. J. 1982, 204, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.R.; Jones, O.T.; Harper, A.M.; Segal, A.W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem. J. 1981, 194, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Ambasta, R.K.; Kumar, P.; Griendling, K.K.; Schmidt, H.H.; Busse, R.; Brandes, R.P. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004, 279, 45935–45941. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Takeda, Y.; Iwasaki, Y.; Yoshizaki, F. Simultaneous estimation of geniposide and genipin in mouse plasma using high-performance liquid chromatography. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2001, 17, 1237–1239. [Google Scholar] [CrossRef]
- Rae, J.; Noack, D.; Heyworth, P.G.; Ellis, B.A.; Curnutte, J.T.; Cross, A.R. Molecular analysis of 9 new families with chronic granulomatous disease caused by mutations in cyba, the gene encoding p22(phox). Blood 2000, 96, 1106–1112. [Google Scholar] [CrossRef]
- Dahan, I.; Issaeva, I.; Gorzalczany, Y.; Sigal, N.; Hirshberg, M.; Pick, E. Mapping of functional domains in the p22(phox) subunit of flavocytochrome b(559) participating in the assembly of the NADPH oxidase complex by “peptide walking”. J. Biol. Chem. 2002, 277, 8421–8432. [Google Scholar] [CrossRef]
- Zhu, Y.; Marchal, C.C.; Casbon, A.J.; Stull, N.; von Lohneysen, K.; Knaus, U.G.; Jesaitis, A.J.; McCormick, S.; Nauseef, W.M.; Dinauer, M.C. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: Identification of regions critical for gp91phox maturation and NADPH oxidase activity. J. Biol. Chem. 2006, 281, 30336–30346. [Google Scholar] [CrossRef]
- Groemping, Y.; Lapouge, K.; Smerdon, S.J.; Rittinger, K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 2003, 113, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Nobuhisa, I.; Takeya, R.; Ogura, K.; Ueno, N.; Kohda, D.; Inagaki, F.; Sumimoto, H. Activation of the superoxide-producing phagocyte NADPH oxidase requires co-operation between the tandem sh3 domains of p47phox in recognition of a polyproline type ii helix and an adjacent alpha-helix of p22phox. Biochem. J. 2006, 396, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Prior, K.K.; Leisegang, M.S.; Josipovic, I.; Lowe, O.; Shah, A.M.; Weissmann, N.; Schroder, K.; Brandes, R.P. Crispr/cas9-mediated knockout of p22phox leads to loss of nox1 and nox4, but not nox5 activity. Redox Biol. 2016, 9, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. The NADPH oxidase and chronic granulomatous disease. Mol. Med. Today 1996, 2, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Maly, F.E.; Schuerer-Maly, C.C.; Quilliam, L.; Cochrane, C.G.; Newburger, P.E.; Curnutte, J.T.; Gifford, M.; Dinauer, M.C. Restitution of superoxide generation in autosomal cytochrome-negative chronic granulomatous disease (a22(0) cgd)-derived b lymphocyte cell lines by transfection with p22phax cdna. J. Exp. Med. 1993, 178, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Banfi, B.; Jesaitis, A.J.; Dinauer, M.C.; Allen, L.A.; Nauseef, W.M. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. Biochem. J. 2007, 403, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Martyn, K.D.; Frederick, L.M.; von Loehneysen, K.; Dinauer, M.C.; Knaus, U.G. Functional analysis of nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 2006, 18, 69–82. [Google Scholar] [CrossRef]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Forro, L.; Schlegel, W.; Krause, K.H. Nox4 activity is determined by mrna levels and reveals a unique pattern of Ros generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef]
- Von Lohneysen, K.; Noack, D.; Jesaitis, A.J.; Dinauer, M.C.; Knaus, U.G. Mutational analysis reveals distinct features of the nox4-p22 phox complex. J. Biol. Chem. 2008, 283, 35273–35282. [Google Scholar] [CrossRef]
- Magnani, F.; Nenci, S.; Millana Fananas, E.; Ceccon, M.; Romero, E.; Fraaije, M.W.; Mattevi, A. Crystal structures and atomic model of NADPH oxidase. Proc. Natl. Acad. Sci. USA 2017, 114, 6764–6769. [Google Scholar] [CrossRef]
- Maehara, Y.; Miyano, K.; Yuzawa, S.; Akimoto, R.; Takeya, R.; Sumimoto, H. A conserved region between the TPR and activation domains of p67phox participates in activation of the phagocyte NADPH oxidase. J. Biol. Chem. 2010, 285, 31435–31445. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.R. P40(phox) participates in the activation of NADPH oxidase by increasing the affinity of p47(phox) for flavocytochrome b(558). Biochem. J. 2000, 349, 113–117. [Google Scholar] [CrossRef]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef]
- Clark, R.A.; Epperson, T.K.; Valente, A.J. Mechanisms of activation of NADPH oxidases. Jpn. J. Infect. Dis. 2004, 57, S22–S23. [Google Scholar]
- Roos, D.; de Boer, M.; Kuribayashi, F.; Meischl, C.; Weening, R.S.; Segal, A.W.; Ahlin, A.; Nemet, K.; Hossle, J.P.; Bernatowska-Matuszkiewicz, E.; et al. Mutations in the x-linked and autosomal recessive forms of chronic granulomatous disease. Blood 1996, 87, 1663–1681. [Google Scholar] [CrossRef]
- Nunoi, H.; Rotrosen, D.; Gallin, J.I.; Malech, H.L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science 1988, 242, 1298–1301. [Google Scholar] [CrossRef]
- Volpp, B.D.; Nauseef, W.M.; Clark, R.A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science 1988, 242, 1295–1297. [Google Scholar] [CrossRef]
- Leto, T.L.; Lomax, K.J.; Volpp, B.D.; Nunoi, H.; Sechler, J.M.; Nauseef, W.M.; Clark, R.A.; Gallin, J.I.; Malech, H.L. Cloning of a 67-kd neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science 1990, 248, 727–730. [Google Scholar] [CrossRef]
- Han, C.H.; Freeman, J.L.; Lee, T.; Motalebi, S.A.; Lambeth, J.D. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox). J. Biol. Chem. 1998, 273, 16663–16668. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y.; Motalebi, S.; Han, C.H.; Lambeth, J.D. The p67(phox) activation domain regulates electron flow from nadph to flavin in flavocytochrome b(558). J. Biol. Chem. 1999, 274, 22999–23005. [Google Scholar] [CrossRef] [PubMed]
- Someya, A.; Nagaoka, I.; Yamashita, T. Purification of the 260 kda cytosolic complex involved in the superoxide production of guinea pig neutrophils. FEBS Lett. 1993, 330, 215–218. [Google Scholar] [CrossRef]
- Wientjes, F.B.; Hsuan, J.J.; Totty, N.F.; Segal, A.W. P40phox, a third cytosolic component of the activation complex of the nadph oxidase to contain src homology 3 domains. Biochem. J. 1993, 296 Pt 3, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Sathyamoorthy, M.; de Mendez, I.; Adams, A.G.; Leto, T.L. P40(phox) down-regulates nadph oxidase activity through interactions with its sh3 domain. J. Biol. Chem. 1997, 272, 9141–9146. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, F.; Nunoi, H.; Wakamatsu, K.; Tsunawaki, S.; Sato, K.; Ito, T.; Sumimoto, H. The adaptor protein p40(phox) as a positive regulator of the superoxide-producing phagocyte oxidase. EMBO J. 2002, 21, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Banfi, B.; Clark, R.A.; Steger, K.; Krause, K.H. Two novel proteins activate superoxide generation by the nadph oxidase nox1. J. Biol. Chem. 2003, 278, 3510–3513. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Kuribayashi, F.; Hiroaki, H.; Takeya, R.; Ito, T.; Kohda, D.; Sumimoto, H. Phosphorylation of p47phox directs phox homology domain from sh3 domain toward phosphoinositides, leading to phagocyte nadph oxidase activation. Proc. Natl. Acad. Sci. USA 2003, 100, 4474–4479. [Google Scholar] [CrossRef] [PubMed]
- Abo, A.; Pick, E.; Hall, A.; Totty, N.; Teahan, C.G.; Segal, A.W. Activation of the nadph oxidase involves the small gtp-binding protein p21rac1. Nature 1991, 353, 668–670. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho gtpases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Heasman, S.J.; Ridley, A.J. Mammalian rho gtpases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008, 9, 690–701. [Google Scholar] [CrossRef]
- Ago, T.; Nunoi, H.; Ito, T.; Sumimoto, H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox). Triple replacement of serines 303, 304, and 328 with aspartates disrupts the sh3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J. Biol. Chem. 1999, 274, 33644–33653. [Google Scholar]
- Yuzawa, S.; Ogura, K.; Horiuchi, M.; Suzuki, N.N.; Fujioka, Y.; Kataoka, M.; Sumimoto, H.; Inagaki, F. Solution structure of the tandem src homology 3 domains of p47phox in an autoinhibited form. J. Biol. Chem. 2004, 279, 29752–29760. [Google Scholar] [CrossRef]
- Inanami, O.; Johnson, J.L.; McAdara, J.K.; Benna, J.E.; Faust, L.R.; Newburger, P.E.; Babior, B.M. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47phox on serine 303 or 304. J. Biol. Chem. 1998, 273, 9539–9543. [Google Scholar] [CrossRef]
- Johnson, J.L.; Park, J.W.; Benna, J.E.; Faust, L.P.; Inanami, O.; Babior, B.M. Activation of p47(phox), a cytosolic subunit of the leukocyte NADPH oxidase. Phosphorylation of ser-359 or ser-370 precedes phosphorylation at other sites and is required for activity. J. Biol. Chem. 1998, 273, 35147–35152. [Google Scholar] [CrossRef]
- El-Benna, J.; Dang, P.M.; Gougerot-Pocidalo, M.A.; Marie, J.C.; Braut-Boucher, F. P47phox, the phagocyte NADPH oxidase/nox2 organizer: Structure, phosphorylation and implication in diseases. Exp. Mol. Med. 2009, 41, 217–225. [Google Scholar] [CrossRef]
- Huang, J.; Kleinberg, M.E. Activation of the phagocyte NADPH oxidase protein p47(phox). Phosphorylation controls sh3 domain-dependent binding to p22(phox). J. Biol. Chem. 1999, 274, 19731–19737. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Babior, B.M. Activation of the leukocyte NADPH oxidase subunit p47phox by protein kinase c. A phosphorylation-dependent change in the conformation of the c-terminal end of p47phox. Biochemistry 1997, 36, 7474–7480. [Google Scholar] [CrossRef] [PubMed]
- Leto, T.L.; Adams, A.G.; de Mendez, I. Assembly of the phagocyte NADPH oxidase: Binding of src homology 3 domains to proline-rich targets. Proc. Natl. Acad. Sci. USA 1994, 91, 10650–10654. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H.; Hata, K.; Mizuki, K.; Ito, T.; Kage, Y.; Sakaki, Y.; Fukumaki, Y.; Nakamura, M.; Takeshige, K. Assembly and activation of the phagocyte NADPH oxidase. Specific interaction of the n-terminal Src homology 3 domain of p47phox with p22phox is required for activation of the NADPH oxidase. J. Biol. Chem. 1996, 271, 22152–22158. [Google Scholar] [CrossRef] [PubMed]
- Shiose, A.; Sumimoto, H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J. Biol. Chem. 2000, 275, 13793–13801. [Google Scholar] [CrossRef]
- Ogura, K.; Nobuhisa, I.; Yuzawa, S.; Takeya, R.; Torikai, S.; Saikawa, K.; Sumimoto, H.; Inagaki, F. Nmr solution structure of the tandem src homology 3 domains of p47phox complexed with a p22phox-derived proline-rich peptide. J. Biol. Chem. 2006, 281, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- De Mendez, I.; Homayounpour, N.; Leto, T.L. Specificity of p47phox sh3 domain interactions in NADPH oxidase assembly and activation. Mol. Cell. Biol. 1997, 17, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Jacobson, J.; Wientjes, F.; Hothersall, J.; Canevari, L.; Duchen, M.R. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 9176–9184. [Google Scholar] [CrossRef]
- Dekker, L.V.; Leitges, M.; Altschuler, G.; Mistry, N.; McDermott, A.; Roes, J.; Segal, A.W. Protein kinase c-beta contributes to NADPH oxidase activation in neutrophils. Biochem. J. 2000, 347 Pt 1, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.M.; Cross, A.R.; Babior, B.M. Assembly of the neutrophil respiratory burst oxidase: A direct interaction between p67phox and cytochrome b558. Proc. Natl. Acad. Sci. USA 2001, 98, 3001–3005. [Google Scholar] [CrossRef]
- Frey, R.S.; Rahman, A.; Kefer, J.C.; Minshall, R.D.; Malik, A.B. Pkczeta regulates tnf-alpha-induced activation of NADPH oxidase in endothelial cells. Circ. Res. 2002, 90, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Leusen, J.H.; de Boer, M.; Bolscher, B.G.; Hilarius, P.M.; Weening, R.S.; Ochs, H.D.; Roos, D.; Verhoeven, A.J. A point mutation in gp91-phox of cytochrome b558 of the human NADPH oxidase leading to defective translocation of the cytosolic proteins p47-phox and p67-phox. J. Clin. Investig. 1994, 93, 2120–2126. [Google Scholar] [CrossRef]
- Massenet, C.; Chenavas, S.; Cohen-Addad, C.; Dagher, M.C.; Brandolin, G.; Pebay-Peyroula, E.; Fieschi, F. Effects of p47phox c terminus phosphorylations on binding interactions with p40phox and p67phox. Structural and functional comparison of p40phox and p67phox sh3 domains. J. Biol. Chem. 2005, 280, 13752–13761. [Google Scholar] [CrossRef]
- Mizuki, K.; Takeya, R.; Kuribayashi, F.; Nobuhisa, I.; Kohda, D.; Nunoi, H.; Takeshige, K.; Sumimoto, H. A region c-terminal to the proline-rich core of p47phox regulates activation of the phagocyte NADPH oxidase by interacting with the c-terminal sh3 domain of p67phox. Arch. Biochem. Biophys. 2005, 444, 185–194. [Google Scholar] [CrossRef]
- Finan, P.; Shimizu, Y.; Gout, I.; Hsuan, J.; Truong, O.; Butcher, C.; Bennett, P.; Waterfield, M.D.; Kellie, S. An sh3 domain and proline-rich sequence mediate an interaction between two components of the phagocyte NADPH oxidase complex. J. Biol. Chem. 1994, 269, 13752–13755. [Google Scholar] [CrossRef]
- Leusen, J.H.; Fluiter, K.; Hilarius, P.M.; Roos, D.; Verhoeven, A.J.; Bolscher, B.G. Interactions between the cytosolic components p47phox and p67phox of the human neutrophil NADPH oxidase that are not required for activation in the cell-free system. J. Biol. Chem. 1995, 270, 11216–11221. [Google Scholar] [CrossRef]
- Doussiere, J.; Pilloud, M.C.; Vignais, P.V. Cytosolic factors in bovine neutrophil oxidase activation. Partial purification and demonstration of translocation to a membrane fraction. Biochemistry 1990, 29, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Volpp, B.D.; Leidal, K.G.; Nauseef, W.M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J. Clin. Investig. 1990, 85, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Kami, K.; Takeya, R.; Sumimoto, H.; Kohda, D. Diverse recognition of non-pxxp peptide ligands by the sh3 domains from p67(phox), grb2 and pex13p. EMBO J. 2002, 21, 4268–4276. [Google Scholar] [CrossRef]
- Koga, H.; Terasawa, H.; Nunoi, H.; Takeshige, K.; Inagaki, F.; Sumimoto, H. Tetratricopeptide repeat (tpr) motifs of p67(phox) participate in interaction with the small gtpase Rac and activation of the phagocyte NADPH oxidase. J. Biol. Chem. 1999, 274, 25051–25060. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Freeman, J.L.; Motalebi, S.A.; Hirshberg, M.; Lambeth, J.D. Rac binding to p67(phox). Structural basis for interactions of the rac1 effector region and insert region with components of the respiratory burst oxidase. J. Biol. Chem. 1997, 272, 18834–18841. [Google Scholar] [CrossRef]
- Dusi, S.; Rossi, F. Activation of NADPH oxidase of human neutrophils involves the phosphorylation and the translocation of cytosolic p67phox. Biochem. J. 1993, 296 Pt 2, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.V.; Moss, S.J.; Segal, A.W. Phosphorylation of p67phox in the neutrophil occurs in the cytosol and is independent of p47phox. FEBS Lett. 1999, 449, 225–229. [Google Scholar] [CrossRef]
- Forbes, L.V.; Truong, O.; Wientjes, F.B.; Moss, S.J.; Segal, A.W. The major phosphorylation site of the NADPH oxidase component p67phox is thr233. Biochem. J. 1999, 338 Pt 1, 99–105. [Google Scholar] [CrossRef]
- Dang, P.M.; Morel, F.; Gougerot-Pocidalo, M.A.; El Benna, J. Phosphorylation of the NADPH oxidase component p67(phox) by erk2 and p38mapk: Selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry 2003, 42, 4520–4526. [Google Scholar] [CrossRef]
- Heyworth, P.G.; Curnutte, J.T.; Nauseef, W.M.; Volpp, B.D.; Pearson, D.W.; Rosen, H.; Clark, R.A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J. Clin. Investig. 1991, 87, 352–356. [Google Scholar] [CrossRef]
- Freeman, J.L.; Lambeth, J.D. NADPH oxidase activity is independent of p47phox in vitro. J. Biol. Chem. 1996, 271, 22578–22582. [Google Scholar] [CrossRef] [PubMed]
- Dusi, S.; Donini, M.; Rossi, F. Mechanisms of NADPH oxidase activation: Translocation of p40phox, rac1 and rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem. J. 1996, 314 Pt 2, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, T.; Geiszt, M.; Leto, T.L. Involvement of rac1 in activation of multicomponent nox1- and nox3-based NADPH oxidases. Mol. Cell. Biol. 2006, 26, 2160–2174. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Koga, H.; Minakami, R.; Sumimoto, H. The insert region of the Rac gtpases is dispensable for activation of superoxide-producing NADPH oxidases. Biochem. J. 2009, 422, 373–382. [Google Scholar] [CrossRef] [PubMed]
- De Leo, F.R.; Ulman, K.V.; Davis, A.R.; Jutila, K.L.; Quinn, M.T. Assembly of the human neutrophil NADPH oxidase involves binding of p67phox and flavocytochrome b to a common functional domain in p47phox. J. Biol. Chem. 1996, 271, 17013–17020. [Google Scholar] [CrossRef]
- Grizot, S.; Faure, J.; Fieschi, F.; Vignais, P.V.; Dagher, M.C.; Pebay-Peyroula, E. Crystal structure of the rac1-rhogdi complex involved in NADPH oxidase activation. Biochemistry 2001, 40, 10007–10013. [Google Scholar] [CrossRef]
- Bokoch, G.M.; Bohl, B.P.; Chuang, T.H. Guanine nucleotide exchange regulates membrane translocation of rac/rho gtp-binding proteins. J. Biol. Chem. 1994, 269, 31674–31679. [Google Scholar] [CrossRef]
- Le Cabec, V.; Mohn, H.; Gacon, G.; Maridonneau-Parini, I. The small gtp-binding protein Rac is not recruited to the plasma membrane upon NADPH oxidase activation in human neutrophils. Biochem. Biophys. Res. Commun. 1994, 198, 1216–1224. [Google Scholar] [CrossRef]
- Kreck, M.L.; Freeman, J.L.; Abo, A.; Lambeth, J.D. Membrane association of Rac is required for high activity of the respiratory burst oxidase. Biochemistry 1996, 35, 15683–15692. [Google Scholar] [CrossRef]
- Vergnaud, S.; Paclet, M.H.; El Benna, J.; Pocidalo, M.A.; Morel, F. Complementation of NADPH oxidase in p67-phox-deficient cgd patients p67-phox/p40-phox interaction. Eur. J. Biochem. 2000, 267, 1059–1067. [Google Scholar] [CrossRef]
- Paclet, M.H.; Coleman, A.W.; Vergnaud, S.; Morel, F. P67-phox-mediated NADPH oxidase assembly: Imaging of cytochrome b558 liposomes by atomic force microscopy. Biochemistry 2000, 39, 9302–9310. [Google Scholar] [CrossRef]
- Sumimoto, H.; Kage, Y.; Nunoi, H.; Sasaki, H.; Nose, T.; Fukumaki, Y.; Ohno, M.; Minakami, S.; Takeshige, K. Role of src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc. Natl. Acad. Sci. USA 1994, 91, 5345–5349. [Google Scholar] [CrossRef]
- Sumimoto, H.; Kamakura, S.; Ito, T. Structure and function of the pb1 domain, a protein interaction module conserved in animals, fungi, amoebas, and plants. Sci. STKE Signal Transduct. Knowl. Environ. 2007, 2007, re6. [Google Scholar] [CrossRef]
- Katsuyama, M.; Matsuno, K.; Yabe-Nishimura, C. Physiological roles of nox/NADPH oxidase, the superoxide-generating enzyme. J. Clin. Biochem. Nutr. 2012, 50, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Bagaitkar, J.; Matute, J.D.; Austin, A.; Arias, A.A.; Dinauer, M.C. Activation of neutrophil respiratory burst by fungal particles requires phosphatidylinositol 3-phosphate binding to p40(phox) in humans but not in mice. Blood 2012, 120, 3385–3387. [Google Scholar] [CrossRef] [PubMed]
- Van de Geer, A.; Nieto-Patlan, A.; Kuhns, D.B.; Tool, A.T.; Arias, A.A.; Bouaziz, M.; de Boer, M.; Franco, J.L.; Gazendam, R.P.; van Hamme, J.L.; et al. Inherited p40phox deficiency differs from classic chronic granulomatous disease. J. Clin. Investig. 2018, 128, 3957–3975. [Google Scholar] [CrossRef]
- Ellson, C.D.; Davidson, K.; Ferguson, G.J.; O’Connor, R.; Stephens, L.R.; Hawkins, P.T. Neutrophils from p40phox-/- mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J. Exp. Med. 2006, 203, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, S.A.; Glazier, C.M.; Stewart, M.Q.; Brown, G.E.; Ellson, C.D.; Yaffe, M.B. Phosphatidylinositol 3-phosphate-dependent and -independent functions of p40phox in activation of the neutrophil NADPH oxidase. J. Biol. Chem. 2008, 283, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Benard, V.; Bohl, B.P.; Bokoch, G.M. The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J. Clin. Investig. 2003, 112, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, I. Functions of Rac gtpases during neuronal development. Dev. Neurosci. 2008, 30, 47–58. [Google Scholar] [CrossRef]
- Abo, A.; Webb, M.R.; Grogan, A.; Segal, A.W. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory gdp/gtp exchange protein (rhogdi) followed by its translocation to the plasma membrane. Biochem. J. 1994, 298 Pt 3, 585–591. [Google Scholar] [CrossRef]
- Roberts, A.W.; Kim, C.; Zhen, L.; Lowe, J.B.; Kapur, R.; Petryniak, B.; Spaetti, A.; Pollock, J.D.; Borneo, J.B.; Bradford, G.B.; et al. Deficiency of the hematopoietic cell-specific rho family gtpase rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 1999, 10, 183–196. [Google Scholar] [CrossRef]
- Kim, C.; Dinauer, M.C. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J. Immunol. 2001, 166, 1223–1232. [Google Scholar] [CrossRef]
- Knaus, U.G.; Heyworth, P.G.; Kinsella, B.T.; Curnutte, J.T.; Bokoch, G.M. Purification and characterization of Rac 2. A cytosolic gtp-binding protein that regulates human neutrophil NADPH oxidase. J. Biol. Chem. 1992, 267, 23575–23582. [Google Scholar] [CrossRef]
- Knaus, U.G.; Heyworth, P.G.; Evans, T.; Curnutte, J.T.; Bokoch, G.M. Regulation of phagocyte oxygen radical production by the gtp-binding protein Rac 2. Science 1991, 254, 1512–1515. [Google Scholar] [CrossRef]
- Mizuno, T.; Kaibuchi, K.; Ando, S.; Musha, T.; Hiraoka, K.; Takaishi, K.; Asada, M.; Nunoi, H.; Matsuda, I.; Takai, Y. Regulation of the superoxide-generating NADPH oxidase by a small gtp-binding protein and its stimulatory and inhibitory gdp/gtp exchange proteins. J. Biol. Chem. 1992, 267, 10215–10218. [Google Scholar] [CrossRef] [PubMed]
- Heyworth, P.G.; Knaus, U.G.; Settleman, J.; Curnutte, J.T.; Bokoch, G.M. Regulation of NADPH oxidase activity by Rac gtpase activating protein(s). Mol. Biol. Cell 1993, 4, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A.; Zheng, Y.; Cancelas, J.A. Rho gtpases and regulation of hematopoietic stem cell localization. Methods Enzymol. 2008, 439, 365–393. [Google Scholar] [PubMed]
- Miyano, K.; Ueno, N.; Takeya, R.; Sumimoto, H. Direct involvement of the small gtpase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J. Biol. Chem. 2006, 281, 21857–21868. [Google Scholar] [CrossRef] [PubMed]
- Ambruso, D.R.; Knall, C.; Abell, A.N.; Panepinto, J.; Kurkchubasche, A.; Thurman, G.; Gonzalez-Aller, C.; Hiester, A.; deBoer, M.; Harbeck, R.J.; et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc. Natl. Acad. Sci. USA 2000, 97, 4654–4659. [Google Scholar] [CrossRef]
- Lapouge, K.; Smith, S.J.; Walker, P.A.; Gamblin, S.J.; Smerdon, S.J.; Rittinger, K. Structure of the tpr domain of p67phox in complex with rac.Gtp. Mol. Cell 2000, 6, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Serrander, L.; Lin, K.; Harris, R.J.; Cawley, J.C.; Allsup, D.J.; Slupsky, J.R.; Krause, K.H.; Zuzel, M. Expression and activity of nox5 in the circulating malignant b cells of hairy cell leukemia. J. Immunol. 2005, 175, 8424–8430. [Google Scholar] [CrossRef]
- Fortemaison, N.; Miot, F.; Dumont, J.E.; Dremier, S. Regulation of h2o2 generation in thyroid cells does not involve rac1 activation. Eur. J. Endocrinol. 2005, 152, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Glogauer, M.; Marchal, C.C.; Zhu, F.; Worku, A.; Clausen, B.E.; Foerster, I.; Marks, P.; Downey, G.P.; Dinauer, M.; Kwiatkowski, D.J. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J. Immunol. 2003, 170, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Filippi, M.D.; Cancelas, J.A.; Siefring, J.E.; Williams, E.P.; Jasti, A.C.; Harris, C.E.; Lee, A.W.; Prabhakar, R.; Atkinson, S.J.; et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 2003, 302, 445–449. [Google Scholar] [CrossRef]
- Diekmann, D.; Abo, A.; Johnston, C.; Segal, A.W.; Hall, A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science 1994, 265, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, D.; Nobes, C.D.; Burbelo, P.D.; Abo, A.; Hall, A. Rac gtpase interacts with gaps and target proteins through multiple effector sites. EMBO J. 1995, 14, 5297–5305. [Google Scholar] [CrossRef] [PubMed]
- Kwong, C.H.; Adams, A.G.; Leto, T.L. Characterization of the effector-specifying domain of Rac involved in NADPH oxidase activation. J. Biol. Chem. 1995, 270, 19868–19872. [Google Scholar] [CrossRef]
- Freeman, J.L.; Abo, A.; Lambeth, J.D. Rac “insert region” is a novel effector region that is implicated in the activation of NADPH oxidase, but not pak65. J. Biol. Chem. 1996, 271, 19794–19801. [Google Scholar] [CrossRef]
- Heyworth, P.G.; Bohl, B.P.; Bokoch, G.M.; Curnutte, J.T. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b558. J. Biol. Chem. 1994, 269, 30749–30752. [Google Scholar] [CrossRef]
- Sarfstein, R.; Gorzalczany, Y.; Mizrahi, A.; Berdichevsky, Y.; Molshanski-Mor, S.; Weinbaum, C.; Hirshberg, M.; Dagher, M.C.; Pick, E. Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: A study based on mutagenesis of p67phox-rac1 chimeras. J. Biol. Chem. 2004, 279, 16007–16016. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Takeshige, K.; Sumimoto, H. Roles for proline-rich regions of p47phox and p67phox in the phagocyte NADPH oxidase activation in vitro. Biochem. Biophys. Res. Commun. 1997, 241, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Diebold, B.A.; Bokoch, G.M. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2001, 2, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, Y.; Mizrahi, A.; Ugolev, Y.; Molshanski-Mor, S.; Pick, E. Tripartite chimeras comprising functional domains derived from the cytosolic NADPH oxidase components p47phox, p67phox, and rac1 elicit activator-independent superoxide production by phagocyte membranes: An essential role for anionic membrane phospholipids. J. Biol. Chem. 2007, 282, 22122–22139. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.Y.; Gianni, D.; Bohl, B.; Taylor, R.M.; Bokoch, G.M. Identification of a conserved Rac-binding site on NADPH oxidases supports a direct gtpase regulatory mechanism. J. Biol. Chem. 2008, 283, 12736–12746. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, P.L. Regulation of NADPH oxidases: The role of Rac proteins. Circ. Res. 2006, 98, 453–462. [Google Scholar] [CrossRef]
- Iyer, S.S.; Pearson, D.W.; Nauseef, W.M.; Clark, R.A. Evidence for a readily dissociable complex of p47phox and p67phox in cytosol of unstimulated human neutrophils. J. Biol. Chem. 1994, 269, 22405–22411. [Google Scholar] [CrossRef]
- Wientjes, F.B.; Panayotou, G.; Reeves, E.; Segal, A.W. Interactions between cytosolic components of the NADPH oxidase: P40phox interacts with both p67phox and p47phox. Biochem. J. 1996, 317 Pt 3, 919–924. [Google Scholar] [CrossRef]
- Zhan, S.; Vazquez, N.; Zhan, S.; Wientjes, F.B.; Budarf, M.L.; Schrock, E.; Ried, T.; Green, E.D.; Chanock, S.J. Genomic structure, chromosomal localization, start of transcription, and tissue expression of the human p40-phox, a new component of the nicotinamide adenine dinucleotide phosphate-oxidase complex. Blood 1996, 88, 2714–2721. [Google Scholar] [CrossRef]
- Lapouge, K.; Smith, S.J.; Groemping, Y.; Rittinger, K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. A central role for p67phox. J. Biol. Chem. 2002, 277, 10121–10128. [Google Scholar] [CrossRef]
- Quinn, M.T.; Evans, T.; Loetterle, L.R.; Jesaitis, A.J.; Bokoch, G.M. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J. Biol. Chem. 1993, 268, 20983–20987. [Google Scholar] [CrossRef]
- Park, J.W.; Babior, B.M. The translocation of respiratory burst oxidase components from cytosol to plasma-membrane is regulated by guanine-nucleotides and diacylglycerol. J. Biol. Chem. 1992, 267, 19901–19906. [Google Scholar] [CrossRef]
- Dang, P.M.; Johnson, J.L.; Babior, B.M. Binding of nicotinamide adenine dinucleotide phosphate to the tetratricopeptide repeat domains at the n-terminus of p67phox, a subunit of the leukocyte nicotinamide adenine dinucleotide phosphate oxidase. Biochemistry 2000, 39, 3069–3075. [Google Scholar] [CrossRef]
- Uhlinger, D.J.; Tyagi, S.R.; Inge, K.L.; Lambeth, J.D. The respiratory burst oxidase of human neutrophils. Guanine nucleotides and arachidonate regulate the assembly of a multicomponent complex in a semirecombinant cell-free system. J. Biol. Chem. 1993, 268, 8624–8631. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Kaibuchi, K.; Sasaki, T.; Hiraoka, K.; Nishiyama, T.; Mizuno, T.; Asada, M.; Nunoi, H.; Matsuda, I.; Matsuura, Y. Post-translational processing of Rac p21s is important both for their interaction with the gdp/gtp exchange proteins and for their activation of NADPH oxidase. J. Biol. Chem. 1992, 267, 25709–25713. [Google Scholar] [CrossRef]
- Freeman, J.L.; Kreck, M.L.; Uhlinger, D.J.; Lambeth, J.D. Ras effector-homologue region on Rac regulates protein associations in the neutrophil respiratory burst oxidase complex. Biochemistry 1994, 33, 13431–13435. [Google Scholar] [CrossRef]
- Koshkin, V.; Lotan, O.; Pick, E. The cytosolic component p47(phox) is not a sine qua non participant in the activation of NADPH oxidase but is required for optimal superoxide production. J. Biol. Chem. 1996, 271, 30326–30329. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Ogawa, H.; Miyano, K.; Tamura, M. Activation of the flavoprotein domain of gp91phox upon interaction with n-terminal p67phox (1–210) and the Rac complex. Biochemistry 2004, 43, 9567–9575. [Google Scholar] [CrossRef] [PubMed]
- Wallach, T.M.; Segal, A.W. Stoichiometry of the subunits of flavocytochrome b558 of the NADPH oxidase of phagocytes. Biochem. J. 1996, 320 Pt 1, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Hashida, S.; Yuzawa, S.; Suzuki, N.N.; Fujioka, Y.; Takikawa, T.; Sumimoto, H.; Inagaki, F.; Fujii, H. Binding of fad to cytochrome b558 is facilitated during activation of the phagocyte NADPH oxidase, leading to superoxide production. J. Biol. Chem. 2004, 279, 26378–26386. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef]
- Takac, I.; Schroder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The e-loop is involved in hydrogen peroxide formation by the NADPH oxidase nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef] [PubMed]
- Shiose, A.; Kuroda, J.; Tsuruya, K.; Hirai, M.; Hirakata, H.; Naito, S.; Hattori, M.; Sakaki, Y.; Sumimoto, H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001, 276, 1417–1423. [Google Scholar] [CrossRef]
- Ago, T.; Kitazono, T.; Ooboshi, H.; Iyama, T.; Han, Y.H.; Takada, J.; Wakisaka, M.; Ibayashi, S.; Utsumi, H.; Iida, M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 2004, 109, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ellmark, S.H.; Dusting, G.J.; Fui, M.N.; Guzzo-Pernell, N.; Drummond, G.R. The contribution of nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 2005, 65, 495–504. [Google Scholar] [CrossRef]
- Kawahara, T.; Ritsick, D.; Cheng, G.; Lambeth, J.D. Point mutations in the proline-rich region of p22phox are dominant inhibitors of nox1- and nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005, 280, 31859–31869. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Nakagawa, K.; Yamasaki, T.; Nakamura, K.; Takeya, R.; Kuribayashi, F.; Imajoh-Ohmi, S.; Igarashi, K.; Shibata, Y.; Sueishi, K.; et al. The superoxide-producing NAD(P)H oxidase nox4 in the nucleus of human vascular endothelial cells. Genes Cells Devoted Mol. Cell. Mech. 2005, 10, 1139–1151. [Google Scholar] [CrossRef]
- Amanso, A.M.; Debbas, V.; Laurindo, F.R. Proteasome inhibition represses unfolded protein response and nox4, sensitizing vascular cells to endoplasmic reticulum stress-induced death. PLoS ONE 2011, 6, e14591. [Google Scholar] [CrossRef]
- Lu, X.; Guo, X.; Wassall, C.D.; Kemple, M.D.; Unthank, J.L.; Kassab, G.S. Reactive oxygen species cause endothelial dysfunction in chronic flow overload. J. Appl. Physiol. 2011, 110, 520–527. [Google Scholar] [CrossRef]
- Ahmad, M.; Kelly, M.R.; Zhao, X.; Kandhi, S.; Wolin, M.S. Roles for nox4 in the contractile response of bovine pulmonary arteries to hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1879–H1888. [Google Scholar] [CrossRef]
- Pendyala, S.; Moitra, J.; Kalari, S.; Kleeberger, S.R.; Zhao, Y.; Reddy, S.P.; Garcia, J.G.; Natarajan, V. Nrf2 regulates hyperoxia-induced nox4 expression in human lung endothelium: Identification of functional antioxidant response elements on the nox4 promoter. Free Radic. Biol. Med. 2011, 50, 1749–1759. [Google Scholar] [CrossRef]
- Ben Mkaddem, S.; Pedruzzi, E.; Werts, C.; Coant, N.; Bens, M.; Cluzeaud, F.; Goujon, J.M.; Ogier-Denis, E.; Vandewalle, A. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell Death Differ. 2010, 17, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Hou, X.; Jourd’heuil, D.; Weisbrod, R.M.; Cohen, R.A. Upregulation of nox4 by tgfbeta1 oxidizes serca and inhibits no in arterial smooth muscle of the prediabetic zucker rat. Circ. Res. 2010, 107, 975–983. [Google Scholar] [CrossRef]
- St Hilaire, C.; Koupenova, M.; Carroll, S.H.; Smith, B.D.; Ravid, K. Tnf-alpha upregulates the a2b adenosine receptor gene: The role of NAD(P)H oxidase 4. Biochem. Biophys. Res. Commun. 2008, 375, 292–296. [Google Scholar] [CrossRef]
- Mahadev, K.; Motoshima, H.; Wu, X.; Ruddy, J.M.; Arnold, R.S.; Cheng, G.; Lambeth, J.D.; Goldstein, B.J. The NAD(P)H oxidase homolog nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 2004, 24, 1844–1854. [Google Scholar] [CrossRef]
- Park, H.S.; Jung, H.Y.; Park, E.Y.; Kim, J.; Lee, W.J.; Bae, Y.S. Cutting edge: Direct interaction of tlr4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of nf-kappa b. J. Immunol. 2004, 173, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rodriguez-Belmonte, E.M.; Mazloum, N.; Xie, B.; Lee, M.Y. Identification of a novel protein, pdip38, that interacts with the p50 subunit of DNA polymerase delta and proliferating cell nuclear antigen. J. Biol. Chem. 2003, 278, 10041–10047. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Li, H.; Wang, Q.; Xie, S.; Rahmeh, A.; Dai, W.; Lee, M.Y. Further characterization of human DNA polymerase delta interacting protein 38. J. Biol. Chem. 2005, 280, 22375–22384. [Google Scholar] [CrossRef]
- Miller, F.J., Jr. NADPH oxidase 4: Walking the walk with poldip2. Circ. Res. 2009, 105, 209–210. [Google Scholar] [CrossRef]
- Lyle, A.N.; Deshpande, N.N.; Taniyama, Y.; Seidel-Rogol, B.; Pounkova, L.; Du, P.; Papaharalambus, C.; Lassegue, B.; Griendling, K.K. Poldip2, a novel regulator of nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009, 105, 249–259. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hattori, K.; Hamanaka, J.; Murase, T.; Egashira, Y.; Mishiro, K.; Ishiguro, M.; Tsuruma, K.; Hirose, Y.; Tanaka, H.; et al. Pharmacological inhibition of tlr4-nox4 signal protects against neuronal death in transient focal ischemia. Sci. Rep. 2012, 2, 896. [Google Scholar] [CrossRef] [PubMed]
- Janiszewski, M.; Lopes, L.R.; Carmo, A.O.; Pedro, M.A.; Brandes, R.P.; Santos, C.X.; Laurindo, F.R. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J. Biol. Chem. 2005, 280, 40813–40819. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Anagnostopoulou, A.; Rios, F.; Montezano, A.C.; Camargo, L.L. Nox5: Molecular biology and pathophysiology. Exp. Physiol. 2019, 104, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Banfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.H. Nox3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004, 279, 46065–46072. [Google Scholar] [CrossRef] [PubMed]
- Luxen, S.; Belinsky, S.A.; Knaus, U.G. Silencing of duox NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008, 68, 1037–1045. [Google Scholar] [CrossRef]
- Grasberger, H.; Refetoff, S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J. Biol. Chem. 2006, 281, 18269–18272. [Google Scholar] [CrossRef] [PubMed]
- De Deken, X.; Wang, D.; Dumont, J.E.; Miot, F. Characterization of thox proteins as components of the thyroid h(2)o(2)-generating system. Exp. Cell Res. 2002, 273, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the roscue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12951. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Hartlein, I.; Smith, S.M.E.; Fieschi, F. NADPH oxidases (nox): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Lambeth, J.D.; Cheng, G.; Arnold, R.S.; Edens, W.A. Novel homologs of gp91phox. Trends Biochem. Sci. 2000, 25, 459–461. [Google Scholar] [CrossRef]
- Kikuchi, H.; Hikage, M.; Miyashita, H.; Fukumoto, M. NADPH oxidase subunit, gp91(phox) homologue, preferentially expressed in human colon epithelial cells. Gene 2000, 254, 237–243. [Google Scholar] [CrossRef]
- Dupuy, C.; Pomerance, M.; Ohayon, R.; Noel-Hudson, M.S.; Deme, D.; Chaaraoui, M.; Francon, J.; Virion, A. Thyroid oxidase (thox2) gene expression in the rat thyroid cell line frtl-5. Biochem. Biophys. Res. Commun. 2000, 277, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Lalucque, H.; Silar, P. NADPH oxidase: An enzyme for multicellularity? Trends Microbiol. 2003, 11, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.G.; Ansorena, E.; Izal, I.; Zalba, G.; de Miguel, C.; Milagro, F.I. Structure, regulation, and physiological functions of NADPH oxidase 5 (nox5). J. Physiol. Biochem. 2023, 79, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Lekstrom, K.; Witta, J.; Leto, T.L. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem. 2003, 278, 20006–20012. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.C.; Bikker, H.; Kempers, M.J.; van Trotsenburg, A.S.; Baas, F.; de Vijlder, J.J.; Vulsma, T.; Ris-Stalpers, C. Inactivating mutations in the gene for thyroid oxidase 2 (thox2) and congenital hypothyroidism. N. Engl. J. Med. 2002, 347, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Lambeth, J.D. Noxo1, regulation of lipid binding, localization, and activation of nox1 by the phox homology (px) domain. J. Biol. Chem. 2004, 279, 4737–4742. [Google Scholar] [CrossRef] [PubMed]
- Paffenholz, R.; Bergstrom, R.A.; Pasutto, F.; Wabnitz, P.; Munroe, R.J.; Jagla, W.; Heinzmann, U.; Marquardt, A.; Bareiss, A.; Laufs, J.; et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004, 18, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Takeya, R.; Sumimoto, H. Regulation of novel superoxide-producing NAD(P)H oxidases. Antioxid. Redox Signal. 2006, 8, 1523–1532. [Google Scholar] [CrossRef]
- Cheng, G.; Ritsick, D.; Lambeth, J.D. Nox3 regulation by noxo1, p47phox, and p67phox. J. Biol. Chem. 2004, 279, 34250–34255. [Google Scholar] [CrossRef]
- Babior, B.M.; Curnutte, J.T.; McMurrich, B.J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J. Clin. Investig. 1976, 58, 989–996. [Google Scholar] [CrossRef]
- Dewald, B.; Baggiolini, M.; Curnutte, J.T.; Babior, B.M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J. Clin. Investig. 1979, 63, 21–29. [Google Scholar] [CrossRef]
- Hiroaki, H.; Ago, T.; Ito, T.; Sumimoto, H.; Kohda, D. Solution structure of the px domain, a target of the sh3 domain. Nat. Struct. Biol. 2001, 8, 526–530. [Google Scholar] [CrossRef]
- Bravo, J.; Karathanassis, D.; Pacold, C.M.; Pacold, M.E.; Ellson, C.D.; Anderson, K.E.; Butler, P.J.; Lavenir, I.; Perisic, O.; Hawkins, P.T.; et al. The crystal structure of the px domain from p40(phox) bound to phosphatidylinositol 3-phosphate. Mol. Cell 2001, 8, 829–839. [Google Scholar] [CrossRef]
- Ponting, C.P. Novel domains in NADPH oxidase subunits, sorting nexins, and Ptdins 3-kinases: Binding partners of SH3 domains? Protein Sci. A Publ. Protein Soc. 1996, 5, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Sumimoto, H. Role of the small gtpase Rac in p22phox-dependent NADPH oxidases. Biochimie 2007, 89, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- De Mendez, I.; Garrett, M.C.; Adams, A.G.; Leto, T.L. Role of p67-phox sh3 domains in assembly of the NADPH oxidase system. J. Biol. Chem. 1994, 269, 16326–16332. [Google Scholar] [CrossRef] [PubMed]
- Maehara, Y.; Miyano, K.; Sumimoto, H. Role for the first sh3 domain of p67phox in activation of superoxide-producing NADPH oxidases. Biochem. Biophys. Res. Commun. 2009, 379, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Taura, M.; Miyano, K.; Minakami, R.; Kamakura, S.; Takeya, R.; Sumimoto, H. A region n-terminal to the tandem sh3 domain of p47phox plays a crucial role in the activation of the phagocyte NADPH oxidase. Biochem. J. 2009, 419, 329–338. [Google Scholar] [CrossRef]
- Ueno, N.; Takeya, R.; Miyano, K.; Kikuchi, H.; Sumimoto, H. The NADPH oxidase nox3 constitutively produces superoxide in a p22phox-dependent manner: Its regulation by oxidase organizers and activators. J. Biol. Chem. 2005, 280, 23328–23339. [Google Scholar] [CrossRef]
- Ueyama, T.; Lekstrom, K.; Tsujibe, S.; Saito, N.; Leto, T.L. Subcellular localization and function of alternatively spliced noxo1 isoforms. Free Radic. Biol. Med. 2007, 42, 180–190. [Google Scholar] [CrossRef]
- Miyano, K.; Sumimoto, H. N-linked glycosylation of the superoxide-producing NADPH oxidase Nox1. Biochem. Biophys. Res. Commun. 2014, 443, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Gianni, D.; Diaz, B.; Taulet, N.; Fowler, B.; Courtneidge, S.A.; Bokoch, G.M. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci. Signal. 2009, 2, ra54. [Google Scholar] [CrossRef] [PubMed]
- Lock, P.; Abram, C.L.; Gibson, T.; Courtneidge, S.A. A new method for isolating tyrosine kinase substrates used to identify fish, an sh3 and px domain-containing protein, and src substrate. EMBO J. 1998, 17, 4346–4357. [Google Scholar] [CrossRef]
- Courtneidge, S.A. Isolation of novel src substrates. Biochem. Soc. Trans. 2003, 31, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Courtneidge, S.A.; Azucena, E.F.; Pass, I.; Seals, D.F.; Tesfay, L. The Src substrate tks5, podosomes (invadopodia), and cancer cell invasion. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 167–171. [Google Scholar] [CrossRef]
- Diaz, B.; Shani, G.; Pass, I.; Anderson, D.; Quintavalle, M.; Courtneidge, S.A. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci. Signal. 2009, 2, ra53. [Google Scholar] [CrossRef]
- Leto, T.L.; Morand, S.; Hurt, D.; Ueyama, T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid. Redox Signal. 2009, 11, 2607–2619. [Google Scholar] [CrossRef]
- Geiszt, M. NADPH oxidases: New kids on the block. Cardiovasc. Res. 2006, 71, 289–299. [Google Scholar] [CrossRef]
- Rousset, F.; Kokje, V.B.C.; Sipione, R.; Schmidbauer, D.; Nacher-Soler, G.; Ilmjarv, S.; Coelho, M.; Fink, S.; Voruz, F.; El Chemaly, A.; et al. Intrinsically self-renewing neuroprogenitors from the a/j mouse spiral ganglion as virtually unlimited source of mature auditory neurons. Front. Cell. Neurosci. 2020, 14, 395. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Wiegmann, K.; Farid, A.; Wolf, A.; Utermohlen, O.; Krut, O.; Kronke, M.; Schramm, M. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of nemo. Sci. Signal. 2019, 12, eaar5926. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ros in macrophages and antimicrobial immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, P.; Srivastava, A.; Jiang, G.; Zhang, X.; Lee, P.J. An endothelial hsp70-tlr4 axis limits Nox3 expression and protects against oxidant injury in lungs. Antioxid. Redox Signal. 2016, 24, 991–1012. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Huang, X.; Man, Y.; Zhou, Y.; Wang, S.; Wang, J.; Li, J. Nox3-derived reactive oxygen species promote tnf-alpha-induced reductions in hepatocyte glycogen levels via a JNK pathway. FEBS Lett. 2010, 584, 995–1000. [Google Scholar] [CrossRef]
- Delmaghani, S.; El-Amraoui, A. Inner ear gene therapies take off: Current promises and future challenges. J. Clin. Med. 2020, 9, 2309. [Google Scholar] [CrossRef]
- Frolenkov, G.I.; Belyantseva, I.A.; Friedman, T.B.; Griffith, A.J. Genetic insights into the morphogenesis of inner ear hair cells. Nat. Rev. Genet. 2004, 5, 489–498. [Google Scholar] [CrossRef] [PubMed]
- LeMasurier, M.; Gillespie, P.G. Hair-cell mechanotransduction and cochlear amplification. Neuron 2005, 48, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Driver, E.C.; Kelley, M.W. Development of the cochlea. Development 2020, 147, dev162263. [Google Scholar] [CrossRef] [PubMed]
- Anniko, M. Development of otoconia. Am. J. Otolaryngol. 1980, 1, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chang, R. Anatomy of the vestibular system: A review. NeuroRehabilitation 2013, 32, 437–443. [Google Scholar] [CrossRef]
- Fife, T.D. Chapter 2—overview of anatomy and physiology of the vestibular system. In Handbook of Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 9, pp. 5–17. [Google Scholar]
- Rousset, F.; Nacher-Soler, G.; Coelho, M.; Ilmjarv, S.; Kokje, V.B.C.; Marteyn, A.; Cambet, Y.; Perny, M.; Roccio, M.; Jaquet, V.; et al. Redox activation of excitatory pathways in auditory neurons as mechanism of age-related hearing loss. Redox Biol. 2020, 30, 101434. [Google Scholar] [CrossRef]
- Chen, P.; Chai, Y.; Liu, H.; Li, G.; Wang, L.; Yang, T.; Wu, H. Postnatal development of microglia-like cells in mouse cochlea. Neural Plast. 2018, 2018, 1970150. [Google Scholar] [CrossRef]
- Sun, S.; Yu, H.; Yu, H.; Honglin, M.; Ni, W.; Zhang, Y.; Guo, L.; He, Y.; Xue, Z.; Ni, Y.; et al. Inhibition of the activation and recruitment of microglia-like cells protects against neomycin-induced ototoxicity. Mol. Neurobiol. 2015, 51, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Morioka, S.; Sakaguchi, H.; Yamaguchi, T.; Ninoyu, Y.; Mohri, H.; Nakamura, T.; Hisa, Y.; Ogita, K.; Saito, N.; Ueyama, T. Hearing vulnerability after noise exposure in a mouse model of reactive oxygen species overproduction. J. Neurochem. 2018, 146, 459–473. [Google Scholar] [CrossRef]
- Jones, S.M.; Erway, L.C.; Bergstrom, R.A.; Schimenti, J.C.; Jones, T.A. Vestibular responses to linear acceleration are absent in otoconia-deficient c57bl/6jei-het mice. Hear. Res. 1999, 135, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Jones, S.M. Short latency compound action potentials from mammalian gravity receptor organs. Hear. Res. 1999, 136, 75–85. [Google Scholar] [CrossRef]
- Jones, S.M.; Erway, L.C.; Johnson, K.R.; Yu, H.; Jones, T.A. Gravity receptor function in mice with graded otoconial deficiencies. Hear. Res. 2004, 191, 34–40. [Google Scholar] [CrossRef]
- Thalmann, R.; Ignatova, E.; Kachar, B.; Ornitz, D.M.; Thalmann, I. Development and maintenance of otoconia: Biochemical considerations. Ann. N. Y. Acad. Sci. 2001, 942, 162–178. [Google Scholar] [CrossRef]
- Fernandez, C.; Goldberg, J.M. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J. Neurophysiol. 1976, 39, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.M.; Fernandez, C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J. Neurophysiol. 1971, 34, 635–660. [Google Scholar] [CrossRef]
- Jones, T.A.; Jones, S.M. Spontaneous activity in the statoacoustic ganglion of the chicken embryo. J. Neurophysiol. 2000, 83, 1452–1468. [Google Scholar] [CrossRef]
- Adrian, E.D. Discharges from vestibular receptors in the cat. J. Physiol. 1943, 101, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Annoni, J.M.; Cochran, S.L.; Precht, W. Pharmacology of the vestibular hair cell-afferent fiber synapse in the frog. J. Neurosci. Off. J. Soc. Neurosci. 1984, 4, 2106–2116. [Google Scholar] [CrossRef] [PubMed]
- Flock, A.; Russell, I. Inhibition by efferent nerve fibres: Action on hair cells and afferent synaptic transmission in the lateral line canal organ of the burbot lota lota. J. Physiol. 1976, 257, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Ishii, Y. Neurophysiological studies on hearing in goldfish. J. Neurophysiol. 1967, 30, 1377–1403. [Google Scholar] [CrossRef]
- Furukawa, T.; Ishii, Y.; Matsuura, S. Synaptic delay and time course of postsynaptic potentials at the junction between hair cells and eighth nerve fibers in the goldfish. Jpn. J. Physiol. 1972, 22, 617–635. [Google Scholar] [CrossRef]
- Fuchs, P.A.; Glowatzki, E. Synaptic studies inform the functional diversity of cochlear afferents. Hear. Res. 2015, 330, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sondag, H.N.; de Jong, H.A.; van Marle, J.; Oosterveld, W.J. Effects of sustained acceleration on the morphological properties of otoconia in hamsters. Acta Oto-Laryngol. 1995, 115, 227–230. [Google Scholar] [CrossRef]
- Suzuki, H.; Ikeda, K.; Takasaka, T. Biological characteristics of the globular substance in the otoconial membrane of the guinea pig. Hear. Res. 1995, 90, 212–218. [Google Scholar] [CrossRef]
- Tu, Y.H.; Cooper, A.J.; Teng, B.; Chang, R.B.; Artiga, D.J.; Turner, H.N.; Mulhall, E.M.; Ye, W.; Smith, A.D.; Liman, E.R. An evolutionarily conserved gene family encodes proton-selective ion channels. Science 2018, 359, 1047–1050. [Google Scholar] [CrossRef]
- Ramsey, I.S.; DeSimone, J.A. Otopetrin-1: A sour-tasting proton channel. J. Gen. Physiol. 2018, 150, 379–382. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Bohne, B.A.; Thalmann, I.; Harding, G.W.; Thalmann, R. Otoconial agenesis in tilted mutant mice. Hear. Res. 1998, 122, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.C.; Hurle, B.; Wang, Y.; Bohne, B.A.; Wuerffel, M.K.; Ornitz, D.M. High-resolution mapping of tlt, a mouse mutant lacking otoconia. Mamm. Genome 1999, 10, 544–548. [Google Scholar] [CrossRef]
- Hurle, B.; Ignatova, E.; Massironi, S.M.; Mashimo, T.; Rios, X.; Thalmann, I.; Thalmann, R.; Ornitz, D.M. Non-syndromic vestibular disorder with otoconial agenesis in tilted/mergulhador mice caused by mutations in otopetrin 1. Hum. Mol. Genet. 2003, 12, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Hurle, B.; Lane, K.; Kenney, J.; Tarantino, L.M.; Bucan, M.; Brownstein, B.H.; Ornitz, D.M. Physical mapping of the mouse tilted locus identifies an association between human deafness loci dfna6/14 and vestibular system development. Genomics 2001, 77, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Everett, L.A.; Belyantseva, I.A.; Noben-Trauth, K.; Cantos, R.; Chen, A.; Thakkar, S.I.; Hoogstraten-Miller, S.L.; Kachar, B.; Wu, D.K.; Green, E.D. Targeted disruption of mouse pds provides insight about the inner-ear defects encountered in pendred syndrome. Hum. Mol. Genet. 2001, 10, 153–161. [Google Scholar] [CrossRef]
- Huang, S.; Qian, S. Advances in otolith-related protein research. Front. Neurosci. 2022, 16, 956200. [Google Scholar] [CrossRef]
- Lundberg, Y.W.; Xu, Y. Proteins involved in otoconia formation and maintenance. Otolaryngology 2012, 1, 3–22. [Google Scholar]
- Simmler, M.C.; Cohen-Salmon, M.; El-Amraoui, A.; Guillaud, L.; Benichou, J.C.; Petit, C.; Panthier, J.J. Targeted disruption of otog results in deafness and severe imbalance. Nat. Genet. 2000, 24, 139–143. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Yang, H.; Zhao, X.; Lovas, S.; Lundberg, Y.W. Expression, functional, and structural analysis of proteins critical for otoconia development. Dev. Dyn. 2010, 239, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thalmann, I.; Thalmann, R.; Ornitz, D.M. Mapping the mouse otoconin-90 (oc90) gene to chromosome 15. Genomics 1999, 58, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kowalski, P.E.; Thalmann, I.; Ornitz, D.M.; Mager, D.L.; Thalmann, R. Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase a2. Proc. Natl. Acad. Sci. USA 1998, 95, 15345–15350. [Google Scholar] [CrossRef] [PubMed]
- Verpy, E.; Leibovici, M.; Petit, C. Characterization of otoconin-95, the major protein of murine otoconia, provides insights into the formation of these inner ear biominerals. Proc. Natl. Acad. Sci. USA 1999, 96, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.M.; Zheng, Y.L.; Hu, B.; Zhang, Z.F. Purple sweet potato color alleviates d-galactose-induced brain aging in old mice by promoting survival of neurons via pi3k pathway and inhibiting cytochrome c-mediated apoptosis. Brain Pathol. 2010, 20, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, H.; Yamoah, E.N.; Lundberg, Y.W. Gene targeting reveals the role of oc90 as the essential organizer of the otoconial organic matrix. Dev. Biol. 2007, 304, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhou, D.; Freeman, J.J.; Thalmann, I.; Ornitz, D.M.; Thalmann, R. In vitro effects of recombinant otoconin 90 upon calcite crystal growth. Significance of tertiary structure. Hear. Res. 2010, 268, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jones, S.M.; Yamoah, E.N.; Lundberg, Y.W. Otoconin-90 deletion leads to imbalance but normal hearing: A comparison with other otoconia mutants. Neuroscience 2008, 153, 289–299. [Google Scholar] [CrossRef]
- Lundberg, Y.W.; Xu, Y.; Thiessen, K.D.; Kramer, K.L. Mechanisms of otoconia and otolith development. Dev. Dyn. 2015, 244, 239–253. [Google Scholar] [CrossRef]
- Ignatova, E.G.; Thalmann, I.; Xu, B.; Ornitz, D.M.; Thalmann, R. Molecular mechanisms underlying ectopic otoconia-like particles in the endolymphatic sac of embryonic mice. Hear. Res. 2004, 194, 65–72. [Google Scholar] [CrossRef]
- Deans, M.R.; Peterson, J.M.; Wong, G.W. Mammalian otolin: A multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein otoconin-90 and cerebellin-1. PLoS ONE 2010, 5, e12765. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Salmon, M.; El-Amraoui, A.; Leibovici, M.; Petit, C. Otogelin: A glycoprotein specific to the acellular membranes of the inner ear. Proc. Natl. Acad. Sci. USA 1997, 94, 14450–14455. [Google Scholar] [CrossRef]
- El-Amraoui, A.; Cohen-Salmon, M.; Petit, C.; Simmler, M.C. Spatiotemporal expression of otogelin in the developing and adult mouse inner ear. Hear. Res. 2001, 158, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zwaenepoel, I.; Mustapha, M.; Leibovici, M.; Verpy, E.; Goodyear, R.; Liu, X.Z.; Nouaille, S.; Nance, W.E.; Kanaan, M.; Avraham, K.B.; et al. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness dfnb22. Proc. Natl. Acad. Sci. USA 2002, 99, 6240–6245. [Google Scholar] [CrossRef]
- Anniko, M.; Wenngren, B.I.; Wroblewski, R. Aberrant elemental composition of otoconia in the dancer mouse mutant with a semidominant gene causing a morphogenetic type of inner ear defect. Acta Oto-Laryngol. 1988, 106, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, X.; Xu, Y.; Wang, L.; He, Q.; Lundberg, Y.W. Matrix recruitment and calcium sequestration for spatial specific otoconia development. PLoS ONE 2011, 6, e20498. [Google Scholar] [CrossRef]
- Wenngren, B.I.; Anniko, M. Vestibular hair cell pathology in the dancer mouse mutant. Acta Oto-Laryngol. 1989, 107, 182–190. [Google Scholar] [CrossRef]
- Kozel, P.J.; Friedman, R.A.; Erway, L.C.; Yamoah, E.N.; Liu, L.H.; Riddle, T.; Duffy, J.J.; Doetschman, T.; Miller, M.L.; Cardell, E.L.; et al. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-atpase isoform 2. J. Biol. Chem. 1998, 273, 18693–18696. [Google Scholar] [CrossRef] [PubMed]
- Sondag, H.N.; de Jong, H.A.; van Marle, J.; Oosterveld, W.J. Behavioural changes in hamsters with otoconial malformations. Acta Oto-Laryngol. 1998, 118, 86–89. [Google Scholar]
- Trune, D.R.; Lim, D.J. The behavior and vestibular nuclear morphology of otoconia-deficient pallid mutant mice. J. Neurogenet. 1983, 1, 53–69. [Google Scholar] [CrossRef]
- de Caprona, M.D.; Beisel, K.W.; Nichols, D.H.; Fritzsch, B. Partial behavioral compensation is revealed in balance tasked mutant mice lacking otoconia. Brain Res. Bull. 2004, 64, 289–301. [Google Scholar] [CrossRef]
- Graf, E.; Kelly, R.; Jedin, H. “A history of the council of trent” 1 (book review). Theol. Stud. 1958, 19, 293. [Google Scholar]
- Sweet, H. Head tilt. Mouse News Lett. 1980, 63, 19. [Google Scholar]
- Bergstrom, R.A.; You, Y.; Erway, L.C.; Lyon, M.F.; Schimenti, J.C. Deletion mapping of the head tilt (het) gene in mice: A vestibular mutation causing specific absence of otoliths. Genetics 1998, 150, 815–822. [Google Scholar] [CrossRef]
- Flaherty, J.P.; Fairfield, H.E.; Spruce, C.A.; McCarty, C.M.; Bergstrom, D.E. Molecular characterization of an allelic series of mutations in the mouse Nox3 gene. Mamm. Genome 2011, 22, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Munroe, R.J.; Bergstrom, R.A.; Zheng, Q.Y.; Libby, B.; Smith, R.; John, S.W.; Schimenti, K.J.; Browning, V.L.; Schimenti, J.C. Mouse mutants from chemically mutagenized embryonic stem cells. Nat. Genet. 2000, 24, 318–321. [Google Scholar] [CrossRef]
- Goldowitz, D.; Frankel, W.N.; Takahashi, J.S.; Holtz-Vitaterna, M.; Bult, C.; Kibbe, W.A.; Snoddy, J.; Li, Y.; Pretel, S.; Yates, J.; et al. Large-scale mutagenesis of the mouse to understand the genetic bases of nervous system structure and function. Brain Res. Mol. Brain Res. 2004, 132, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, R.; Gorg, B.; Becker, S.; Qvartskhava, N.; Bidmon, H.J.; Selbach, O.; Haas, H.L.; Schliess, F.; Haussinger, D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia 2007, 55, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Jones, T.A.; Johnson, K.R.; Yu, H.; Erway, L.C.; Zheng, Q.Y. A comparison of vestibular and auditory phenotypes in inbred mouse strains. Brain Res. 2006, 1091, 40–46. [Google Scholar] [CrossRef]
- Mohri, H.; Ninoyu, Y.; Sakaguchi, H.; Hirano, S.; Saito, N.; Ueyama, T. Nox3-derived superoxide in cochleae induces sensorineural hearing loss. J. Neurosci. 2021, 41, 4716–4731. [Google Scholar] [CrossRef]
- Hudspeth, A.J. Integrating the active process of hair cells with cochlear function. Nat. Rev. Neurosci. 2014, 15, 600–614. [Google Scholar] [CrossRef]
- Kiss, P.J.; Knisz, J.; Zhang, Y.; Baltrusaitis, J.; Sigmund, C.D.; Thalmann, R.; Smith, R.J.; Verpy, E.; Banfi, B. Inactivation of NADPH oxidase organizer 1 results in severe imbalance. Curr. Biol. CB 2006, 16, 208–213. [Google Scholar] [CrossRef]
- Ward, B.K.; Lee, Y.H.; Roberts, D.C.; Naylor, E.; Migliaccio, A.A.; Della Santina, C.C. Mouse magnetic-field nystagmus in strong static magnetic fields is dependent on the presence of Nox3. Otol. Neurotol. 2018, 39, e1150–e1159. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, L.; Zhao, X.; Zhang, Y.; Jones, T.A.; Jones, S.M.; Lundberg, Y.W. Functional cooperation between two otoconial proteins oc90 and Nox3. J. Vestib. Res. Equilib. Orientat. 2021, 31, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Darrat, I.; Ahmad, N.; Seidman, K.; Seidman, M.D. Auditory research involving antioxidants. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W. Aminoglycoside induced ototoxicity. Toxicology 2008, 249, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; McFadden, S.L.; Liu, C.C.; Hight, N.; Zheng, X.Y. The role of antioxidants in protection from impulse noise. Ann. N. Y. Acad. Sci. 1999, 884, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M. A higher oxidative status accelerates senescence and aggravates age-dependent disorders in samp strains of mice. Mech. Ageing Dev. 2002, 123, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Kopke, R.; Allen, K.A.; Henderson, D.; Hoffer, M.; Frenz, D.; Van de Water, T. A radical demise. Toxins and trauma share common pathways in hair cell death. Ann. N. Y. Acad. Sci. 1999, 884, 171–191. [Google Scholar] [CrossRef]
- Rybak, L.P.; Ramkumar, V. Ototoxicity. Kidney Int. 2007, 72, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.H.; Beijnen, J.H.; Balm, A.J.; Schellens, J.H. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat. Rev. 2006, 32, 390–397. [Google Scholar] [CrossRef]
- Du, Z.; Li, S.; Liu, L.; Yang, Q.; Zhang, H.; Gao, C. NADPH oxidase 3-associated oxidative stress and caspase 3-dependent apoptosis in the cochleae of d-galactose-induced aged rats. Mol. Med. Rep. 2015, 12, 7883–7890. [Google Scholar] [CrossRef] [PubMed]
- Mukherjea, D.; Jajoo, S.; Kaur, T.; Sheehan, K.E.; Ramkumar, V.; Rybak, L.P. Transtympanic administration of short interfering (si)rna for the nox3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid. Redox Signal. 2010, 13, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Mukherjea, D.; Whitworth, C.A.; Nandish, S.; Dunaway, G.A.; Rybak, L.P.; Ramkumar, V. Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience 2006, 139, 733–740. [Google Scholar] [CrossRef]
- Rousset, F.; Nacher-Soler, G.; Kokje, V.B.C.; Sgroi, S.; Coelho, M.; Krause, K.H.; Senn, P. NADPH oxidase 3 deficiency protects from noise-induced sensorineural hearing loss. Front. Cell Dev. Biol. 2022, 10, 832314. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Lin, S.C.; Wong, A.C.; Wackrow, B.; Thorne, P.R. Noise-induced changes in expression levels of NADPH oxidases in the cochlea. Hear. Res. 2013, 304, 145–152. [Google Scholar] [CrossRef]
- Besser, S.; Sicker, M.; Marx, G.; Winkler, U.; Eulenburg, V.; Hulsmann, S.; Hirrlinger, J. A transgenic mouse line expressing the red fluorescent protein tdtomato in gabaergic neurons. PLoS ONE 2015, 10, e0129934. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.J.; Chung, T.; Youn, H.; Kang, K.W.; Lee, D.S.; Chung, J.K. A novel hnis/tdtomato fusion reporter for visualizing the relationship between the cellular localization of sodium iodide symporter and its iodine uptake function under heat shock treatment. Mol. Imaging 2015, 14, 1–10. [Google Scholar]
- Fu, P.; Mohan, V.; Mansoor, S.; Tiruppathi, C.; Sadikot, R.T.; Natarajan, V. Role of nicotinamide adenine dinucleotide phosphate-reduced oxidase proteins in pseudomonas aeruginosa-induced lung inflammation and permeability. Am. J. Respir. Cell Mol. Biol. 2013, 48, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shan, P.; Jiang, G.; Cohn, L.; Lee, P.J. Toll-like receptor 4 deficiency causes pulmonary emphysema. J. Clin. Investig. 2006, 116, 3050–3059. [Google Scholar] [CrossRef]
- Morimoto, H.; Iwata, K.; Ogonuki, N.; Inoue, K.; Atsuo, O.; Kanatsu-Shinohara, M.; Morimoto, T.; Yabe-Nishimura, C.; Shinohara, T. Ros are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell 2013, 12, 774–786. [Google Scholar] [CrossRef]
- Gupta, A.P.; Syed, A.A.; Garg, R.; Goand, U.K.; Singh, P.; Riyazuddin, M.; Valicherla, G.R.; Husain, A.; Gayen, J.R. Pancreastatin inhibitor psti8 attenuates hyperinsulinemia induced obesity and inflammation mediated insulin resistance via mapk/nox3-jnk pathway. Eur. J. Pharmacol. 2019, 864, 172723. [Google Scholar] [CrossRef]
- Kashiwabara, Y.; Ambe, K.; Nakagawa, T.; Watanabe, H. Immunohistochemical localization of nox in mouse circumvallate papillae. Tissue Cell 2015, 47, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Carpentier, J.L.; Foti, M.; Szanto, I. Insulin-induced vascular endothelial growth factor expression is mediated by the NADPH oxidase Nox3. Exp. Cell Res. 2006, 312, 3413–3424. [Google Scholar] [CrossRef] [PubMed]
- Maru, Y.; Nishino, T.; Kakinuma, K. Expression of nox genes in rat organs, mouse oocytes, and sea urchin eggs. DNA Seq. 2005, 16, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Michihara, A.; Oda, A.; Mido, M. High expression levels of NADPH oxidase 3 in the cerebrum of ten-week-old stroke-prone spontaneously hypertensive rats. Biol. Pharm. Bull. 2016, 39, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Mizukami, H.; Kurosaki, K.; Kuriiwa, F.; Mukai, T. Existence of a threshold for hydroxyl radical generation independent of hypoxia in rat striatum during carbon monoxide poisoning. Arch. Toxicol. 2011, 85, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Cooney, S.J.; Zhao, Y.; Byrnes, K.R. Characterization of the expression and inflammatory activity of NADPH oxidase after spinal cord injury. Free Radic. Res. 2014, 48, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhong, H.; Yanamadala, S.; Campese, V.M. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension 2006, 48, 309–315. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Expression of genes that encode cellular oxidant/antioxidant systems are affected by heat stress. Mol. Biol. Rep. 2018, 45, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Polettini, J.; Silva, M.G.; Kacerovsky, M.; Syed, T.A.; Saade, G.; Menon, R. Expression profiles of fetal membrane nicotinamide adenine dinucleotide phosphate oxidases (nox) 2 and 3 differentiates spontaneous preterm birth and pprom pathophysiologies. Placenta 2014, 35, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.W.; Xu, X.D.; Zong, K.; Qiu, X.G. Overexpression of duox2 mediates doxorubicin resistance and predicts prognosis of pancreatic cancer. Gland. Surg. 2022, 11, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Li, T.; Wang, C.; Shi, X.; Li, Y.; Zhang, S.; Zhao, Z.; Dong, H.; Qiao, Y. Berberine exerts antioxidant effects via protection of spiral ganglion cells against cytomegalovirus-induced apoptosis. Free Radic. Biol. Med. 2018, 121, 127–135. [Google Scholar] [CrossRef]
- Hamernik, R.P.; Turrentine, G.; Roberto, M.; Salvi, R.; Henderson, D. Anatomical correlates of impulse noise-induced mechanical damage in the cochlea. Hear. Res. 1984, 13, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P. Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 2006, 576, 11–21. [Google Scholar] [CrossRef]
- Kasahara, Y.; Tuder, R.M.; Cool, C.D.; Lynch, D.A.; Flores, S.C.; Voelkel, N.F. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir. Crit. Care Med. 2001, 163, 737–744. [Google Scholar] [CrossRef]
- Lu, Q.; Harrington, E.O.; Rounds, S. Apoptosis and lung injury. Keio J. Med. 2005, 54, 184–189. [Google Scholar] [CrossRef]
- Toborek, M.; Hennig, B. Vitamin e attenuates induction of elastase-like activity by tumor necrosis factor-alpha, cholestan-3 beta,5 alpha,6 beta-triol and linoleic acid in cultured endothelial cells. Clin. Chim. Acta Int. J. Clin. Chem. 1993, 215, 201–211. [Google Scholar] [CrossRef]
- Yasuoka, H.; Garrett, S.M.; Nguyen, X.X.; Artlett, C.M.; Feghali-Bostwick, C.A. NADPH oxidase-mediated induction of reactive oxygen species and extracellular matrix deposition by insulin-like growth factor binding protein-5. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L644–L655. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Zhao, W.; Zhou, X.; Miao, G.; Sun, C.; Zhang, H. NADPH oxidase activation contributes to heavy ion irradiation-induced cell death. Dose-Response A Publ. Int. Hormesis Soc. 2017, 15, 1559325817699697. [Google Scholar] [CrossRef]
- Nakayama, N.; Nakamura, T.; Okada, H.; Iwaki, S.; Sobel, B.E.; Fujii, S. Modulators of induction of plasminogen activator inhibitor type-1 in hepg2 cells by transforming growth factor-beta. Coron. Artery Dis. 2011, 22, 468–478. [Google Scholar] [CrossRef]
- Li, H.; Liu, Q.; Wang, N.; Xu, J. Correlation of different NADPH oxidase homologues with late endothelial progenitor cell senescence induced by angiotensin ii: Effect of telmisartan. Intern. Med. 2011, 50, 1631–1642. [Google Scholar] [CrossRef]
- Moon, Y.M.; Kang, H.J.; Cho, J.S.; Park, I.H.; Lee, H.M. Nox4 mediates hypoxia-stimulated myofibroblast differentiation in nasal polyp-derived fibroblasts. Int. Arch. Allergy Immunol. 2012, 159, 399–409. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Wu, L.C.; Dai, T.; Chen, S.Y.; Wang, A.Y.; Lin, K.; Lin, D.M.; Yang, J.Q.; Cheng, B.; Zhang, L.; et al. NADPH oxidase-2 is a key regulator of human dermal fibroblasts: A potential therapeutic strategy for the treatment of skin fibrosis. Exp. Dermatol. 2014, 23, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Van Buul, J.D.; Fernandez-Borja, M.; Anthony, E.C.; Hordijk, P.L. Expression and localization of nox2 and nox4 in primary human endothelial cells. Antioxid. Redox Signal. 2005, 7, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.D.; Shah, M.H.; Liu, G.S.; Chan, E.C.; Crowston, J.G.; Peshavariya, H.M. Transforming growth factor beta1-induced NADPH oxidase-4 expression and fibrotic response in conjunctival fibroblasts. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3011–3017. [Google Scholar] [CrossRef]
- O’Brien, W.J.; Heimann, T.; Rizvi, F. NADPH oxidase expression and production of superoxide by human corneal stromal cells. Mol. Vis. 2009, 15, 2535–2543. [Google Scholar] [PubMed]
- Olguin-Albuerne, M.; Dominguez, G.; Moran, J. Effect of staurosporine in the morphology and viability of cerebellar astrocytes: Role of reactive oxygen species and NADPH oxidase. Oxidative Med. Cell. Longev. 2014, 2014, 678371. [Google Scholar] [CrossRef] [PubMed]
- Accetta, R.; Damiano, S.; Morano, A.; Mondola, P.; Paterno, R.; Avvedimento, E.V.; Santillo, M. Reactive oxygen species derived from nox3 and nox5 drive differentiation of human oligodendrocytes. Front. Cell. Neurosci. 2016, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Lee, L.; Iordanskaia, T.; Ramos-Alvarez, I.; Moreno, P.; Boudreau, H.E.; Leto, T.L.; Jensen, R.T. Pac1 regulates receptor tyrosine kinase transactivation in a reactive oxygen species-dependent manner. Peptides 2019, 120, 170017. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Han, Y.M.; Jeong, M.; Chung, M.H.; Kwon, C.I.; Ko, K.H.; Hahm, K.B. Synthetic 8-hydroxydeoxyguanosine inhibited metastasis of pancreatic cancer through concerted inhibitions of erm and rho-gtpase. Free Radic. Biol. Med. 2017, 110, 151–161. [Google Scholar] [CrossRef]
- Malleter, M.; Tauzin, S.; Bessede, A.; Castellano, R.; Goubard, A.; Godey, F.; Leveque, J.; Jezequel, P.; Campion, L.; Campone, M.; et al. Cd95l cell surface cleavage triggers a prometastatic signaling pathway in triple-negative breast cancer. Cancer Res. 2013, 73, 6711–6721. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wu, Z.; Hu, X.; Zhang, J.; Gao, Z.; Han, X.; Qin, J.; Li, C.; Wang, Y. The pi3k/akt/gsk-3beta/ros/eif2b pathway promotes breast cancer growth and metastasis via suppression of nk cell cytotoxicity and tumor cell susceptibility. Cancer Biol. Med. 2019, 16, 38–54. [Google Scholar] [PubMed]
- Ito, K.; Ota, A.; Ono, T.; Nakaoka, T.; Wahiduzzaman, M.; Karnan, S.; Konishi, H.; Furuhashi, A.; Hayashi, T.; Yamada, Y.; et al. Inhibition of nox1 induces apoptosis by attenuating the akt signaling pathway in oral squamous cell carcinoma cell lines. Oncol. Rep. 2016, 36, 2991–2998. [Google Scholar] [CrossRef]
- Kitamoto, K.; Miura, Y.; Karnan, S.; Ota, A.; Konishi, H.; Hosokawa, Y.; Sato, K. Inhibition of NADPH oxidase 2 induces apoptosis in osteosarcoma: The role of reactive oxygen species in cell proliferation. Oncol. Lett. 2018, 15, 7955–7962. [Google Scholar] [CrossRef]
- Tanaka, M.; Miura, Y.; Numanami, H.; Karnan, S.; Ota, A.; Konishi, H.; Hosokawa, Y.; Hanyuda, M. Inhibition of NADPH oxidase 4 induces apoptosis in malignant mesothelioma: Role of reactive oxygen species. Oncol. Rep. 2015, 34, 1726–1732. [Google Scholar] [CrossRef]
- Kim, M.Y.; Bang, E.; Hwangbo, H.; Ji, S.Y.; Kim, D.H.; Lee, H.; Park, C.; Hong, S.H.; Kim, G.Y.; Choi, Y.H. Diallyl trisulfide inhibits monosodium urate-induced nlrp3 inflammasome activation via nox3/4-dependent mitochondrial oxidative stress in raw 264.7 and bone marrow-derived macrophages. Phytomed. Int. J. Phytother. Phytopharm. 2023, 112, 154705. [Google Scholar] [CrossRef] [PubMed]
- Khanal, T.; Kim, H.G.; Do, M.T.; Choi, J.H.; Chung, Y.C.; Kim, H.S.; Park, Y.J.; Jeong, T.C.; Jeong, H.G. Genipin induces cyclooxygenase-2 expression via NADPH oxidase, mapks, ap-1, and nf-kappab in raw 264.7 cells. Food Chem. Toxicol. Int. 2014, 64, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Tang, M.; Suzuki, M.; Gunasekara, C.; Anbe, Y.; Hiraoka, Y.; Liu, J.; Grasberger, H.; Ohkita, M.; Matsumura, Y.; et al. Essential role of NADPH oxidase-dependent production of reactive oxygen species in maintenance of sustained b cell receptor signaling and b cell proliferation. J. Immunol. 2019, 202, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, R.; Bewtra, A.K.; Agrawal, D.K. Chloride channel 3 channels in the activation and migration of human blood eosinophils in allergic asthma. Am. J. Respir. Cell Mol. Biol. 2015, 53, 235–245. [Google Scholar] [CrossRef]
- Li, Z.Y.; Jiang, W.Y.; Cui, Z.J. An essential role of NAD(P)H oxidase 2 in uva-induced calcium oscillations in mast cells. Photochem. Photobiol. Sci. 2015, 14, 414–428. [Google Scholar] [CrossRef]
- Cui, X.L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH oxidase isoform 1 (nox1) in human placenta: Involvement in preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef]
- Matsubara, S.; Sato, I. Enzyme histochemically detectable NAD(P)H oxidase in human placental trophoblasts: Normal, preeclamptic, and fetal growth restriction-complicated pregnancy. Histochem. Cell Biol. 2001, 116, 1–7. [Google Scholar] [CrossRef]
- Manes, C. Human placental NAD(P)H oxidase: Solubilization and properties. Placenta 2001, 22, 58–63. [Google Scholar] [CrossRef]
- Morimoto, H.; Kanatsu-Shinohara, M.; Shinohara, T. Ros-generating oxidase Nox3 regulates the self-renewal of mouse spermatogonial stem cells. Biol. Reprod. 2015, 92, 147. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.; Lachance, G.; Bellmann, K.; Laplante, M.; Stadler, K.; Marette, A. Cytokines promote lipolysis in 3t3-l1 adipocytes through induction of NADPH oxidase 3 expression and superoxide production. J. Lipid Res. 2018, 59, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Brault, J.; Vigne, B.; Meunier, M.; Beaumel, S.; Mollin, M.; Park, S.; Stasia, M.J. Nox4 is the main NADPH oxidase involved in the early stages of hematopoietic differentiation from human induced pluripotent stem cells. Free Radic. Biol. Med. 2020, 146, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.M.; Saed, M.G.; Abuanzeh, S.; Abu-Soud, H.M.; Al-Hendy, A.; Diamond, M.P.; Saed, G.M. Nicotinamide adenine dinucleotide phosphate oxidase is differentially regulated in normal myometrium versus leiomyoma. Reprod. Sci. 2014, 21, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gharib, T.G.; Huang, C.C.; Taylor, J.M.; Misek, D.E.; Kardia, S.L.; Giordano, T.J.; Iannettoni, M.D.; Orringer, M.B.; Hanash, S.M.; et al. Discordant protein and mrna expression in lung adenocarcinomas. Mol. Cell Proteom. 2002, 1, 304–313. [Google Scholar] [CrossRef]
- Pascal, L.E.; True, L.D.; Campbell, D.S.; Deutsch, E.W.; Risk, M.; Coleman, I.M.; Eichner, L.J.; Nelson, P.S.; Liu, A.Y. Correlation of mrna and protein levels: Cell type-specific gene expression of cluster designation antigens in the prostate. BMC Genom. 2008, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Gluschko, A.; Herb, M.; Wiegmann, K.; Krut, O.; Neiss, W.F.; Utermohlen, O.; Kronke, M.; Schramm, M. The β2 integrin mac-1 induces protective lc3-associated phagocytosis of listeria monocytogenes. Cell Host Microbe 2018, 23, 324–337.e325. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C. What are the bona fide gsk3 substrates? Int. J. Alzheimer’s Dis. 2011, 2011, 505607. [Google Scholar]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. Gsk-3 as potential target for therapeutic intervention in cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Nong, S.; Huang, X.; Lu, Y.; Zhao, H.; Lin, Y.; Man, Y.; Wang, S.; Yang, J.; Li, J. The effects of palmitate on hepatic insulin resistance are mediated by NADPH oxidase 3-derived reactive oxygen species through jnk and p38mapk pathways. J. Biol. Chem. 2010, 285, 29965–29973. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and prevention. Semin. Hear. 2019, 40, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.; Mukherjea, D.; Dhukhwa, A.; Rybak, L.P. Oxidative stress and inflammation caused by cisplatin ototoxicity. Antioxidants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Kim, S.J.; Oh, G.S.; Moon, H.D.; Kwon, K.B.; Park, C.; Park, B.H.; Lee, H.K.; Chung, S.Y.; et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 3933–3946. [Google Scholar] [CrossRef]
- Mukherjea, D.; Jajoo, S.; Whitworth, C.; Bunch, J.R.; Turner, J.G.; Rybak, L.P.; Ramkumar, V. Short interfering rna against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 13056–13065. [Google Scholar] [CrossRef]
- Shin, Y.S.; Song, S.J.; Kang, S.U.; Hwang, H.S.; Choi, J.W.; Lee, B.H.; Jung, Y.S.; Kim, C.H. A novel synthetic compound, 3-amino-3-(4-fluoro-phenyl)-1h-quinoline-2,4-dione, inhibits cisplatin-induced hearing loss by the suppression of reactive oxygen species: In vitro and in vivo study. Neuroscience 2013, 232, 1–12. [Google Scholar] [CrossRef]
- Hara, S.; Kobayashi, M.; Kuriiwa, F.; Ikematsu, K.; Mizukami, H. Hydroxyl radical production via NADPH oxidase in rat striatum due to carbon monoxide poisoning. Toxicology 2018, 394, 63–71. [Google Scholar] [CrossRef]
- Zahra, G.; Esmaeil, K.; Mohammad, F.; Rashidy-Pour, A.; Mahdi, M.; Mahdi, A.; Ali, K. Combined effects of the exposure to silver nanoparticles and noise on hearing function and cochlea structure of the male rats. Life Sci. 2022, 304, 120724. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.R.; Park, J.H.; Khanal, T.; Choi, J.H.; Do, M.T.; Jin, S.W.; Han, E.H.; Chung, Y.H.; Jeong, H.G. Endosulfan induces cox-2 expression via NADPH oxidase and the ros, mapk, and akt pathways. Arch. Toxicol. 2015, 89, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.D.; Hussain, Q.Z. Effect of sub-chronic endosulfan exposure on humoral and cell-mediated immune responses in albino rats. Arch. Toxicol. 1986, 59, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Omurtag, G.Z.; Tozan, A.; Sehirli, A.O.; Sener, G. Melatonin protects against endosulfan-induced oxidative tissue damage in rats. J. Pineal Res. 2008, 44, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.Z.; Nisar, M.A.; Alshwmi, M.; Din, S.R.U.; Gamallat, Y.; Khan, M.; Ma, T. Brevilin a inhibits stat3 signaling and induces ros-dependent apoptosis, mitochondrial stress and endoplasmic reticulum stress in mcf-7 breast cancer cells. OncoTargets Ther. 2020, 13, 435–450. [Google Scholar] [CrossRef]
- Zhuang, W.; Wang, C.; Shi, X.; Qiu, S.; Zhang, S.; Xu, B.; Chen, M.; Jiang, W.; Dong, H.; Qiao, Y. Mcmv triggers ros/nlrp3-associated inflammasome activation in the inner ear of mice and cultured spiral ganglion neurons, contributing to sensorineural hearing loss. Int. J. Mol. Med. 2018, 41, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Owens, K.M.; Kulawiec, M.; Desouki, M.M.; Vanniarajan, A.; Singh, K.K. Impaired oxphos complex iii in breast cancer. PLoS ONE 2011, 6, e23846. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, F.; Kovalenkov, Y.; Pandey, D.; Moseley, M.A.; Foster, M.W.; Black, S.M.; Venema, R.C.; Stepp, D.W.; Fulton, D.J. Nitric oxide reduces NADPH oxidase 5 (nox5) activity by reversible s-nitrosylation. Free Radic. Biol. Med. 2012, 52, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, K.; Wu, X.; Zilbering, A.; Zhu, L.; Lawrence, J.T.; Goldstein, B.J. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3t3-l1 adipocytes. J. Biol. Chem. 2001, 276, 48662–48669. [Google Scholar] [CrossRef]
- Krawczyk, S.A.; Haller, J.F.; Ferrante, T.; Zoeller, R.A.; Corkey, B.E. Reactive oxygen species facilitate translocation of hormone sensitive lipase to the lipid droplet during lipolysis in human differentiated adipocytes. PLoS ONE 2012, 7, e34904. [Google Scholar] [CrossRef]
- Maeda, H.; Rajesh, K.G.; Maeda, H.; Suzuki, R.; Sasaguri, S. Epidermal growth factor and insulin inhibit cell death in pancreatic beta cells by activation of pi3-kinase/akt signaling pathway under oxidative stress. Transplant. Proc. 2004, 36, 1163–1165. [Google Scholar] [CrossRef]
- Stiehl, D.P.; Jelkmann, W.; Wenger, R.H.; Hellwig-Burgel, T. Normoxic induction of the hypoxia-inducible factor 1alpha by insulin and interleukin-1beta involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 2002, 512, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Babior, B.M.; Hunt, T.K.; Ellison, E.C.; Roy, S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J. Biol. Chem. 2002, 277, 33284–33290. [Google Scholar] [CrossRef] [PubMed]
- Berra, E.; Pages, G.; Pouyssegur, J. Map kinases and hypoxia in the control of vegf expression. Cancer Metastasis Rev. 2000, 19, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, Y.; Gao, W.; Jiang, Q.; Geng, L.; Ding, J.; Li, S.; Li, J. Transcription factor sp1 transcriptionally enhances gsdme expression for pyroptosis. Cell Death Dis. 2024, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Pages, G.; Pouyssegur, J. Transcriptional regulation of the vascular endothelial growth factor gene—A concert of activating factors. Cardiovasc. Res. 2005, 65, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Milanini-Mongiat, J.; Pouyssegur, J.; Pages, G. Identification of two sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: Their implication in vascular endothelial growth factor gene transcription. J. Biol. Chem. 2002, 277, 20631–20639. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Maddux, B.A.; Goldfine, I.D. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal. 2005, 7, 1040–1052. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Da Silva Morais, A.; Schroyen, B.; Van Hul, N.; Geerts, A. Insulin resistance in hepatocytes and sinusoidal liver cells: Mechanisms and consequences. J. Hepatol. 2007, 47, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yin, B.; Zhang, H.; Zhang, S.; Zeng, Q.; Wang, J.; Jiang, X.; Yuan, L.; Wang, C.Y.; Li, Z. Blockade of tumor necrosis factor (tnf) receptor type 1-mediated tnf-alpha signaling protected wistar rats from diet-induced obesity and insulin resistance. Endocrinology 2008, 149, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Honda, S.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote tnfalpha-induced death and sustained jnk activation by inhibiting map kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Gorgun, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for jnk in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Nakatani, Y.; Miyatsuka, T.; Kawamori, D.; Matsuoka, T.A.; Matsuhisa, M.; Kajimoto, Y.; Ichijo, H.; Yamasaki, Y.; Hori, M. Possible novel therapy for diabetes with cell-permeable jnk-inhibitory peptide. Nat. Med. 2004, 10, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Fridlyand, L.E.; Philipson, L.H. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes. Metab. 2006, 8, 136–145. [Google Scholar] [CrossRef]
- Wei, Y.; Sowers, J.R.; Clark, S.E.; Li, W.; Ferrario, C.M.; Stump, C.S. Angiotensin ii-induced skeletal muscle insulin resistance mediated by nf-kappab activation via NADPH oxidase. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E345–E351. [Google Scholar] [CrossRef]
- Nakamura, S.; Takamura, T.; Matsuzawa-Nagata, N.; Takayama, H.; Misu, H.; Noda, H.; Nabemoto, S.; Kurita, S.; Ota, T.; Ando, H.; et al. Palmitate induces insulin resistance in h4iiec3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 2009, 284, 14809–14818. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646, viii—ix. [Google Scholar] [CrossRef]
- McGarry, J.D. Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef]
- Azevedo-Martins, A.K.; Monteiro, A.P.; Lima, C.L.; Lenzen, S.; Curi, R. Fatty acid-induced toxicity and neutral lipid accumulation in insulin-producing rinm5f cells. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2006, 20, 1106–1113. [Google Scholar] [CrossRef]
- Mohammed-Ali, Z.; Carlisle, R.E.; Nademi, S.; Dickhout, J.G. Chapter 16—Animal models of kidney disease. In Animal Models for the Study of Human Disease, 2nd ed.; Conn, P.M., Ed.; Academic Press: New York, NY, USA, 2017; pp. 379–417. [Google Scholar]
- Nakatani, Y.; Kaneto, H.; Kawamori, D.; Hatazaki, M.; Miyatsuka, T.; Matsuoka, T.A.; Kajimoto, Y.; Matsuhisa, M.; Yamasaki, Y.; Hori, M. Modulation of the jnk pathway in liver affects insulin resistance status. J. Biol. Chem. 2004, 279, 45803–45809. [Google Scholar] [CrossRef]
- Davis, J.E.; Gabler, N.K.; Walker-Daniels, J.; Spurlock, M.E. The c-jun n-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3t3-l1 adipocytes. Horm. Metab. Res. Horm. 2009, 41, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef]
- Delbosc, S.; Paizanis, E.; Magous, R.; Araiz, C.; Dimo, T.; Cristol, J.P.; Cros, G.; Azay, J. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis 2005, 179, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, S.; Debure, L.; Moreau, J.F.; Legembre, P. Cd95-mediated cell signaling in cancer: Mutations and post-translational modulations. Cell. Mol. Life Sci. CMLS 2012, 69, 1261–1277. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Holler, N.; Bodmer, J.L.; Hahne, M.; Frei, K.; Fontana, A.; Tschopp, J. Conversion of membrane-bound fas(cd95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 1998, 187, 1205–1213. [Google Scholar] [CrossRef]
- Suda, T.; Hashimoto, H.; Tanaka, M.; Ochi, T.; Nagata, S. Membrane fas ligand kills human peripheral blood t lymphocytes, and soluble fas ligand blocks the killing. J. Exp. Med. 1997, 186, 2045–2050. [Google Scholar] [CrossRef]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-dependent apo-1 (fas/cd95)-associated proteins form a death-inducing signaling complex (disc) with the receptor. EMBO J. 1995, 14, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, S.; Chaigne-Delalande, B.; Selva, E.; Khadra, N.; Daburon, S.; Contin-Bordes, C.; Blanco, P.; Le Seyec, J.; Ducret, T.; Counillon, L.; et al. The naturally processed cd95l elicits a c-yes/calcium/pi3k-driven cell migration pathway. PLoS Biol. 2011, 9, e1001090. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.H.; Falke, J.J. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 16176–16181. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, M.D. Stimulating store-operated Ca2+ entry. Nat. Cell Biol. 2009, 11, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Kleber, S.; Sancho-Martinez, I.; Wiestler, B.; Beisel, A.; Gieffers, C.; Hill, O.; Thiemann, M.; Mueller, W.; Sykora, J.; Kuhn, A.; et al. Yes and pi3k bind cd95 to signal invasion of glioblastoma. Cancer Cell 2008, 13, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Yamamoto, H.; Tominaga, K.; Masuda, K.; Kawai, T.; Teshima-Kondo, S.; Rokutan, K. NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (raw264.7) into osteoclasts. J. Med. Investig. JMI 2009, 56, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, M.J.; Appel, W.H., Jr.; Verhoeven, A.J.; Karnovsky, M.J. NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J. Cell Biol. 1994, 126, 765–772. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Ries, W.; Key, L. Expression of nox4 in osteoclasts. J. Cell. Biochem. 2004, 92, 238–248. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in rankl-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef]
- Gavazzi, G.; Banfi, B.; Deffert, C.; Fiette, L.; Schappi, M.; Herrmann, F.; Krause, K.H. Decreased blood pressure in nox1-deficient mice. FEBS Lett. 2006, 580, 497–504. [Google Scholar] [CrossRef]
- Morrison, S.J. Neuronal potential and lineage determination by neural stem cells. Curr. Opin. Cell Biol. 2001, 13, 666–672. [Google Scholar] [CrossRef]
- Kageyama, R.; Ohtsuka, T.; Hatakeyama, J.; Ohsawa, R. Roles of bhlh genes in neural stem cell differentiation. Exp. Cell Res. 2005, 306, 343–348. [Google Scholar] [CrossRef]
- Kennedy, K.A.; Sandiford, S.D.; Skerjanc, I.S.; Li, S.S. Reactive oxygen species and the neuronal fate. Cell. Mol. Life Sci. CMLS 2012, 69, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Katoh, S.; Mitsui, Y.; Kitani, K.; Suzuki, T. Hyperoxia induces the differentiated neuronal phenotype of pc12 cells by producing reactive oxygen species. Biochem. Biophys. Res. Commun. 1997, 241, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Urra, O.; Alberdi, E.; Matute, C. Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell Death Dis. 2012, 3, e268. [Google Scholar] [CrossRef] [PubMed]
- Nissen, C.; Ciesielski-Treska, J.; Hertz, L.; Mandel, P. Regulation of oxygen consumption in neuroblastoma cells: Effects of differentiation and of potassium. J. Neurochem. 1973, 20, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Ladi, E.; Mayer-Proschel, M.; Noble, M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc. Natl. Acad. Sci. USA 2000, 97, 10032–10037. [Google Scholar] [CrossRef] [PubMed]
- Heumuller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schroder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef]
- Riganti, C.; Costamagna, C.; Bosia, A.; Ghigo, D. The NADPH oxidase inhibitor apocynin (acetovanillone) induces oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 179–187. [Google Scholar] [CrossRef]
- Vejrazka, M.; Micek, R.; Stipek, S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ros production in non-phagocytic cells. Biochim. Biophys. Acta 2005, 1722, 143–147. [Google Scholar] [CrossRef]
- Li, Y.; Trush, M.A. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem. Biophys. Res. Commun. 1998, 253, 295–299. [Google Scholar] [CrossRef]
- Hutchinson, D.S.; Csikasz, R.I.; Yamamoto, D.L.; Shabalina, I.G.; Wikstrom, P.; Wilcke, M.; Bengtsson, T. Diphenylene iodonium stimulates glucose uptake in skeletal muscle cells through mitochondrial complex i inhibition and activation of amp-activated protein kinase. Cell. Signal. 2007, 19, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.J.; Anderson, D.J.; McDonald, B.J.; Narayanasami, R.; Bennett, B.M. Inhibition of NADPH-cytochrome p450 reductase and glyceryl trinitrate biotransformation by diphenyleneiodonium sulfate. Biochem. Pharmacol. 1998, 56, 881–893. [Google Scholar] [CrossRef]
- Lu, Q.R.; Sun, T.; Zhu, Z.; Ma, N.; Garcia, M.; Stiles, C.D.; Rowitch, D.H. Common developmental requirement for olig function indicates a motor neuron/oligodendrocyte connection. Cell 2002, 109, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Kessler, J.A. Interactions between id and olig proteins mediate the inhibitory effects of bmp4 on oligodendroglial differentiation. Development 2004, 131, 4131–4142. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef] [PubMed]
- Mazzonetto, P.C.; Ariza, C.B.; Ocanha, S.G.; de Souza, T.A.; Ko, G.M.; Menck, C.F.M.; Massironi, S.M.G.; Porcionatto, M.A. Mutation in NADPH oxidase 3 (nox3) impairs shh signaling and increases cerebellar neural stem/progenitor cell proliferation. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1502–1515. [Google Scholar] [CrossRef]
- Wechsler-Reya, R.J.; Scott, M.P. Control of neuronal precursor proliferation in the cerebellum by sonic hedgehog. Neuron 1999, 22, 103–114. [Google Scholar] [CrossRef]
- Dahmane, N.; Ruiz i Altaba, A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 1999, 126, 3089–3100. [Google Scholar] [CrossRef]
- Corrales, J.D.; Rocco, G.L.; Blaess, S.; Guo, Q.; Joyner, A.L. Spatial pattern of sonic hedgehog signaling through gli genes during cerebellum development. Development 2004, 131, 5581–5590. [Google Scholar] [CrossRef]
- Lewis, P.M.; Gritli-Linde, A.; Smeyne, R.; Kottmann, A.; McMahon, A.P. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev. Biol. 2004, 270, 393–410. [Google Scholar] [CrossRef]
- Vaillant, C.; Monard, D. Shh pathway and cerebellar development. Cerebellum 2009, 8, 291–301. [Google Scholar] [CrossRef]
- Salman, M.S. Epidemiology of cerebellar diseases and therapeutic approaches. Cerebellum 2018, 17, 4–11. [Google Scholar] [CrossRef]
- Massironi, S.M.; Reis, B.L.; Carneiro, J.G.; Barbosa, L.B.; Ariza, C.B.; Santos, G.C.; Guenet, J.L.; Godard, A.L. Inducing mutations in the mouse genome with the chemical mutagen ethylnitrosourea. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2006, 39, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Nagamani, S.C.; Erez, A.; Eng, C.; Ou, Z.; Chinault, C.; Workman, L.; Coldwell, J.; Stankiewicz, P.; Patel, A.; Lupski, J.R.; et al. Interstitial deletion of 6q25.2-q25.3: A novel microdeletion syndrome associated with microcephaly, developmental delay, dysmorphic features and hearing loss. Eur. J. Hum. Genet. EJHG 2009, 17, 573–581. [Google Scholar] [CrossRef]
- Ahlfeld, J.; Favaro, R.; Pagella, P.; Kretzschmar, H.A.; Nicolis, S.; Schuller, U. Sox2 requirement in sonic hedgehog-associated medulloblastoma. Cancer Res. 2013, 73, 3796–3807. [Google Scholar] [CrossRef] [PubMed]
- Kenney, A.M.; Rowitch, D.H. Sonic hedgehog promotes g(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol. Cell. Biol. 2000, 20, 9055–9067. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Zhang, A.; Li, H.; Jin, Y.; Sun, X.; Li, H.; Gao, J. Lkb1 regulates cerebellar development by controlling sonic hedgehog-mediated granule cell precursor proliferation and granule cell migration. Sci. Rep. 2015, 5, 16232. [Google Scholar] [CrossRef] [PubMed]
- Pogoriler, J.; Millen, K.; Utset, M.; Du, W. Loss of cyclin d1 impairs cerebellar development and suppresses medulloblastoma formation. Development 2006, 133, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.L.; Zhu, S.Y.; Xia, Y.P.; Mao, L.; Mei, Y.W.; Yao, Y.F.; Xue, Y.M.; Hu, B. Sonic hedgehog protects cortical neurons against oxidative stress. Neurochem. Res. 2011, 36, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Shinohara, H.; Baba, Y. B cell signaling and fate decision. Annu. Rev. Immunol. 2010, 28, 21–55. [Google Scholar] [CrossRef]
- Kurosaki, T.; Hikida, M. Tyrosine kinases and their substrates in b lymphocytes. Immunol. Rev. 2009, 228, 132–148. [Google Scholar] [CrossRef]
- Weber, M.; Treanor, B.; Depoil, D.; Shinohara, H.; Harwood, N.E.; Hikida, M.; Kurosaki, T.; Batista, F.D. Phospholipase c-gamma2 and vav cooperate within signaling microclusters to propagate b cell spreading in response to membrane-bound antigen. J. Exp. Med. 2008, 205, 853–868. [Google Scholar] [CrossRef]
- Choi, M.S.; Brines, R.D.; Holman, M.J.; Klaus, G.G. Induction of nf-at in normal b lymphocytes by anti-immunoglobulin or cd40 ligand in conjunction with il-4. Immunity 1994, 1, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wienands, J.; Larbolette, O.; Reth, M. Evidence for a preformed transducer complex organized by the b cell antigen receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 7865–7870. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.M.; Clark, E.A. Bcr-induced superoxide negatively regulates b-cell proliferation and t-cell-independent type 2 ab responses. Eur. J. Immunol. 2009, 39, 3395–3403. [Google Scholar] [CrossRef]
- Singh, D.K.; Kumar, D.; Siddiqui, Z.; Basu, S.K.; Kumar, V.; Rao, K.V. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 2005, 121, 281–293. [Google Scholar] [CrossRef]
- Capasso, M.; Bhamrah, M.K.; Henley, T.; Boyd, R.S.; Langlais, C.; Cain, K.; Dinsdale, D.; Pulford, K.; Khan, M.; Musset, B.; et al. Hvcn1 modulates bcr signal strength via regulation of bcr-dependent generation of reactive oxygen species. Nat. Immunol. 2010, 11, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Defranco, A.L. Prolonged production of reactive oxygen species in response to b cell receptor stimulation promotes b cell activation and proliferation. J. Immunol. 2012, 189, 4405–4416. [Google Scholar] [CrossRef]
- Musarrat, J.; Arezina-Wilson, J.; Wani, A.A. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur. J. Cancer 1996, 32A, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, P.; Yarlagadda, P.; Vadjunec, N.M.; Proia, A.D.; Harris, R.A.; Portilla, D. Ppar alpha ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal fao and pdc activity. Am. J. Physiol. Ren. Physiol. 2004, 286, F572–F580. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-ohdg): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.Y.; Hong, K.S.; Choi, K.S.; Chung, M.H.; Kim, Y.; Kim, J.H.; Hahm, K.B. A novel approach for stress-induced gastritis based on paradoxical anti-oxidative and anti-inflammatory action of exogenous 8-hydroxydeoxyguanosine. Biochem. Pharmacol. 2011, 81, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabbagh, M.; Fusi, L.; Higham, J.; Lee, Y.; Lei, K.; Hanyaloglu, A.C.; Lam, E.W.; Christian, M.; Brosens, J.J. NADPH oxidase-derived reactive oxygen species mediate decidualization of human endometrial stromal cells in response to cyclic amp signaling. Endocrinology 2011, 152, 730–740. [Google Scholar] [CrossRef]
- Carter, R.J.; Morton, J.; Dunnett, S.B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001, 15, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Dupureur, C.M.; Zhang, X.; Tsai, M.D. Phospholipase a2 engineering. The roles of disulfide bonds in structure, conformational stability, and catalytic function. Biochemistry 1995, 34, 15307–15314. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase a2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. The growing phospholipase a2 superfamily of signal transduction enzymes. Trends Biochem. Sci. 1997, 22, 1–2. [Google Scholar] [CrossRef]
- Jones, T.A.; Jones, S.M.; Hoffman, L.F. Resting discharge patterns of macular primary afferents in otoconia-deficient mice. J. Assoc. Res. Otolaryngol. JARO 2008, 9, 490–505. [Google Scholar] [CrossRef]
- Maklad, A.; Feng, F.; Fritzsch, B. Vestibular primary afferent pathways in mammals. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; p. 4199. [Google Scholar]
- Basaldella, E.; Takeoka, A.; Sigrist, M.; Arber, S. Multisensory signaling shapes vestibulo-motor circuit specificity. Cell 2015, 163, 301–312. [Google Scholar] [CrossRef]
- Hernandez, E.; Das, J.M. Neuroanatomy, nucleus vestibular. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lim, R.; Brichta, A.M. Chapter 27—Vestibular system. In The Mouse Nervous System; Watson, C., Paxinos, G., Puelles, L., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 661–681. [Google Scholar]
- Houpt, T.A.; Houpt, C.E. Circular swimming in mice after exposure to a high magnetic field. Physiol. Behav. 2010, 100, 284–290. [Google Scholar] [CrossRef]
- Snyder, D.J.; Jahng, J.W.; Smith, J.C.; Houpt, T.A. C-fos induction in visceral and vestibular nuclei of the rat brain stem by a 9.4 t magnetic field. Neuroreport 2000, 11, 2681–2685. [Google Scholar] [CrossRef]
- Cason, A.M.; Kwon, B.; Smith, J.C.; Houpt, T.A. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol. Behav. 2009, 97, 36–43. [Google Scholar] [CrossRef]
- Lavinsky, J.; Crow, A.L.; Pan, C.; Wang, J.; Aaron, K.A.; Ho, M.K.; Li, Q.; Salehide, P.; Myint, A.; Monges-Hernadez, M.; et al. Genome-wide association study identifies Nox3 as a critical gene for susceptibility to noise-induced hearing loss. PLoS Genet. 2015, 11, e1005094. [Google Scholar]
- Rousset, F.; Carnesecchi, S.; Senn, P.; Krause, K.H. Nox3-targeted therapies for inner ear pathologies. Curr. Pharm. Des. 2015, 21, 5977–5987. [Google Scholar] [CrossRef]
- Rybak, L.P. Cis-platinum associated hearing loss. J. Laryngol. Otol. 1981, 95, 745–747. [Google Scholar] [CrossRef]
- Clerici, W.J.; Hensley, K.; DiMartino, D.L.; Butterfield, D.A. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear. Res. 1996, 98, 116–124. [Google Scholar] [CrossRef]
- Kopke, R.D.; Liu, W.; Gabaizadeh, R.; Jacono, A.; Feghali, J.; Spray, D.; Garcia, P.; Steinman, H.; Malgrange, B.; Ruben, R.J.; et al. Use of organotypic cultures of corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol. 1997, 18, 559–571. [Google Scholar] [PubMed]
- Dhukhwa, A.; Bhatta, P.; Sheth, S.; Korrapati, K.; Tieu, C.; Mamillapalli, C.; Ramkumar, V.; Mukherjea, D. Targeting inflammatory processes mediated by trpvi and tnf-alpha for treating noise-induced hearing loss. Front. Cell. Neurosci. 2019, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.R.; Nuttall, A.L.; Scheibe, F.; Miller, J.M. Sound-induced artifact in cochlear blood flow measurements using the laser doppler flowmeter. Hear. Res. 1987, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Nakai, Y.; Takayama, M.; Konishi, K.; Iguchi, H.; Nakagawa, T.; Shibata, S.; Kato, A.; Sunami, K.; Kawakatsu, C. The emergence of free radicals after acoustic trauma and strial blood flow. Acta Oto-Laryngol. Suppl. 1995, 519, 87–92. [Google Scholar] [CrossRef]
- McAlpine, D.; Johnstone, B.M. The ototoxic mechanism of cisplatin. Hear. Res. 1990, 47, 191–203. [Google Scholar] [CrossRef]
- Rybak, L.P.; Husain, K.; Whitworth, C.; Somani, S.M. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: Antioxidant defense system. Toxicol. Sci. Off. J. Soc. Toxicol. 1999, 47, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Campbell, K.C.; Meech, R.P.; Rybak, L.P.; Hughes, L.F. D-methionine protects against cisplatin damage to the stria vascularis. Hear. Res. 1999, 138, 13–28. [Google Scholar] [CrossRef]
- Korver, K.D.; Rybak, L.P.; Whitworth, C.; Campbell, K.M. Round window application of d-methionine provides complete cisplatin otoprotection. Otolaryngol. Head Neck Surg. 2002, 126, 683–689. [Google Scholar] [CrossRef]
- Choe, W.T.; Chinosornvatana, N.; Chang, K.W. Prevention of cisplatin ototoxicity using transtympanic n-acetylcysteine and lactate. Otol. Neurotol. 2004, 25, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Thomas Dickey, D.; Muldoon, L.L.; Kraemer, D.F.; Neuwelt, E.A. Protection against cisplatin-induced ototoxicity by n-acetylcysteine in a rat model. Hear. Res. 2004, 193, 25–30. [Google Scholar] [CrossRef]
- Feghali, J.G.; Liu, W.; Van De Water, T.R. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope 2001, 111, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Kelly, T. Ototoxicity: Bioprotective mechanisms. Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 328–333. [Google Scholar] [CrossRef]
- Yamashita, D.; Jiang, H.Y.; Schacht, J.; Miller, J.M. Delayed production of free radicals following noise exposure. Brain Res. 2004, 1019, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, D.; Jiang, H.Y.; Le Prell, C.G.; Schacht, J.; Miller, J.M. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience 2005, 134, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G.; Yamashita, D.; Minami, S.B.; Yamasoba, T.; Miller, J.M. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear. Res. 2007, 226, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Schacht, J. Emerging treatments for noise-induced hearing loss. Expert Opin. Emerg. Drugs 2011, 16, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Muller, U.; Barr-Gillespie, P.G. New treatment options for hearing loss. Nat. Rev. Drug Discov. 2015, 14, 346–365. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Bonnet, C.; Safieddine, S. Deafness: From genetic architecture to gene therapy. Nat. Rev. Genet. 2023, 24, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Kamogashira, T.; Fujimoto, C.; Yamasoba, T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. BioMed Res. Int. 2015, 2015, 617207. [Google Scholar] [CrossRef]
- Pittman, C.A.; Ward, B.K.; Nieman, C.L. A review of adult-onset hearing loss: A primer for neurologists. Curr. Treat Options Neurol. 2021, 23, 20. [Google Scholar] [CrossRef]
- Le, T.N.; Straatman, L.V.; Lea, J.; Westerberg, B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol. Head Neck Surg. Le J. D’oto-Rhino-Laryngol. Chir. Cervico-Faciale 2017, 46, 41. [Google Scholar] [CrossRef]
- Wang, J.; Puel, J.L. Presbycusis: An update on cochlear mechanisms and therapies. J. Clin. Med. 2020, 9, 218. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Wu, P.Z.; Liberman, L.D.; Bennett, K.; de Gruttola, V.; O’Malley, J.T.; Liberman, M.C. Primary neural degeneration in the human cochlea: Evidence for hidden hearing loss in the aging ear. Neuroscience 2019, 407, 8–20. [Google Scholar] [CrossRef]
- Peineau, T.; Belleudy, S.; Pietropaolo, S.; Bouleau, Y.; Dulon, D. Synaptic release potentiation at aging auditory ribbon synapses. Front. Aging Neurosci. 2021, 13, 756449. [Google Scholar] [CrossRef]
- Schwander, M.; Kachar, B.; Muller, U. Review series: The cell biology of hearing. J. Cell Biol. 2010, 190, 9–20. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Huarcaya Najarro, E.; Sayyid, Z.N.; Cheng, A.G. Sensory hair cell development and regeneration: Similarities and differences. Development 2015, 142, 1561–1571. [Google Scholar] [CrossRef]
- Furness, D.N. Molecular basis of hair cell loss. Cell Tissue Res. 2015, 361, 387–399. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear. Res. 2019, 377, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Clerici, W.J.; Yang, L. Direct effects of intraperilymphatic reactive oxygen species generation on cochlear function. Hear. Res. 1996, 101, 14–22. [Google Scholar] [CrossRef]
- Bielefeld, E.C.; Hu, B.H.; Harris, K.C.; Henderson, D. Damage and threshold shift resulting from cochlear exposure to paraquat-generated superoxide. Hear. Res. 2005, 207, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Ryan, A.F. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 2015, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: Oxidative stress and ros signaling. Free Radic. Biol. Med. 2019, 135, 46–59. [Google Scholar] [CrossRef]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kaygusuz, I.; Ozturk, A.; Ustundag, B.; Yalcin, S. Role of free oxygen radicals in noise-related hearing impairment. Hear. Res. 2001, 162, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Oto-Rhino-Laryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neuro-Otol. 1999, 4, 229–236. [Google Scholar] [CrossRef]
- Priuska, E.M.; Schacht, J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem. Pharmacol. 1995, 50, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Hockenbery, D.M.; Rubel, E.W. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear. Res. 1997, 104, 1–14. [Google Scholar] [CrossRef]
- Dehne, N.; Lautermann, J.; Petrat, F.; Rauen, U.; de Groot, H. Cisplatin ototoxicity: Involvement of iron and enhanced formation of superoxide anion radicals. Toxicol. Appl. Pharmacol. 2001, 174, 27–34. [Google Scholar] [CrossRef]
- Paparella, M.M.; Oda, M.; Hiraide, F.; Brady, D. Pathology of sensorineural hearing loss in otitis media. Ann. Otol. Rhinol. Laryngol. 1972, 81, 632–647. [Google Scholar] [CrossRef]
- Merchant, S.N.; Gopen, Q. A human temporal bone study of acute bacterial meningogenic labyrinthitis. Am. J. Otol. 1996, 17, 375–385. [Google Scholar]
- Fujimoto, C.; Yamasoba, T. Oxidative stresses and mitochondrial dysfunction in age-related hearing loss. Oxidative Med. Cell. Longev. 2014, 2014, 582849. [Google Scholar] [CrossRef]
- Warraich, U.E.; Hussain, F.; Kayani, H.U.R. Aging—Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Kornatowski, M.; Krzywinska, O.; Kedziora-Kornatowska, K. Changes in the blood antioxidant defense of advanced age people. Clin. Interv. Aging 2019, 14, 763–771. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; McFadden, S.L.; Ding, D.L.; Lear, P.M.; Ho, Y.S. Targeted mutation of the gene for cellular glutathione peroxidase (gpx1) increases noise-induced hearing loss in mice. J. Assoc. Res. Otolaryngol. JARO 2000, 1, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.H.; Henderson, D.; Nicotera, T.M. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear. Res. 2002, 166, 62–71. [Google Scholar] [CrossRef]
- Bohne, B. Mechanisms of Noise Damage in the Inner Ear; John H. Mills Raven Press: New York, NY, USA, 1976; pp. 41–68. [Google Scholar]
- Bohne, B.A.; Harding, G.W.; Lee, S.C. Death pathways in noise-damaged outer hair cells. Hear. Res. 2007, 223, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Murai, N.; Kirkegaard, M.; Jarlebark, L.; Risling, M.; Suneson, A.; Ulfendahl, M. Activation of jnk in the inner ear following impulse noise exposure. J. Neurotrauma 2008, 25, 72–77. [Google Scholar] [CrossRef]
- Seidman, M.D.; Shivapuja, B.G.; Quirk, W.S. The protective effects of allopurinol and superoxide dismutase on noise-induced cochlear damage. Otolaryngol. Head Neck Surg. 1993, 109, 1052–1056. [Google Scholar] [CrossRef]
- Liu, Z. Experimental study on the mechanism of free radical in blast trauma induced hearing loss. Zhonghua Er Bi Yan Hou Ke Za Zhi 1992, 27, 24–26. [Google Scholar]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. Tlr signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L.; Harris, C.M.; Choe, K.M.; Brennan, C.A. Inflammatory production of reactive oxygen species by drosophila hemocytes activates cellular immune defenses. Biochem. Biophys. Res. Commun. 2018, 505, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Romagna, C.D.; Pereira, J.L.; Souza-Pinto, N.C. The role of mitochondrial DNA damage in the citotoxicity of reactive oxygen species. J. Bioenerg. Biomembr. 2011, 43, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, J.; Jones, D.P. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta 1998, 1366, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, S.; Wang, L.; Li, H.; Wang, Y.; Liu, H.; Wang, X.; Zhu, X.; Liu, Z.; Ye, F.; et al. Mitochondrial dysfunction in hearing loss: Oxidative stress, autophagy and nlrp3 inflammasome. Front. Cell Dev. Biol. 2023, 11, 1119773. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant therapy against oxidative damage of the inner ear: Protection and preconditioning. Antioxidants 2020, 9, 1076. [Google Scholar] [CrossRef]
- Kishimoto-Urata, M.; Urata, S.; Fujimoto, C.; Yamasoba, T. Role of oxidative stress and antioxidants in acquired inner ear disorders. Antioxidants 2022, 11, 1469. [Google Scholar] [CrossRef]
- Fay, R.R. Comparative psychoacoustics. Hear. Res. 1988, 34, 295–305. [Google Scholar] [CrossRef]
- Nacher-Soler, G.; Lenglet, S.; Coelho, M.; Thomas, A.; Voruz, F.; Krause, K.H.; Senn, P.; Rousset, F. Local cisplatin delivery in mouse reliably models sensorineural ototoxicity without systemic adverse effects. Front. Cell. Neurosci. 2021, 15, 701783. [Google Scholar] [CrossRef]
- Milosevic, J.; Kalicanin, P. Long arm deletion of chromosome no. 6 in a mentally retarded boy with multiple physical malformations. J. Ment. Defic. Res. 1975, 19, 139–144. [Google Scholar] [PubMed]
- Hopkin, R.J.; Schorry, E.; Bofinger, M.; Milatovich, A.; Stern, H.J.; Jayne, C.; Saal, H.M. New insights into the phenotypes of 6q deletions. Am. J. Med. Genet. 1997, 70, 377–386. [Google Scholar] [CrossRef]
- Sukumar, S.; Wang, S.; Hoang, K.; Vanchiere, C.M.; England, K.; Fick, R.; Pagon, B.; Reddy, K.S. Subtle overlapping deletions in the terminal region of chromosome 6q24.2–q26: Three cases studied using fish. Am. J. Med. Genet. 1999, 87, 17–22. [Google Scholar] [CrossRef]
- Pandya, A.; Braverman, N.; Pyeritz, R.E.; Ying, K.L.; Kline, A.D.; Falk, R.E. Interstitial deletion of the long arm of chromosome 6 associated with unusual limb anomalies: Report of two new patients and review of the literature. Am. J. Med. Genet. 1995, 59, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Lohscheller, J.; Kummer, P.; Eysholdt, U.; Rosanowski, F. Severe sensory hearing loss in del(6q)-syndrome. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Stucken, E.Z.; Hong, R.S. Noise-induced hearing loss: An occupational medicine perspective. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Dobie, R.A. The burdens of age-related and occupational noise-induced hearing loss in the united states. Ear Hear. 2008, 29, 565–577. [Google Scholar] [CrossRef]
- Yankaskas, K. Prelude: Noise-induced tinnitus and hearing loss in the military. Hear. Res. 2013, 295, 3–8. [Google Scholar] [CrossRef]
- Guski, R.; Schreckenberg, D.; Schuemer, R. Who environmental noise guidelines for the european region: A systematic review on environmental noise and annoyance. Int. J. Environ. Res. Public Health 2017, 14, 1539. [Google Scholar] [CrossRef]
- Saffree Jeffree, M.; Ismail, N.; Awang Lukman, K. Hearing impairment and contributing factors among fertilizer factory workers. J. Occup. Health 2016, 58, 434–443. [Google Scholar] [CrossRef]
- Habybabady, R.H.; Mohammadi, M.; Mortazavi, S.B.; Khavanin, A.; Mirzaei, R.; Malvajerdi, M.S. The effect of simultaneous exposure to cigarette smoke and noise on distortion product otoacoustic emissions in rats. Toxicol. Ind. Health 2019, 35, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Kaur, T.; Clayman, A.C.; Nash, A.J.; Schrader, A.D.; Warchol, M.E.; Ohlemiller, K.K. Lack of fractalkine receptor on macrophages impairs spontaneous recovery of ribbon synapses after moderate noise trauma in c57bl/6 mice. Front. Neurosci. 2019, 13, 620. [Google Scholar] [CrossRef] [PubMed]
- Pourbakht, A.; Yamasoba, T. Cochlear damage caused by continuous and intermittent noise exposure. Hear. Res. 2003, 178, 70–78. [Google Scholar] [CrossRef]
- Op de Beeck, K.; Schacht, J.; Van Camp, G. Apoptosis in acquired and genetic hearing impairment: The programmed death of the hair cell. Hear. Res. 2011, 281, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Shargorodsky, J.; Curhan, S.G.; Curhan, G.C.; Eavey, R. Change in prevalence of hearing loss in us adolescents. JAMA 2010, 304, 772–778. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Li, P.; Wang, Z.; Zhao, F.; Gao, Z.; Xu, L.; Luo, Y.J.; Fan, J.; Liu, P. Alterations in gray matter volume due to unilateral hearing loss. Sci. Rep. 2016, 6, 25811. [Google Scholar] [CrossRef]
- Nakajima, K.; Kanda, E.; Hosobuchi, A.; Suwa, K. Subclinical hearing loss, longer sleep duration, and cardiometabolic risk factors in japanese general population. Int. J. Otolaryngol. 2014, 2014, 218218. [Google Scholar] [CrossRef] [PubMed]
- Lie, A.; Skogstad, M.; Johannessen, H.A.; Tynes, T.; Mehlum, I.S.; Nordby, K.C.; Engdahl, B.; Tambs, K. Occupational noise exposure and hearing: A systematic review. Int. Arch. Occup. Environ. Health 2016, 89, 351–372. [Google Scholar] [CrossRef]
- Sliwinska-Kowalska, M.; Pawelczyk, M. Contribution of genetic factors to noise-induced hearing loss: A human studies review. Mutat. Res. 2013, 752, 61–65. [Google Scholar] [CrossRef]
- Heinonen-Guzejev, M.; Vuorinen, H.S.; Mussalo-Rauhamaa, H.; Heikkilä, K.; Koskenvuo, M.; Kaprio, J. Genetic component of noise sensitivity. Twin Res. Hum. Genet. 2012, 8, 245–249. [Google Scholar] [CrossRef]
- Erway, L.C.; Shiau, Y.W.; Davis, R.R.; Krieg, E.F. Genetics of age-related hearing loss in mice. Iii. Susceptibility of inbred and f1 hybrid strains to noise-induced hearing loss. Hear. Res. 1996, 93, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kozel, P.J.; Davis, R.R.; Krieg, E.F.; Shull, G.E.; Erway, L.C. Deficiency in plasma membrane calcium atpase isoform 2 increases susceptibility to noise-induced hearing loss in mice. Hear. Res. 2002, 164, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Holme, R.H.; Steel, K.P. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a cdh23 but not a myo7a mutation. J. Assoc. Res. Otolaryngol. JARO 2004, 5, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.; Whitworth, C.A.; Pingle, S.C.; Hughes, L.F.; Rybak, L.P. Noise induces a1 adenosine receptor expression in the chinchilla cochlea. Hear. Res. 2004, 188, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Vethanayagam, R.R.; Dong, Y.; Cai, Q.; Hu, B.H. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience 2015, 303, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Billings, P.; Harris, J.P.; Firestein, G.S.; Keithley, E.M. Innate immunity contributes to cochlear adaptive immune responses. Audiol. Neuro-Otol. 2005, 10, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Fujioka, M.; Kanzaki, S.; Okano, H.J.; Shibata, S.; Yamashita, D.; Masuda, M.; Mihara, M.; Ohsugi, Y.; Ogawa, K.; et al. Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci. Res. 2010, 66, 345–352. [Google Scholar] [CrossRef]
- Keithley, E.M.; Wang, X.; Barkdull, G.C. Tumor necrosis factor alpha can induce recruitment of inflammatory cells to the cochlea. Otol. Neurotol. 2008, 29, 854–859. [Google Scholar] [CrossRef]
- Paciello, F.; Di Pino, A.; Rolesi, R.; Troiani, D.; Paludetti, G.; Grassi, C.; Fetoni, A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: In Vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020, 143, 111555. [Google Scholar] [CrossRef]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef]
- Bennett, B.J.; Farber, C.R.; Orozco, L.; Kang, H.M.; Ghazalpour, A.; Siemers, N.; Neubauer, M.; Neuhaus, I.; Yordanova, R.; Guan, B.; et al. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res. 2010, 20, 281–290. [Google Scholar] [CrossRef]
- Farber, C.R.; Bennett, B.J.; Orozco, L.; Zou, W.; Lira, A.; Kostem, E.; Kang, H.M.; Furlotte, N.; Berberyan, A.; Ghazalpour, A.; et al. Mouse genome-wide association and systems genetics identify asxl2 as a regulator of bone mineral density and osteoclastogenesis. PLoS Genet. 2011, 7, e1002038. [Google Scholar] [CrossRef]
- Park, C.C.; Gale, G.D.; de Jong, S.; Ghazalpour, A.; Bennett, B.J.; Farber, C.R.; Langfelder, P.; Lin, A.; Khan, A.H.; Eskin, E.; et al. Gene networks associated with conditional fear in mice identified using a systems genetics approach. BMC Syst. Biol. 2011, 5, 43. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Y.; Li, Z.; Xu, X.; Lei, S.; Huang, L.; Xu, L.; Zhang, M.; Yang, L. Associations of noise kurtosis, genetic variations in nox3 and lifestyle factors with noise-induced hearing loss. Environ. Health A Glob. Access Sci. Source 2020, 19, 13. [Google Scholar] [CrossRef]
- Lei, S.F.; Ahroon, W.A.; Hamernik, R.P. The application of frequency and time domain kurtosis to the assessment of hazardous noise exposures. J. Acoust. Soc. Am. 1994, 96, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Qiu, W.; Zeng, L.; Chen, S.S.; Cheng, X.R.; Davis, R.I.; Hamernik, R.P. Application of the kurtosis statistic to the evaluation of the risk of hearing loss in workers exposed to high-level complex noise. Ear Hear. 2010, 31, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kitamura, T.; Sakai, H.; Ando, Y. The loudness of “complex noise” in relation to the factors extracted from the auto-correlation function. J. Sound Vib. 2001, 241, 97–103. [Google Scholar] [CrossRef]
- Erdreich, J. A distribution based definition of impulse noise. J. Acoust. Soc. Am. 1986, 79, 990–998. [Google Scholar] [CrossRef]
- Xie, H.W.; Qiu, W.; Heyer, N.J.; Zhang, M.B.; Zhang, P.; Zhao, Y.M.; Hamernik, R.P. The use of the kurtosis-adjusted cumulative noise exposure metric in evaluating the hearing loss risk for complex noise. Ear Hear. 2016, 37, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.I.; Qiu, W.; Heyer, N.J.; Zhao, Y.; Qiuling Yang, M.S.; Li, N.; Tao, L.; Zhu, L.; Zeng, L.; Yao, D. The use of the kurtosis metric in the evaluation of occupational hearing loss in workers in china: Implications for hearing risk assessment. Noise Health 2012, 14, 330–342. [Google Scholar] [CrossRef]
- Kaur, T.; Borse, V.; Sheth, S.; Sheehan, K.; Ghosh, S.; Tupal, S.; Jajoo, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine a1 receptor protects against cisplatin ototoxicity by suppressing the nox3/stat1 inflammatory pathway in the cochlea. J. Neurosci. 2016, 36, 3962–3977. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Zhao, L.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef]
- Roda, E.; Bottone, M.G.; Biggiogera, M.; Milanesi, G.; Coccini, T. Pulmonary and hepatic effects after low dose exposure to nanosilver: Early and long-lasting histological and ultrastructural alterations in rat. Toxicol. Rep. 2019, 6, 1047–1060. [Google Scholar] [CrossRef]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2015, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, X.; Takemura, T.; Hanagata, N.; Wu, G.; Chou, L.L. Genotoxicity and molecular response of silver nanoparticle (np)-based hydrogel. J. Nanobiotechnol. 2012, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kawata, K.; Osawa, M.; Okabe, S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to hepg2 human hepatoma cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, M.J.; Lee, S.Y.; Oh, S.M.; Chung, K.H. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (beas-2b) cells. Mutat. Res. 2011, 726, 129–135. [Google Scholar] [CrossRef]
- Neely, S.T.; Johnson, T.A.; Kopun, J.; Dierking, D.M.; Gorga, M.P. Distortion-product otoacoustic emission input/output characteristics in normal-hearing and hearing-impaired human ears. J. Acoust. Soc. Am. 2009, 126, 728–738. [Google Scholar] [CrossRef]
- Martin, G.K.; Stagner, B.B.; Chung, Y.S.; Lonsbury-Martin, B.L. Characterizing distortion-product otoacoustic emission components across four species. J. Acoust. Soc. Am. 2011, 129, 3090–3103. [Google Scholar] [CrossRef]
- Shahtaheri, S.J.; Goodarzi, Z.; Karami, E.; Khavanin, A.; Khansari, M.G.; Kiani, M.; Rashidy-Pour, A. Effects of acute exposure to al(2)o(3)-nps (alpha and gamma) and white noise and their combination on cochlea structure and function in wistar rats. Environ. Sci. Pollut. Res. Int. 2023, 30, 89859–89876. [Google Scholar] [CrossRef]
- Ates, M.; Demir, V.; Arslan, Z.; Daniels, J.; Farah, I.O.; Bogatu, C. Evaluation of alpha and gamma aluminum oxide nanoparticle accumulation, toxicity, and depuration in artemia salina larvae. Environ. Toxicol. 2015, 30, 109–118. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Kumari, S.A.; Madhusudhanachary, P.; Turner, T.; Tchounwou, P.B. Biochemical and histopathological evaluation of al(2)o(3) nanomaterials in kidney of wistar rats. Curr. Top. Biochem. Res. 2018, 19, 1–12. [Google Scholar] [PubMed]
- Jacukowicz-Sobala, I.; Ocinski, D.; Kociolek-Balawejder, E. Iron and aluminium oxides containing industrial wastes as adsorbents of heavy metals: Application possibilities and limitations. Waste Manag. Res. 2015, 33, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Brown, A.M.; Willitsford, A.H.; Dinello-Fass, R.; Airola, M.B.; Siegrist, K.M.; Thomas, M.E.; Chang, Y. Lidar measurements of solid rocket propellant fire particle plumes. Appl. Opt. 2016, 55, 4657–4669. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.; Milton, R.; Perry, K.M.A.; Thompson, D.R. Effect of aluminium and alumina on the lung in grinders of duralumin aeroplane propellers. Br. J. Ind. Med. 1944, 1, 159–164. [Google Scholar] [CrossRef]
- Sikkeland, L.; Alexis, N.E.; Fry, R.C.; Martin, E.; Danielsen, T.E.; Sostrand, P.; Kongerud, J. Inflammation in induced sputum after aluminium oxide exposure: An experimental chamber study. Occup. Environ. Med. 2016, 73, 199–205. [Google Scholar] [CrossRef]
- Xing, M.; Zou, H.; Gao, X.; Chang, B.; Tang, S.; Zhang, M. Workplace exposure to airborne alumina nanoparticles associated with separation and packaging processes in a pilot factory. Environ. Sci. Process Impacts 2015, 17, 656–666. [Google Scholar] [CrossRef]
- Lu, J.; Li, W.; Du, X.; Ewert, D.L.; West, M.B.; Stewart, C.; Floyd, R.A.; Kopke, R.D. Antioxidants reduce cellular and functional changes induced by intense noise in the inner ear and cochlear nucleus. J. Assoc. Res. Otolaryngol. JARO 2014, 15, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Fram, R.J. Cisplatin and platinum analogues: Recent advances. Curr. Opin. Oncol. 1992, 4, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Boulikas, T.; Vougiouka, M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review). Oncol. Rep. 2004, 11, 559–595. [Google Scholar] [CrossRef] [PubMed]
- Laurell, G. Pharmacological intervention in the field of ototoxicity. Hno 2019, 67, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.A.; Schwade, N.D.; Truelson, J.M. Fosfomycin does not inhibit the tumoricidal efficacy of cisplatinum. Laryngoscope 1999, 109, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; El-Demerdash, E. Protective effects of l-arginine against cisplatin-induced renal oxidative stress and toxicity: Role of nitric oxide. Basic Clin. Pharmacol. Toxicol. 2005, 97, 91–97. [Google Scholar] [CrossRef]
- Bodenner, D.L.; Dedon, P.C.; Keng, P.C.; Borch, R.F. Effect of diethyldithiocarbamate on cis-diamminedichloroplatinum(ii)-induced cytotoxicity, DNA cross-linking, and gamma-glutamyl transpeptidase inhibition. Cancer Res. 1986, 46, 2745–2750. [Google Scholar]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Lipp, H.P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat. Rec. 2012, 295, 1837–1850. [Google Scholar] [CrossRef]
- Koike, N.; Sasaki, A.; Murakami, T.; Suzuki, K. Effect of edaravone against cisplatin-induced chronic renal injury. Drug Chem. Toxicol. 2021, 44, 437–446. [Google Scholar] [CrossRef]
- Thadhani, R.; Pascual, M.; Bonventre, J.V. Acute renal failure. N. Engl. J. Med. 1996, 334, 1448–1460. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Jajoo, S.; Ramkumar, V. Cisplatin ototoxicity and protection: Clinical and experimental studies. Tohoku J. Exp. Med. 2009, 219, 177–186. [Google Scholar] [CrossRef] [PubMed]
- McKeage, M.J. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995, 13, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, C.; Berger, C.C.; Hartmann, J.T.; Kollmannsberger, C.; Schmoll, H.J.; Kuczyk, M.A.; Kanz, L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br. J. Cancer 1998, 77, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Teh, B.S.; Strother, D.R.; Davis, Q.G.; Chiu, J.K.; Lu, H.H.; Carpenter, L.S.; Mai, W.Y.; Chintagumpala, M.M.; South, M.; et al. Intensity-modulated radiation therapy for pediatric medulloblastoma: Early report on the reduction of ototoxicity. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Anniko, M.; Sobin, A. Cisplatin: Evaluation of its ototoxic potential. Am. J. Otolaryngol. 1986, 7, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, N.; Whitworth, C.A.; Rybak, L.P. Acute changes in cochlear potentials due to cisplatin. Hear. Res. 2000, 149, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.I.; Hess, A.; Bloch, W.; Michel, O. Nitric oxide synthase inhibitor suppresses the ototoxic side effect of cisplatin in guinea pigs. Anticancer Drugs 2000, 11, 401–406. [Google Scholar] [CrossRef]
- Hong, O.; Kerr, M.J.; Poling, G.L.; Dhar, S. Understanding and preventing noise-induced hearing loss. Dis.-A-Mon. DM 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Waissbluth, S.; Salehi, P.; He, X.; Daniel, S.J. Systemic dexamethasone for the prevention of cisplatin-induced ototoxicity. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 1597–1605. [Google Scholar] [CrossRef]
- Fleischman, R.W.; Stadnicki, S.W.; Ethier, M.F.; Schaeppi, U. Ototoxicity of cis-dichlorodiammine platinum (ii) in the guinea pig. Toxicol. Appl. Pharmacol. 1975, 33, 320–332. [Google Scholar] [CrossRef]
- Stadnicki, S.W.; Fleischman, R.W.; Schaeppi, U.; Merriam, P. Cis-dichlorodiammineplatinum (ii) (nsc-119875): Hearing loss and other toxic effects in rhesus monkeys. Cancer Chemother. Rep. 1975, 59, 467–480. [Google Scholar]
- Staecker, H.; Liu, W.; Malgrange, B.; Lefebvre, P.P.; Van De Water, T.R. Vector-mediated delivery of bcl-2 prevents degeneration of auditory hair cells and neurons after injury. ORL 2007, 69, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cai, J.; Xu, L.; Wang, H.; Liu, W. Cisplatin-induced stria vascularis damage is associated with inflammation and fibrosis. Neural Plast. 2020, 2020, 8851525. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, S.; Klis, S.F.; de Groot, J.C.; Smoorenburg, G.F. Alterations in the stria vascularis in relation to cisplatin ototoxicity and recovery. Hear. Res. 2003, 185, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, J.; Ouchi, T. Steroid-responsive bilateral sensorineural hearing loss and immune complexes. Arch. Oto-Rhino-Laryngol. 1981, 230, 5–9. [Google Scholar] [CrossRef]
- Yoshida, K.; Ichimiya, I.; Suzuki, M.; Mogi, G. Effect of proinflammatory cytokines on cultured spiral ligament fibrocytes. Hear. Res. 1999, 137, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Firestein, G.S.; Billings, P.B.; Harris, J.P.; Keithley, E.M. Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J. Assoc. Res. Otolaryngol. JARO 2003, 4, 139–147. [Google Scholar] [CrossRef]
- Kim, H.J.; So, H.S.; Lee, J.H.; Park, C.; Lee, J.B.; Youn, M.J.; Kim, S.J.; Yang, S.H.; Lee, K.M.; Kwon, K.B.; et al. Role of proinflammatory cytokines in cisplatin-induced vestibular hair cell damage. Head Neck 2008, 30, 1445–1456. [Google Scholar] [CrossRef]
- So, H.; Kim, H.; Kim, Y.; Kim, E.; Pae, H.O.; Chung, H.T.; Kim, H.J.; Kwon, K.B.; Lee, K.M.; Lee, H.Y.; et al. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via nrf2/ho-1. J. Assoc. Res. Otolaryngol. JARO 2008, 9, 290–306. [Google Scholar] [CrossRef]
- Watanabe, K.; Inai, S.; Jinnouchi, K.; Bada, S.; Hess, A.; Michel, O.; Yagi, T. Nuclear-factor kappa b (nf-kappa b)-inducible nitric oxide synthase (inos/nos ii) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer. Res. 2002, 22, 4081–4085. [Google Scholar]
- Ries, F.; Klastersky, J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am. J. Kidney Dis. 1986, 8, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Leibbrandt, M.E.; Wolfgang, G.H.; Metz, A.L.; Ozobia, A.A.; Haskins, J.R. Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int. 1995, 48, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Hayakawa, M.; Kato, T.; Hanaki, Y.; Shimizu, K.; Ozawa, T. Adverse effects of anti-tumor drug, cisplatin, on rat kidney mitochondria: Disturbances in glutathione peroxidase activity. Biochem. Biophys. Res. Commun. 1989, 159, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- So, H.S.; Park, C.; Kim, H.J.; Lee, J.H.; Park, S.Y.; Lee, J.H.; Lee, Z.W.; Kim, H.M.; Kalinec, F.; Lim, D.J.; et al. Protective effect of t-type calcium channel blocker flunarizine on cisplatin-induced death of auditory cells. Hear. Res. 2005, 204, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.S.; Silveira, A.F.; Teixeira, A.R.; Hyppolito, M.A. Mechanisms of cisplatin ototoxicity: Theoretical review. J. Laryngol. Otol. 2013, 127, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, H.; Yonemura, K.; Ohishi, K.; Hishida, A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J. Lab. Clin. Med. 1998, 131, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Whitworth, C.; Somani, S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope 1999, 109, 1740–1744. [Google Scholar] [CrossRef]
- Sergi, B.; Ferraresi, A.; Troiani, D.; Paludetti, G.; Fetoni, A.R. Cisplatin ototoxicity in the guinea pig: Vestibular and cochlear damage. Hear. Res. 2003, 182, 56–64. [Google Scholar] [CrossRef]
- Takumida, M.; Anniko, M. Simultaneous detection of both nitric oxide and reactive oxygen species in guinea pig vestibular sensory cells. ORL 2002, 64, 143–147. [Google Scholar] [CrossRef]
- Darlington, C.L.; Smith, P.F. Vestibulotoxicity following aminoglycoside antibiotics and its prevention. Curr. Opin. Investig. Drugs 2003, 4, 841–846. [Google Scholar] [PubMed]
- Rivolta, M.N.; Grix, N.; Lawlor, P.; Ashmore, J.F.; Jagger, D.J.; Holley, M.C. Auditory hair cell precursors immortalized from the mammalian inner ear. Proceedings. Biol. Sci. 1998, 265, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Benkafadar, N.; Menardo, J.; Bourien, J.; Nouvian, R.; Francois, F.; Decaudin, D.; Maiorano, D.; Puel, J.L.; Wang, J. Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol. Med. 2017, 9, 7–26. [Google Scholar] [CrossRef]
- Fredholm, B.B.; AP, I.J.; Jacobson, K.A.; Linden, J.; Muller, C.E. International union of basic and clinical pharmacology. Lxxxi. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Ramkumar, V.; Ravi, R.; Wilson, M.C.; Gettys, T.W.; Whitworth, C.; Rybak, L.P. Identification of a1 adenosine receptors in rat cochlea coupled to inhibition of adenylyl cyclase. Am. J. Physiol. 1994, 267, C731–C737. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.S.; Nie, Z.; Whitworth, C.; Rybak, L.P.; Ramkumar, V. Up-regulation of adenosine receptors in the cochlea by cisplatin. Hear. Res. 1997, 111, 143–152. [Google Scholar] [CrossRef]
- Ford, M.S.; Maggirwar, S.B.; Rybak, L.P.; Whitworth, C.; Ramkumar, V. Expression and function of adenosine receptors in the chinchilla cochlea. Hear. Res. 1997, 105, 130–140. [Google Scholar] [CrossRef]
- Bryant, G.M.; Barron, S.E.; Norris, C.H.; Guth, P.S. Adenosine is a modulator of hair cell-afferent neurotransmission. Hear. Res. 1987, 30, 231–237. [Google Scholar] [CrossRef]
- Nario, K.; Kitano, I.; Mori, N.; Matsunaga, T. The effect of adenosine on cochlear potentials in the guinea pig. Eur. Arch. Oto-Rhino-Laryngol. 1994, 251, 428–433. [Google Scholar] [CrossRef]
- Hight, N.G.; McFadden, S.L.; Henderson, D.; Burkard, R.F.; Nicotera, T. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and r-pia. Hear. Res. 2003, 179, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Vlajkovic, S.M.; Guo, C.X.; Telang, R.; Wong, A.C.; Paramananthasivam, V.; Boison, D.; Housley, G.D.; Thorne, P.R. Adenosine kinase inhibition in the cochlea delays the onset of age-related hearing loss. Exp. Gerontol. 2011, 46, 905–914. [Google Scholar] [CrossRef]
- Whitworth, C.A.; Ramkumar, V.; Jones, B.; Tsukasaki, N.; Rybak, L.P. Protection against cisplatin ototoxicity by adenosine agonists. Biochem. Pharmacol. 2004, 67, 1801–1807. [Google Scholar] [CrossRef]
- Kaur, T.; Mukherjea, D.; Sheehan, K.; Jajoo, S.; Rybak, L.P.; Ramkumar, V. Short interfering rna against stat1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011, 2, e180. [Google Scholar] [CrossRef]
- Demmler, G.J. Infectious diseases society of america and centers for disease control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev. Infect. Dis. 1991, 13, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Stagno, S.; Pass, R.F.; Cloud, G.; Britt, W.J.; Henderson, R.E.; Walton, P.D.; Veren, D.A.; Page, F.; Alford, C.A. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 1986, 256, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Barbi, M.; Binda, S.; Caroppo, S.; Primache, V. Neonatal screening for congenital cytomegalovirus infection and hearing loss. J. Clin. Virol. 2006, 35, 206–209. [Google Scholar] [CrossRef]
- Dreher, A.M.; Arora, N.; Fowler, K.B.; Novak, Z.; Britt, W.J.; Boppana, S.B.; Ross, S.A. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J. Pediatr. 2014, 164, 855–859. [Google Scholar] [CrossRef]
- Shi, X.; Dong, Y.; Li, Y.; Zhao, Z.; Li, H.; Qiu, S.; Li, Y.; Guo, W.; Qiao, Y. Inflammasome activation in mouse inner ear in response to mcmv induced hearing loss. J. Otol. 2015, 10, 143–149. [Google Scholar] [CrossRef]
- Schachtele, S.J.; Mutnal, M.B.; Schleiss, M.R.; Lokensgard, J.R. Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J. Neurovirol. 2011, 17, 201–211. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverria, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Katsarkas, A.; Ayukawa, H. Hearing loss due to aging (presbycusis). J. Otolaryngol. 1986, 15, 239–244. [Google Scholar]
- Gennis, V.; Garry, P.J.; Haaland, K.Y.; Yeo, R.A.; Goodwin, J.S. Hearing and cognition in the elderly. New findings and a review of the literature. Arch. Intern. Med. 1991, 151, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Logroscino, G. Age-related hearing impairment-a risk factor and frailty marker for dementia and ad. Nat. Rev. Neurol. 2015, 11, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, B.R.; Brewster, K.; Golub, J.S.; Kim, A.H.; Roose, S.P. Sensation and psychiatry: Linking age-related hearing loss to late-life depression and cognitive decline. Am. J. Psychiatry 2018, 175, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Schrepfer, T.; Schacht, J. Age-related hearing impairment and the triad of acquired hearing loss. Front. Cell. Neurosci. 2015, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, A.W.; Kammen-Jolly, K.; Felder, E.; Hussl, B.; Rask-Andersen, H.; Schrott-Fischer, A. Selective aspects of human pathology in high-tone hearing loss of the aging inner ear. Hear. Res. 2001, 157, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, Y.; Peng, W.; Sun, Y.; Kong, W.J. Age-related changes in the central auditory system: Comparison of d-galactose-induced aging rats and naturally aging rats. Brain Res. 2010, 1344, 43–53. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, Y.; Peng, W.; Sun, Y.; Hu, Y.J.; Yang, Y.; Kong, W.J. Increased mitochondrial DNA damage and decreased base excision repair in the auditory cortex of d-galactose-induced aging rats. Mol. Biol. Rep. 2011, 38, 3635–3642. [Google Scholar] [CrossRef]
- Du, Z.; Yang, Y.; Hu, Y.; Sun, Y.; Zhang, S.; Peng, W.; Zhong, Y.; Huang, X.; Kong, W. A long-term high-fat diet increases oxidative stress, mitochondrial damage and apoptosis in the inner ear of d-galactose-induced aging rats. Hear. Res. 2012, 287, 15–24. [Google Scholar] [CrossRef]
- Cui, X.; Zuo, P.; Zhang, Q.; Li, X.; Hu, Y.; Long, J.; Packer, L.; Liu, J. Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of r-alpha-lipoic acid. J. Neurosci. Res. 2006, 83, 1584–1590. [Google Scholar] [CrossRef]
- Hua, X.; Lei, M.; Zhang, Y.; Ding, J.; Han, Q.; Hu, G.; Xiao, M. Long-term d-galactose injection combined with ovariectomy serves as a new rodent model for alzheimer’s disease. Life Sci. 2007, 80, 1897–1905. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, Y.L.; Wu, D.M.; Luo, L.; Sun, D.X.; Shan, Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem. Pharmacol. 2007, 74, 1078–1090. [Google Scholar] [CrossRef]
- Chen, C.F.; Lang, S.Y.; Zuo, P.P.; Yang, N.; Wang, X.Q.; Xia, C. Effects of d-galactose on the expression of hippocampal peripheral-type benzodiazepine receptor and spatial memory performances in rats. Psychoneuroendocrinology 2006, 31, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prakash, A.; Dogra, S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by d-galactose in mice. Food Chem. Toxicol. 2010, 48, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Lu, J.; Zheng, Y.L.; Hu, B.; Fan, S.H.; Wu, D.M.; Zheng, Z.H.; Shan, Q.; Liu, C.M. Purple sweet potato color protects mouse liver against d-galactose-induced apoptosis via inhibiting caspase-3 activation and enhancing pi3k/akt pathway. Food Chem. Toxicol. 2010, 48, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Hua, X.; Xiao, M.; Ding, J.; Han, Q.; Hu, G. Impairments of astrocytes are involved in the d-galactose-induced brain aging. Biochem. Biophys. Res. Commun. 2008, 369, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.M.; Wu, W.M.; Hu, M.L. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and alzheimer’s disease in c57bl/6j mice treated with d-galactose. Food Chem. Toxicol. 2009, 47, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, L.; Zuo, P.; Han, Z.; Fang, Z.; Li, W.; Liu, J. D-galactose-caused life shortening in drosophila melanogaster and musca domestica is associated with oxidative stress. Biogerontology 2004, 5, 317–325. [Google Scholar] [CrossRef]
- Kong, W.J.; Wang, Y.; Wang, Q.; Hu, Y.J.; Han, Y.C.; Liu, J. The relation between d-galactose injection and mitochondrial DNA 4834 bp deletion mutation. Exp. Gerontol. 2006, 41, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Hu, Y.J.; Wang, Q.; Wang, Y.; Han, Y.C.; Cheng, H.M.; Kong, W.; Guan, M.X. The effect of the mtdna4834 deletion on hearing. Biochem. Biophys. Res. Commun. 2006, 344, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Hu, Y.J.; Yang, Y.; Peng, W.; Sun, Y.; Chen, B.; Huang, X.; Kong, W.J. Contribution of common deletion to total deletion burden in mitochondrial DNA from inner ear of d-galactose-induced aging rats. Mutat. Res. 2011, 712, 11–19. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, Y.J.; Chen, B.; Peng, W.; Sun, Y.; Yang, Y.; Zhao, X.Y.; Fan, G.R.; Huang, X.; Kong, W.J. Mitochondrial transcription factor a overexpression and base excision repair deficiency in the inner ear of rats with d-galactose-induced aging. FEBS J. 2011, 278, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Yamasoba, T.; Someya, S.; Yamada, C.; Weindruch, R.; Prolla, T.A.; Tanokura, M. Role of mitochondrial dysfunction and mitochondrial DNA mutations in age-related hearing loss. Hear. Res. 2007, 226, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Druzhyna, N.M.; Wilson, G.L.; LeDoux, S.P. Mitochondrial DNA repair in aging and disease. Mech. Ageing Dev. 2008, 129, 383–390. [Google Scholar] [CrossRef]
- Meissner, C.; Bruse, P.; Mohamed, S.A.; Schulz, A.; Warnk, H.; Storm, T.; Oehmichen, M. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: A useful biomarker or more? Exp. Gerontol. 2008, 43, 645–652. [Google Scholar] [CrossRef]
- Markaryan, A.; Nelson, E.G.; Hinojosa, R. Quantification of the mitochondrial DNA common deletion in presbycusis. Laryngoscope 2009, 119, 1184–1189. [Google Scholar] [CrossRef]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: Implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 2008, 43, 24–33. [Google Scholar] [CrossRef]
- Basu, S.; Xie, X.; Uhler, J.P.; Hedberg-Oldfors, C.; Milenkovic, D.; Baris, O.R.; Kimoloi, S.; Matic, S.; Stewart, J.B.; Larsson, N.G.; et al. Accurate mapping of mitochondrial DNA deletions and duplications using deep sequencing. PLoS Genet. 2020, 16, e1009242. [Google Scholar] [CrossRef]
- Schon, E.A.; Rizzuto, R.; Moraes, C.T.; Nakase, H.; Zeviani, M.; DiMauro, S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science 1989, 244, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Pang, C.Y.; Hsu, H.S.; Wei, Y.H. Differential accumulations of 4,977 bp deletion in mitochondrial DNA of various tissues in human ageing. Biochim. Biophys. Acta 1994, 1226, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Hanke, T.; Erasmi, A.W.; Bechtel, M.J.; Scharfschwerdt, M.; Meissner, C.; Sievers, H.H.; Gosslau, A. Mitochondrial DNA deletions and the aging heart. Exp. Gerontol. 2006, 41, 508–517. [Google Scholar] [CrossRef]
- Nakano, Y.; Longo-Guess, C.M.; Bergstrom, D.E.; Nauseef, W.M.; Jones, S.M.; Banfi, B. Mutation of the cyba gene encoding p22phox causes vestibular and immune defects in mice. J. Clin. Investig. 2008, 118, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F.; Salmon, P.; Bredl, S.; Cherpin, O.; Coelho, M.; Myburgh, R.; Alessandrini, M.; Perny, M.; Roccio, M.; Speck, R.F.; et al. Optimizing synthetic mirna minigene architecture for efficient mirna hairpin concatenation and multi-target gene knockdown. Mol. Ther. Nucleic Acids 2019, 14, 351–363. [Google Scholar] [CrossRef]
- Zhang, S.; Xing, J.; Gong, Y.; Li, P.; Wang, B.; Xu, L. Downregulation of vdr in benign paroxysmal positional vertigo patients inhibits otolith-associated protein expression levels. Mol. Med. Rep. 2021, 24, 591. [Google Scholar] [CrossRef]
- Froehling, D.A.; Silverstein, M.D.; Mohr, D.N.; Beatty, C.W.; Offord, K.P.; Ballard, D.J. Benign positional vertigo: Incidence and prognosis in a population-based study in olmsted county, minnesota. Mayo Clin. Proc. 1991, 66, 596–601. [Google Scholar] [CrossRef]
- Imai, T.; Higashi-Shingai, K.; Takimoto, Y.; Masumura, C.; Hattori, K.; Inohara, H. New scoring system of an interview for the diagnosis of benign paroxysmal positional vertigo. Acta Oto-Laryngol. 2016, 136, 283–288. [Google Scholar] [CrossRef]
- Fife, T.D.; Iverson, D.J.; Lempert, T.; Furman, J.M.; Baloh, R.W.; Tusa, R.J.; Hain, T.C.; Herdman, S.; Morrow, M.J.; Gronseth, G.S.; et al. Practice parameter: Therapies for benign paroxysmal positional vertigo (an evidence-based review): Report of the quality standards subcommittee of the american academy of neurology. Neurology 2008, 70, 2067–2074. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical practice guideline: Benign paroxysmal positional vertigo (update). Otolaryngol.-Head Neck Surg. 2017, 156, S1–S47. [Google Scholar] [CrossRef]
- Jang, Y.S.; Hwang, C.H.; Shin, J.Y.; Bae, W.Y.; Kim, L.S. Age-related changes on the morphology of the otoconia. Laryngoscope 2006, 116, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.W.; Honrubia, V.; Jacobson, K. Benign positional vertigo: Clinical and oculographic features in 240 cases. Neurology 1987, 37, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Pyykko, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.H. Meniere’s disease. Nat. Rev. Dis. Primers 2016, 2, 16028. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, J.S.; Shin, J.W.; Kim, S.; Lee, H.; Lee, A.Y.; Kim, J.M.; Jo, H.; Song, J.; Ghim, Y. Decreased serum vitamin d in idiopathic benign paroxysmal positional vertigo. J. Neurol. 2013, 260, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, C.; Han, W.; Lu, X.; Chen, C.; Fan, Z. Reduction of bone mineral density in native chinese female idiopathic benign paroxysmal positional vertigo patients. Am J Otolaryngol 2018, 39, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Yoda, S.; Cureoglu, S.; Yildirim-Baylan, M.; Morita, N.; Fukushima, H.; Harada, T.; Paparella, M.M. Association between type 1 diabetes mellitus and deposits in the semicircular canals. Otolaryngol. Head Neck Surg. 2011, 145, 458–462. [Google Scholar] [CrossRef]
- Holick, M.F. Resurrection of vitamin d deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin d: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery Chinese Medical Association. Guideline of diagnosis and treatment of benign paroxysmal positional vertigo (2017). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi Chin. J. Otorhinolaryngol. Head Neck Surg. 2017, 52, 173–177. [Google Scholar]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef]
- Lokke, A.; Lange, P.; Scharling, H.; Fabricius, P.; Vestbo, J. Developing copd: A 25 year follow up study of the general population. Thorax 2006, 61, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Cho, C.Y.; Thimmulappa, R.K.; Zhen, L.; Srisuma, S.S.; Kensler, T.W.; Yamamoto, M.; Petrache, I.; Tuder, R.M.; Biswal, S. Genetic ablation of nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Investig. 2004, 114, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 50–60. [Google Scholar] [CrossRef]
- Taggart, C.; Cervantes-Laurean, D.; Kim, G.; McElvaney, N.G.; Wehr, N.; Moss, J.; Levine, R.L. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J. Biol. Chem. 2000, 275, 27258–27265. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, P.; Sasidhar, M.; Chupp, G.L.; Flavell, R.A.; Choi, A.M.; Lee, P.J. Reactive oxygen species and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase mediate hyperoxia-induced cell death in lung epithelium. Am. J. Respir. Cell Mol. Biol. 2003, 28, 305–315. [Google Scholar] [CrossRef]
- Ishii, Y.; Itoh, K.; Morishima, Y.; Kimura, T.; Kiwamoto, T.; Iizuka, T.; Hegab, A.E.; Hosoya, T.; Nomura, A.; Sakamoto, T.; et al. Transcription factor nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J. Immunol. 2005, 175, 6968–6975. [Google Scholar] [CrossRef]
- Pons, J.; Sauleda, J.; Regueiro, V.; Santos, C.; Lopez, M.; Ferrer, J.; Agusti, A.G.; Bengoechea, J.A. Expression of toll-like receptor 2 is up-regulated in monocytes from patients with chronic obstructive pulmonary disease. Respir. Res. 2006, 7, 64. [Google Scholar] [CrossRef]
- Sukkar, M.B.; Xie, S.; Khorasani, N.M.; Kon, O.M.; Stanbridge, R.; Issa, R.; Chung, K.F. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J. Allergy Clin. Immunol. 2006, 118, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Marwick, J.A. Molecular mechanisms of oxidative stress in airways and lungs with reference to asthma and chronic obstructive pulmonary disease. Ann. N. Y. Acad. Sci. 2010, 1203, 85–91. [Google Scholar] [CrossRef]
- Pratico, D.; Basili, S.; Vieri, M.; Cordova, C.; Violi, F.; Fitzgerald, G.A. Chronic obstructive pulmonary disease is associated with an increase in urinary levels of isoprostane f2alpha-iii, an index of oxidant stress. Am. J. Respir. Crit. Care Med. 1998, 158, 1709–1714. [Google Scholar] [CrossRef]
- Lius, E.E.; Syafaah, I. Hyperoxia in the management of respiratory failure: A literature review. Ann. Med. Surg. 2022, 81, 104393. [Google Scholar] [CrossRef] [PubMed]
- Lilien, T.A.; van Meenen, D.M.P.; Schultz, M.J.; Bos, L.D.J.; Bem, R.A. Hyperoxia-induced lung injury in acute respiratory distress syndrome: What is its relative impact? Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L9–L16. [Google Scholar] [CrossRef]
- Minkove, S.; Dhamapurkar, R.; Cui, X.; Li, Y.; Sun, J.; Cooper, D.; Eichacker, P.Q.; Torabi-Parizi, P. Effect of low-to-moderate hyperoxia on lung injury in preclinical animal models: A systematic review and meta-analysis. Intensive Care Med. Exp. 2023, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Kallet, R.H.; Matthay, M.A. Hyperoxic acute lung injury. Respir. Care 2013, 58, 123–141. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, P.; Qureshi, S.; Homer, R.; Medzhitov, R.; Noble, P.W.; Lee, P.J. Cutting edge: Tlr4 deficiency confers susceptibility to lethal oxidant lung injury. J. Immunol. 2005, 175, 4834–4838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Shan, P.; Hunt, C.R.; Pandita, T.K.; Lee, P.J. A protective hsp70-tlr4 pathway in lethal oxidant lung injury. J. Immunol. 2013, 191, 1393–1403. [Google Scholar] [CrossRef]
- Hunt, C.R.; Dix, D.J.; Sharma, G.G.; Pandita, R.K.; Gupta, A.; Funk, M.; Pandita, T.K. Genomic instability and enhanced radiosensitivity in hsp70.1- and hsp70.3-deficient mice. Mol. Cell. Biol. 2004, 24, 899–911. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Palsson-McDermott, E.M.; Bowie, A.G.; Jefferies, C.A.; Mansell, A.S.; Brady, G.; Brint, E.; Dunne, A.; Gray, P.; Harte, M.T.; et al. Mal (myd88-adapter-like) is required for toll-like receptor-4 signal transduction. Nature 2001, 413, 78–83. [Google Scholar] [CrossRef]
- Rajpoot, S.; Wary, K.K.; Ibbott, R.; Liu, D.; Saqib, U.; Thurston, T.L.M.; Baig, M.S. Tirap in the mechanism of inflammation. Front. Immunol. 2021, 12, 697588. [Google Scholar] [CrossRef]
- Ruwanpura, S.M.; McLeod, L.; Lilja, A.R.; Brooks, G.; Dousha, L.F.; Seow, H.J.; Bozinovski, S.; Vlahos, R.; Hertzog, P.J.; Anderson, G.P.; et al. Non-essential role for tlr2 and its signaling adaptor mal/tirap in preserving normal lung architecture in mice. PLoS ONE 2013, 8, e78095. [Google Scholar] [CrossRef] [PubMed]
- Ruwanpura, S.M.; McLeod, L.; Miller, A.; Jones, J.; Bozinovski, S.; Vlahos, R.; Ernst, M.; Armes, J.; Bardin, P.G.; Anderson, G.P.; et al. Interleukin-6 promotes pulmonary emphysema associated with apoptosis in mice. Am. J. Respir. Cell Mol. Biol. 2011, 45, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Ruwanpura, S.M.; McLeod, L.; Miller, A.; Jones, J.; Vlahos, R.; Ramm, G.; Longano, A.; Bardin, P.G.; Bozinovski, S.; Anderson, G.P.; et al. Deregulated stat3 signaling dissociates pulmonary inflammation from emphysema in gp130 mutant mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L627–L639. [Google Scholar] [CrossRef] [PubMed]
- Hantos, Z.; Adamicza, A.; Janosi, T.Z.; Szabari, M.V.; Tolnai, J.; Suki, B. Lung volumes and respiratory mechanics in elastase-induced emphysema in mice. J. Appl. Physiol. 2008, 105, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Schwarz, M.I.; Brown, K.; Tooze, J.A.; Colby, T.V.; Waldron, J.A., Jr.; Flint, A.; Thurlbeck, W.; Cherniack, R.M. Idiopathic pulmonary fibrosis: Relationship between histopathologic features and mortality. Am. J. Respir. Crit. Care Med. 2001, 164, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Navaratnam, V.; Fleming, K.M.; West, J.; Smith, C.J.; Jenkins, R.G.; Fogarty, A.; Hubbard, R.B. The rising incidence of idiopathic pulmonary fibrosis in the UK. Thorax 2011, 66, 462–467. [Google Scholar] [CrossRef]
- Zisman, D.A.; Keane, M.P.; Belperio, J.A.; Strieter, R.M.; Lynch, J.P. Pulmonary fibrosis. In Fibrosis Research: Methods and Protocols; Varga, J., Brenner, D.A., Phan, S.H., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 3–44. [Google Scholar]
- Bocchino, M.; Agnese, S.; Fagone, E.; Svegliati, S.; Grieco, D.; Vancheri, C.; Gabrielli, A.; Sanduzzi, A.; Avvedimento, E.V. Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis. PLoS ONE 2010, 5, e14003. [Google Scholar] [CrossRef]
- Manoury, B.; Nenan, S.; Leclerc, O.; Guenon, I.; Boichot, E.; Planquois, J.M.; Bertrand, C.P.; Lagente, V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir. Res. 2005, 6, 11. [Google Scholar] [CrossRef]
- Murthy, S.; Adamcakova-Dodd, A.; Perry, S.S.; Tephly, L.A.; Keller, R.M.; Metwali, N.; Meyerholz, D.K.; Wang, Y.; Glogauer, M.; Thorne, P.S.; et al. Modulation of reactive oxygen species by rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L846–L855. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Fattman, C.L.; Tan, R.J.; Oury, T.D. Oxidative stress in pulmonary fibrosis: A possible role for redox modulatory therapy. Am. J. Respir. Crit. Care Med. 2005, 172, 417–422. [Google Scholar] [CrossRef]
- Korfei, M.; von der Beck, D.; Henneke, I.; Markart, P.; Ruppert, C.; Mahavadi, P.; Ghanim, B.; Klepetko, W.; Fink, L.; Meiners, S.; et al. Comparative proteome analysis of lung tissue from patients with idiopathic pulmonary fibrosis (ipf), non-specific interstitial pneumonia (nsip) and organ donors. J. Proteom. 2013, 85, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Ciabattoni, G.; Paredi, P.; Pantelidis, P.; du Bois, R.M.; Kharitonov, S.A.; Barnes, P.J. 8-isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am. J. Respir. Crit. Care Med. 1998, 158, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.; Allan, G.J.; Lochrie, J.D.; Flint, D.J. Insulin-like growth factor-binding protein-5 (igfbp-5): A critical member of the igf axis. Biochem. J. 2006, 395, 1–19. [Google Scholar] [CrossRef]
- Han, N.; Zhang, F.; Li, G.; Zhang, X.; Lin, X.; Yang, H.; Wang, L.; Cao, Y.; Du, J.; Fan, Z. Local application of igfbp5 protein enhanced periodontal tissue regeneration via increasing the migration, cell proliferation and osteo/dentinogenic differentiation of mesenchymal stem cells in an inflammatory niche. Stem Cell Res. Ther. 2017, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, K.; Lemire, M.; Potvin, C.; Tremblay, A.; Hunninghake, G.M.; Raby, B.A.; Hudson, T.J.; Perez-Iratxeta, C.; Andrade-Navarro, M.A.; Laprise, C. Genes to diseases (g2d) computational method to identify asthma candidate genes. PLoS ONE 2008, 3, e2907. [Google Scholar] [CrossRef]
- Perez-Iratxeta, C.; Wjst, M.; Bork, P.; Andrade, M.A. G2d: A tool for mining genes associated with disease. BMC Genet 2005, 6, 45. [Google Scholar] [CrossRef]
- Perez-Iratxeta, C.; Bork, P.; Andrade-Navarro, M.A. Update of the g2d tool for prioritization of gene candidates to inherited diseases. Nucleic Acids Res. 2007, 35, W212–W216. [Google Scholar] [CrossRef]
- Heyer, E.; Tremblay, M. Variability of the genetic contribution of quebec population founders associated to some deleterious genes. Am. J. Hum. Genet. 1995, 56, 970–978. [Google Scholar]
- Richter, A.; Rioux, J.D.; Bouchard, J.P.; Mercier, J.; Mathieu, J.; Ge, B.; Poirier, J.; Julien, D.; Gyapay, G.; Weissenbach, J.; et al. Location score and haplotype analyses of the locus for autosomal recessive spastic ataxia of charlevoix-saguenay, in chromosome region 13q11. Am. J. Hum. Genet. 1999, 64, 768–775. [Google Scholar] [CrossRef]
- Yin, C.; Li, K.; Yu, Y.; Huang, H.; Yu, Y.; Wang, Z.; Yan, J.; Pu, Y.; Li, Z.; Li, D.; et al. Genome-wide association study identifies loci and candidate genes for non-idiopathic pulmonary hypertension in eastern chinese han population. BMC Pulm. Med. 2018, 18, 158. [Google Scholar] [CrossRef]
- Barman, S.A.; Chen, F.; Li, X.; Haigh, S.; Stepp, D.W.; Kondrikov, D.; Mahboubi, K.; Bordan, Z.; Traber, P.; Su, Y.; et al. Galectin-3 promotes vascular remodeling and contributes to pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2018, 197, 1488–1492. [Google Scholar] [CrossRef]
- Soubrier, F.; Chung, W.K.; Machado, R.; Grunig, E.; Aldred, M.; Geraci, M.; Loyd, J.E.; Elliott, C.G.; Trembath, R.C.; Newman, J.H.; et al. Genetics and genomics of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2013, 62, D13–D21. [Google Scholar] [CrossRef]
- Cantu, E.; Shah, R.J.; Lin, W.; Daye, Z.J.; Diamond, J.M.; Suzuki, Y.; Ellis, J.H.; Borders, C.F.; Andah, G.A.; Beduhn, B.; et al. Oxidant stress regulatory genetic variation in recipients and donors contributes to risk of primary graft dysfunction after lung transplantation. J. Thorac. Cardiovasc. Surg. 2015, 149, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Charles, E.J.; Kron, I.L. One step closer to the elimination of primary graft dysfunction. J. Thorac. Cardiovasc. Surg. 2015, 149, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Carby, M.; Bag, R.; Corris, P.; Hertz, M.; Weill, D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A consensus statement of the international society for heart and lung transplantation. J. Heart Lung Transplant 2005, 24, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Edwards, L.B.; Kucheryavaya, A.Y.; Aurora, P.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Stehlik, J.; Hertz, M.I. The registry of the international society for heart and lung transplantation: Twenty-seventh official adult lung and heart-lung transplant report—2010. J. Heart Lung Transplant. 2010, 29, 1104–1118. [Google Scholar] [CrossRef]
- Suzuki, Y.; Cantu, E.; Christie, J.D. Primary graft dysfunction. Semin. Respir. Crit. Care Med. 2013, 34, 305–319. [Google Scholar] [CrossRef] [PubMed]
- de Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef]
- Williams, A.; Riise, G.C.; Anderson, B.A.; Kjellstrom, C.; Schersten, H.; Kelly, F.J. Compromised antioxidant status and persistent oxidative stress in lung transplant recipients. Free Radic. Res. 1999, 30, 383–393. [Google Scholar] [CrossRef]
- Rega, F.R.; Wuyts, W.A.; Vanaudenaerde, B.M.; Jannis, N.C.; Neyrinck, A.P.; Verleden, G.M.; Lerut, T.E.; Van Raemdonck, D.E. Nebulized n-acetyl cysteine protects the pulmonary graft inside the non-heart-beating donor. J. Heart Lung Transplant. 2005, 24, 1369–1377. [Google Scholar] [CrossRef]
- Kozower, B.D.; Christofidou-Solomidou, M.; Sweitzer, T.D.; Muro, S.; Buerk, D.G.; Solomides, C.C.; Albelda, S.M.; Patterson, G.A.; Muzykantov, V.R. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003, 21, 392–398. [Google Scholar] [CrossRef]
- Cho, H.Y.; Reddy, S.P.; Kleeberger, S.R. Nrf2 defends the lung from oxidative stress. Antioxid. Redox Signal. 2006, 8, 76–87. [Google Scholar] [CrossRef]
- Lubos, E.; Mahoney, C.E.; Leopold, J.A.; Zhang, Y.Y.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 modulates lipopolysaccharide-induced adhesion molecule expression in endothelial cells by altering cd14 expression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2525–2532. [Google Scholar]
- Hamanishi, T.; Furuta, H.; Kato, H.; Doi, A.; Tamai, M.; Shimomura, H.; Sakagashira, S.; Nishi, M.; Sasaki, H.; Sanke, T.; et al. Functional variants in the glutathione peroxidase-1 (gpx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes 2004, 53, 2455–2460. [Google Scholar] [CrossRef]
- Liu, P.; Shi, L.; Cang, X.; Huang, J.; Wu, X.; Yan, J.; Chen, L.; Cui, S.; Ye, X. Ctbp2 ameliorates palmitate-induced insulin resistance in hepg2 cells through ros mediated jnk pathway. Gen. Comp. Endocrinol. 2017, 247, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Styskal, J.; Van Remmen, H.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic. Biol. Med. 2012, 52, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa-Nagata, N.; Takamura, T.; Ando, H.; Nakamura, S.; Kurita, S.; Misu, H.; Ota, T.; Yokoyama, M.; Honda, M.; Miyamoto, K.; et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metab. Clin. Exp. 2008, 57, 1071–1077. [Google Scholar] [CrossRef]
- Hoehn, K.L.; Salmon, A.B.; Hohnen-Behrens, C.; Turner, N.; Hoy, A.J.; Maghzal, G.J.; Stocker, R.; Van Remmen, H.; Kraegen, E.W.; Cooney, G.J.; et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 17787–17792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Zhou, B.G.; Sheng, J.Q.; Chen, Y.; Cao, Y.Q.; Chen, C. Molecular mechanisms of hepatic insulin resistance in nonalcoholic fatty liver disease and potential treatment strategies. Pharmacol. Res. 2020, 159, 104984. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the paradox of hepatic insulin resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Boden, G.; Shulman, G.I. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 2002, 32, 14–23. [Google Scholar] [CrossRef]
- Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Montonen, J.; Knekt, P.; Harkanen, T.; Jarvinen, R.; Heliovaara, M.; Aromaa, A.; Reunanen, A. Dietary patterns and the incidence of type 2 diabetes. Am. J. Epidemiol. 2005, 161, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.; Yki-Jarvinen, H.; Hawkins, M. Out of the frying pan: Dietary saturated fat influences nonalcoholic fatty liver disease. J. Clin. Investig. 2017, 127, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Magnusson, I.; Rothman, D.L.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Increased rate of gluconeogenesis in type ii diabetes mellitus. A 13c nuclear magnetic resonance study. J. Clin. Investig. 1992, 90, 1323–1327. [Google Scholar] [CrossRef]
- Bock, G.; Chittilapilly, E.; Basu, R.; Toffolo, G.; Cobelli, C.; Chandramouli, V.; Landau, B.R.; Rizza, R.A. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: Role of increased rates of gluconeogenesis. Diabetes 2007, 56, 1703–1711. [Google Scholar] [CrossRef]
- Basu, R.; Barosa, C.; Jones, J.; Dube, S.; Carter, R.; Basu, A.; Rizza, R.A. Pathogenesis of prediabetes: Role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. J. Clin. Endocrinol. Metab. 2013, 98, E409–E417. [Google Scholar] [CrossRef]
- Funatsu, H.; Yamashita, H. Molecular biology in development and progression of diabetic retinopathy. Nihon Rinsho Jpn. J. Clin. Med. 2002, 60, 162–166. [Google Scholar]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- McClung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuniga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef]
- Bettaieb, A.; Vazquez Prieto, M.A.; Rodriguez Lanzi, C.; Miatello, R.M.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (-)-epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress. Free Radic. Biol. Med. 2014, 72, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Cremonini, E.; Kang, H.; Kang, J.; Haj, F.G.; Oteiza, P.I. Anti-inflammatory actions of (-)-epicatechin in the adipose tissue of obese mice. Int. J. Biochem. Cell Biol. 2016, 81, 383–392. [Google Scholar] [CrossRef]
- Cremonini, E.; Wang, Z.; Bettaieb, A.; Adamo, A.M.; Daveri, E.; Mills, D.A.; Kalanetra, K.M.; Haj, F.G.; Karakas, S.; Oteiza, P.I. (-)-epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: Implications for steatosis and insulin resistance. Redox Biol. 2018, 14, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.P.; Cheng, Y.; Mahata, S.K.; Corti, A.; Tota, B. Chromogranin a and derived peptides in health and disease. J. Mol. Neurosci. MN 2012, 48, 347–356. [Google Scholar] [CrossRef]
- Hossain, Z.; Valicherla, G.R.; Gupta, A.P.; Syed, A.A.; Riyazuddin, M.; Chandra, S.; Siddiqi, M.I.; Gayen, J.R. Discovery of pancreastatin inhibitor psti8 for the treatment of insulin resistance and diabetes: Studies in rodent models of diabetes mellitus. Sci. Rep. 2018, 8, 8715. [Google Scholar] [CrossRef]
- Sanchez-Margalet, V.; Gonzalez-Yanes, C. Pancreastatin inhibits insulin action in rat adipocytes. Am. J. Physiol. 1998, 275, E1055–E1060. [Google Scholar] [CrossRef]
- Gayen, J.R.; Saberi, M.; Schenk, S.; Biswas, N.; Vaingankar, S.M.; Cheung, W.W.; Najjar, S.M.; O’Connor, D.T.; Bandyopadhyay, G.; Mahata, S.K. A novel pathway of insulin sensitivity in chromogranin a null mice: A crucial role for pancreastatin in glucose homeostasis. J. Biol. Chem. 2009, 284, 28498–28509. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Efendic, S.; Mutt, V.; Makk, G.; Feistner, G.J.; Barchas, J.D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 1986, 324, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, G.K.; Lu, M.; Avolio, E.; Siddiqui, J.A.; Gayen, J.R.; Wollam, J.; Vu, C.U.; Chi, N.W.; O’Connor, D.T.; Mahata, S.K. Pancreastatin-dependent inflammatory signaling mediates obesity-induced insulin resistance. Diabetes 2015, 64, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Broedbaek, K.; Hilsted, L. Chromogranin a as biomarker in diabetes. Biomark. Med. 2016, 10, 1181–1189. [Google Scholar] [CrossRef]
- Allu, P.K.; Chirasani, V.R.; Ghosh, D.; Mani, A.; Bera, A.K.; Maji, S.K.; Senapati, S.; Mullasari, A.S.; Mahapatra, N.R. Naturally occurring variants of the dysglycemic peptide pancreastatin: Differential potencies for multiple cellular functions and structure-function correlation. J. Biol. Chem. 2014, 289, 4455–4469. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative stress in type 2 diabetes: Impacts from pathogenesis to lifestyle modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Rajan, S.; Shankar, K.; Beg, M.; Varshney, S.; Gupta, A.; Srivastava, A.; Kumar, D.; Mishra, R.K.; Hussain, Z.; Gayen, J.R.; et al. Chronic hyperinsulinemia reduces insulin sensitivity and metabolic functions of brown adipocyte. J. Endocrinol. 2016, 230, 275–290. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, A.P.; Katekar, R.; Verma, S.; Goand, U.K.; Dadge, S.; Gayen, J.R. Pancreastatin inhibitor psti8 prevents free fatty acid-induced oxidative stress and insulin resistance by modulating jnk pathway: In vitro and in vivo findings. Life Sci. 2022, 289, 120221. [Google Scholar] [CrossRef]
- Mouche, S.; Mkaddem, S.B.; Wang, W.; Katic, M.; Tseng, Y.H.; Carnesecchi, S.; Steger, K.; Foti, M.; Meier, C.A.; Muzzin, P.; et al. Reduced expression of the NADPH oxidase nox4 is a hallmark of adipocyte differentiation. Biochim. Biophys. Acta 2007, 1773, 1015–1027. [Google Scholar] [CrossRef]
- Schroder, K.; Wandzioch, K.; Helmcke, I.; Brandes, R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.A.; Acharya, J.D.; Mehendale, N.K.; Kamat, S.S.; Ghaskadbi, S.S. Pterostilbene reverses palmitic acid mediated insulin resistance in hepg2 cells by reducing oxidative stress and triglyceride accumulation. Free Radic. Res. 2019, 53, 815–827. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yanez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. PTR 2008, 22, 169–179. [Google Scholar] [CrossRef]
- Acharya, J.D.; Ghaskadbi, S.S. Protective effect of pterostilbene against free radical mediated oxidative damage. BMC Complement. Altern. Med. 2013, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, M.A.; Pari, L. Effect of pterostilbene on lipids and lipid profiles in streptozotocin-nicotinamide induced type 2 diabetes mellitus. J. Appl. Biomed. 2008, 6, 31–37. [Google Scholar] [CrossRef]
- Elango, B.; Dornadula, S.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene ameliorates streptozotocin-induced diabetes through enhancing antioxidant signaling pathways mediated by nrf2. Chem. Res. Toxicol. 2016, 29, 47–57. [Google Scholar] [CrossRef]
- Gomez-Zorita, S.; Fernandez-Quintela, A.; Aguirre, L.; Macarulla, M.T.; Rimando, A.M.; Portillo, M.P. Pterostilbene improves glycaemic control in rats fed an obesogenic diet: Involvement of skeletal muscle and liver. Food Funct. 2015, 6, 1968–1976. [Google Scholar] [CrossRef]
- Pihlajaniemi, T.; Myllyla, R.; Kivirikko, K.I.; Tryggvason, K. Effects of streptozotocin diabetes, glucose, and insulin on the metabolism of type iv collagen and proteoglycan in murine basement membrane-forming ehs tumor tissue. J. Biol. Chem. 1982, 257, 14914–14920. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ros) and wound healing: The functional role of ros and emerging ros-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, H.Y.; Jang, J.Y.; Lee, H.R.; Shin, Y.S.; Kim, C.H. Redox treatment ameliorates diabetes mellitus-induced skin flap necrosis via inhibiting apoptosis and promoting neoangiogenesis. Exp. Biol. Med. 2021, 246, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, K.; Fujii, K.; Kamouchi, M.; Nakane, H.; Arihiro, S.; Okada, Y.; Ibayashi, S.; Iida, M. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. J. Neurol. Sci. 2004, 221, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, D.; Luo, W.; Yu, J.; Li, J.; Yu, Y.; Zhang, X.; Chen, J.; Wu, X.R.; Huang, C. E3 ligase activity of xiap ring domain is required for xiap-mediated cancer cell migration, but not for its rhogdi binding activity. PLoS ONE 2012, 7, e35682. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Lau, D.C. The obesity epidemic and its impact on hypertension. Can. J. Cardiol. 2012, 28, 326–333. [Google Scholar] [CrossRef]
- Franklin, S.S.; Gustin, W.t.; Wong, N.D.; Larson, M.G.; Weber, M.A.; Kannel, W.B.; Levy, D. Hemodynamic patterns of age-related changes in blood pressure. The framingham heart study. Circulation 1997, 96, 308–315. [Google Scholar] [CrossRef]
- Pravenec, M.; Wallace, C.; Aitman, T.J.; Kurtz, T.W. Gene expression profiling in hypertension research: A critical perspective. Hypertension 2003, 41, 3–8. [Google Scholar] [CrossRef]
- Radkowski, P.; Wator, G.; Skupien, J.; Bogdali, A.; Wolkow, P. Analysis of gene expression to predict dynamics of future hypertension incidence in type 2 diabetic patients. BMC Proc. 2016, 10, 113–117. [Google Scholar] [CrossRef]
- Chen, G.; Adeyemo, A.A.; Zhou, J.; Chen, Y.; Doumatey, A.; Lashley, K.; Huang, H.; Amoah, A.; Agyenim-Boateng, K.; Eghan, B.A., Jr.; et al. A genome-wide search for linkage to renal function phenotypes in west africans with type 2 diabetes. Am. J. Kidney Dis. 2007, 49, 394–400. [Google Scholar] [CrossRef]
- Kwak, S.H.; Hernandez-Cancela, R.B.; DiCorpo, D.A.; Condon, D.E.; Merino, J.; Wu, P.; Brody, J.A.; Yao, J.; Guo, X.; Ahmadizar, F.; et al. Time-to-event genome-wide association study for incident cardiovascular disease in people with type 2 diabetes mellitus. medRxiv 2023. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Talior, I.; Yarkoni, M.; Bashan, N.; Eldar-Finkelman, H. Increased glucose uptake promotes oxidative stress and pkc-delta activation in adipocytes of obese, insulin-resistant mice. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E295–E302. [Google Scholar] [CrossRef] [PubMed]
- Wajngarten, M.; Silva, G.S. Hypertension and stroke: Update on treatment. Eur. Cardiol. 2019, 14, 111–115. [Google Scholar] [CrossRef]
- Johansson, B.B. Hypertension mechanisms causing stroke. Clin. Exp. Pharmacol. Physiol. 1999, 26, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Neaton, J.D.; Blackburn, H.; Jacobs, D.; Kuller, L.; Lee, D.J.; Sherwin, R.; Shih, J.; Stamler, J.; Wentworth, D. Serum cholesterol level and mortality findings for men screened in the multiple risk factor intervention trial. Multiple risk factor intervention trial research group. Arch. Intern. Med. 1992, 152, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Peeling, J.; Yan, H.J.; Chen, S.G.; Campbell, M.; Del Bigio, M.R. Protective effects of free radical inhibitors in intracerebral hemorrhage in rat. Brain Res. 1998, 795, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Yamamoto, K.; Morita, N.; Ohta, Y.; Chikugo, T.; Higashizawa, T.; Suzuki, T. Establishment and use of the m strain of stroke-prone spontaneously hypertensive rat. J. Hypertension. Suppl. 1986, 4, S21–S24. [Google Scholar]
- Iritani, N.; Fukuda, E.; Nara, Y.; Yamori, Y. Lipid metabolism in spontaneously hypertensive rats (shr). Atherosclerosis 1977, 28, 217–222. [Google Scholar] [CrossRef]
- Tanito, M.; Nakamura, H.; Kwon, Y.W.; Teratani, A.; Masutani, H.; Shioji, K.; Kishimoto, C.; Ohira, A.; Horie, R.; Yodoi, J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid. Redox Signal. 2004, 6, 89–97. [Google Scholar] [CrossRef]
- Michihara, A.; Shimatani, M.; Anraku, M.; Tomida, H.; Akasaki, K. High levels of oxidative stress exist in the brain than serum or kidneys in stroke-prone spontaneously hypertensive rats at ten weeks of age. Biol. Pharm. Bull. 2010, 33, 518–521. [Google Scholar] [CrossRef]
- Loft, S.; Vistisen, K.; Ewertz, M.; Tjonneland, A.; Overvad, K.; Poulsen, H.E. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: Influence of smoking, gender and body mass index. Carcinogenesis 1992, 13, 2241–2247. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamamoto, E.; Kataoka, K.; Yamashita, T.; Tokutomi, Y.; Dong, Y.F.; Matsuba, S.; Ogawa, H.; Kim-Mitsuyama, S. Pioglitazone exerts protective effects against stroke in stroke-prone spontaneously hypertensive rats, independently of blood pressure. Stroke 2007, 38, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Borchi, E.; Giordano, C.; Baruzzo, S.; Ponziani, V.; Sebastiani, M.; Nassi, P.; Mugelli, A.; d’Amati, G.; Cerbai, E. NADPH oxidase-dependent redox signaling in human heart failure: Relationship between the left and right ventricle. J. Mol. Cell Cardiol. 2007, 42, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Borchi, E.; Bargelli, V.; Stillitano, F.; Giordano, C.; Sebastiani, M.; Nassi, P.A.; d’Amati, G.; Cerbai, E.; Nediani, C. Enhanced ros production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim. Biophys. Acta 2010, 1802, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Maack, C.; Kartes, T.; Kilter, H.; Schafers, H.J.; Nickenig, G.; Bohm, M.; Laufs, U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-gtpase and represents a target for statin treatment. Circulation 2003, 108, 1567–1574. [Google Scholar] [CrossRef]
- Zhang, M.; Perino, A.; Ghigo, A.; Hirsch, E.; Shah, A.M. NADPH oxidases in heart failure: Poachers or gamekeepers? Antioxid. Redox Signal. 2013, 18, 1024–1041. [Google Scholar] [CrossRef]
- Fenyo, I.M.; Florea, I.C.; Raicu, M.; Manea, A. Tyrphostin ag490 reduces napdh oxidase activity and expression in the aorta of hypercholesterolemic apolipoprotein e-deficient mice. Vasc. Pharmacol. 2011, 54, 100–106. [Google Scholar] [CrossRef]
- Sirker, A.; Murdoch, C.E.; Protti, A.; Sawyer, G.J.; Santos, C.X.; Martin, D.; Zhang, X.; Brewer, A.C.; Zhang, M.; Shah, A.M. Cell-specific effects of nox2 on the acute and chronic response to myocardial infarction. J. Mol. Cell Cardiol. 2016, 98, 11–17. [Google Scholar] [CrossRef]
- Bell, R.M.; Cave, A.C.; Johar, S.; Hearse, D.J.; Shah, A.M.; Shattock, M.J. Pivotal role of nox-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J. 2005, 19, 2037–2039. [Google Scholar] [CrossRef]
- Wind, S.; Beuerlein, K.; Armitage, M.E.; Taye, A.; Kumar, A.H.; Janowitz, D.; Neff, C.; Shah, A.M.; Wingler, K.; Schmidt, H.H. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by nox1/2 is reversed by NADPH oxidase inhibition. Hypertension 2010, 56, 490–497. [Google Scholar] [CrossRef]
- Bkaily, G.; Najibeddine, W.; Jacques, D. Increase of NADPH oxidase 3 in heart failure of hereditary cardiomyopathy (1). Can. J. Physiol. Pharmacol. 2019, 97, 902–908. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Hill, M.F.; Singal, P.K. Role of oxidative stress in transition of hypertrophy to heart failure. J. Am. Coll. Cardiol. 1996, 28, 506–514. [Google Scholar] [CrossRef]
- Wu, R.; Yin, D.; Sadekova, N.; Deschepper, C.F.; de Champlain, J.; Girouard, H. Protective effects of aspirin from cardiac hypertrophy and oxidative stress in cardiomyopathic hamsters. Oxidative Med. Cell. Longev. 2012, 2012, 761710. [Google Scholar] [CrossRef] [PubMed]
- Mollnau, H.; Oelze, M.; August, M.; Wendt, M.; Daiber, A.; Schulz, E.; Baldus, S.; Kleschyov, A.L.; Materne, A.; Wenzel, P.; et al. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Jacques, D.; Bkaily, G. Endocardial endothelial cell hypertrophy takes place during the development of hereditary cardiomyopathy. Mol. Cell Biochem. 2019, 453, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Elmoselhi, A.B.; Hata, T.; Makino, N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 2000, 47, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Jeroudi, M.O.; Patel, B.S.; Aruoma, O.I.; Halliwell, B.; Lai, E.K.; McCay, P.B. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circ. Res. 1989, 65, 607–622. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Nakamura, M.; Wang, N.P.; Wilcox, J.N.; Shearer, S.; Ronson, R.S.; Guyton, R.A.; Vinten-Johansen, J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc. Res. 2000, 45, 651–660. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Kloner, R.A.; Jennings, R.B. Consequences of brief ischemia: Stunning, preconditioning, and their clinical implications: Part 2. Circulation 2001, 104, 3158–3167. [Google Scholar] [CrossRef]
- Hayashidani, S.; Tsutsui, H.; Shiomi, T.; Ikeuchi, M.; Matsusaka, H.; Suematsu, N.; Wen, J.; Egashira, K.; Takeshita, A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 2003, 108, 2134–2140. [Google Scholar] [CrossRef]
- Kaikita, K.; Hayasaki, T.; Okuma, T.; Kuziel, W.A.; Ogawa, H.; Takeya, M. Targeted deletion of cc chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am. J. Pathol. 2004, 165, 439–447. [Google Scholar] [CrossRef]
- Dewald, O.; Zymek, P.; Winkelmann, K.; Koerting, A.; Ren, G.; Abou-Khamis, T.; Michael, L.H.; Rollins, B.J.; Entman, M.L.; Frangogiannis, N.G. Ccl2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 2005, 96, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, H.; Hirose, M.; Takahashi, M.; Kawaguchi, M.; Ise, H.; Kolattukudy, P.E.; Yamada, M.; Ikeda, U. Mcp-1 induces cardioprotection against ischaemia/reperfusion injury: Role of reactive oxygen species. Cardiovasc. Res. 2008, 78, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P.E.; Quach, T.; Bergese, S.; Breckenridge, S.; Hensley, J.; Altschuld, R.; Gordillo, G.; Klenotic, S.; Orosz, C.; Parker-Thornburg, J. Myocarditis induced by targeted expression of the mcp-1 gene in murine cardiac muscle. Am. J. Pathol. 1998, 152, 101–111. [Google Scholar] [PubMed]
- Vats, S.; Sundquist, K.; Sundquist, J.; Zhang, N.; Wang, X.; Acosta, S.; Gottsater, A.; Memon, A.A. Oxidative stress-related genetic variation and antioxidant vitamin intake in intact and ruptured abdominal aortic aneurysm: A swedish population-based retrospective cohort study. Eur. J. Prev. Cardiol. 2024, 31, 61–74. [Google Scholar] [CrossRef]
- Manjer, J.; Elmstahl, S.; Janzon, L.; Berglund, G. Invitation to a population-based cohort study: Differences between subjects recruited using various strategies. Scand. J. Public Health 2002, 30, 103–112. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s choice—European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Handa, A.; Silver, L.E.; Rothwell, P.M.; Oxford Vascular, S. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br. J. Surg. 2015, 102, 907–915. [Google Scholar] [CrossRef]
- Reimerink, J.J.; van der Laan, M.J.; Koelemay, M.J.; Balm, R.; Legemate, D.A. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br. J. Surg. 2013, 100, 1405–1413. [Google Scholar] [CrossRef]
- Song, P.; He, Y.; Adeloye, D.; Zhu, Y.; Ye, X.; Yi, Q.; Rahimi, K.; Rudan, I.; Global Health Epidemiology Research Group. The global and regional prevalence of abdominal aortic aneurysms: A systematic review and modeling analysis. Ann. Surg. 2023, 277, 912–919. [Google Scholar] [CrossRef]
- Sweeting, M.J.; Thompson, S.G.; Brown, L.C.; Powell, J.T.; Collaborators, R. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br. J. Surg. 2012, 99, 655–665. [Google Scholar] [CrossRef]
- Guzik, B.; Sagan, A.; Ludew, D.; Mrowiecki, W.; Chwala, M.; Bujak-Gizycka, B.; Filip, G.; Grudzien, G.; Kapelak, B.; Zmudka, K.; et al. Mechanisms of oxidative stress in human aortic aneurysms--association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol. 2013, 168, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.L.; Gavrila, D.; Weintraub, N.L. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Blomkalns, A.L.; Ogbi, M.; Thomas, M.; Gavrila, D.; Neltner, B.S.; Cassis, L.A.; Thompson, R.W.; Weiss, R.M.; Lindower, P.D.; et al. Role of myeloperoxidase in abdominal aortic aneurysm formation: Mitigation by taurine. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1168–H1179. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Andersson, E.; Ekbom, A.; Feychting, M.; Kim, J.L.; Reuterwall, C.; Heurgren, M.; Olausson, P.O. External review and validation of the swedish national inpatient register. BMC Public Health 2011, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Caramori, M.L.; Fioretto, P.; Mauer, M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesions. Diabetes 2003, 52, 1036–1040. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Hunt, S.C.; Hasstedt, S.J.; Coon, H.; Camp, N.J.; Cawthon, R.M.; Wu, L.L.; Hopkins, P.N. Linkage of creatinine clearance to chromosome 10 in utah pedigrees replicates a locus for end-stage renal disease in humans and renal failure in the fawn-hooded rat. Kidney Int. 2002, 62, 1143–1148. [Google Scholar] [CrossRef]
- Rokutan, K.; Kawahara, T.; Kuwano, Y.; Tominaga, K.; Nishida, K.; Teshima-Kondo, S. Nox enzymes and oxidative stress in the immunopathology of the gastrointestinal tract. Semin. Immunopathol. 2008, 30, 315–327. [Google Scholar] [CrossRef]
- Salles, N.; Szanto, I.; Herrmann, F.; Armenian, B.; Stumm, M.; Stauffer, E.; Michel, J.P.; Krause, K.H. Expression of mrna for ros-generating NADPH oxidases in the aging stomach. Exp. Gerontol. 2005, 40, 353–357. [Google Scholar] [CrossRef]
- Augusto, A.C.; Miguel, F.; Mendonca, S.; Pedrazzoli, J., Jr.; Gurgueira, S.A. Oxidative stress expression status associated to Helicobacter pylori virulence in gastric diseases. Clin. Biochem. 2007, 40, 615–622. [Google Scholar] [CrossRef]
- El Hassani, R.A.; Benfares, N.; Caillou, B.; Talbot, M.; Sabourin, J.C.; Belotte, V.; Morand, S.; Gnidehou, S.; Agnandji, D.; Ohayon, R.; et al. Dual oxidase2 is expressed all along the digestive tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G933–G942. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Arts, P.; Knarren, G.H.; Mulder, A.H.; Wakelkamp, I.M.; Hermus, A.R.; Joosten, L.A.; Netea, M.G.; Bisschop, P.H.; de Herder, W.W.; et al. Rare nox3 variants confer susceptibility to agranulocytosis during thyrostatic treatment of graves’ disease. Clin. Pharmacol. Ther. 2017, 102, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhong, J.; Xiao, X.H.; Zhou, L.Z.; Chen, Y.J.; Liu, J.H.; Cao, R.X.; Wen, G.B. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr. J. 2013, 60, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, N.; Ohnishi, H.; Nishiuma, T.; Doukuni, R.; Umezawa, K.; Oozone, S.; Kuramoto, E.; Yoshimura, S.; Kinami, S. Antithyroid drug-induced agranulocytosis complicated by pneumococcal sepsis and upper airway obstruction. Intern. Med. 2013, 52, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Toh Yoon, E.W. Severe drug-induced agranulocytosis successfully treated with recombinant human granulocyte colony-stimulating factor. Case Rep. Med. 2018, 2018, 8439791. [Google Scholar]
- Andrès, E.; Maloisel, F. Antibiotic-induced agranulocytosis: A monocentric study of 21 cases. Arch. Intern. Med. 2001, 161, 2619. [Google Scholar] [CrossRef]
- di Fonzo, H.; Villegas Gutsch, M.; Castroagudin, A.; Cabrera, M.V.; Mazzei, M.E.; Rueda, D. Agranulocytosis induced by vancomycin. Case report and literature review. Am. J. Case Rep. 2018, 19, 1053–1056. [Google Scholar] [CrossRef]
- Weissel, M. Propylthiouracil: Clinical overview of its efficacy and its side effects more than 50 years after the introduction of its use in thyrostatic treatment. Exp. Clin. Endocrinol. Diabetes 2010, 118, 101–104. [Google Scholar] [CrossRef]
- Sato, S.; Noh, J.Y.; Sato, S.; Suzuki, M.; Yasuda, S.; Matsumoto, M.; Kunii, Y.; Mukasa, K.; Sugino, K.; Ito, K.; et al. Comparison of efficacy and adverse effects between methimazole 15 mg+inorganic iodine 38 mg/day and methimazole 30 mg/day as initial therapy for graves’ disease patients with moderate to severe hyperthyroidism. Thyroid 2015, 25, 43–50. [Google Scholar] [CrossRef]
- Taylor, P.N.; Vaidya, B. Side effects of anti-thyroid drugs and their impact on the choice of treatment for thyrotoxicosis in pregnancy. Eur. Thyroid J. 2012, 1, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Andersohn, F.; Konzen, C.; Garbe, E. Systematic review: Agranulocytosis induced by nonchemotherapy drugs. Ann. Intern. Med. 2007, 146, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Kubota, S.; Fukata, S.; Kudo, T.; Nishihara, E.; Ito, M.; Amino, N.; Miyauchi, A. Methimazole-induced agranulocytosis in patients with graves’ disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid 2009, 19, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Pan, C.M.; Zhao, S.X.; Liang, J.; Gao, G.Q.; Zhang, X.M.; Yuan, G.Y.; Li, C.G.; Xue, L.Q.; Shen, M.; et al. A genome-wide association study identifies two new risk loci for graves’ disease. Nat. Genet. 2011, 43, 897–901. [Google Scholar] [PubMed]
- Liu, W.; Wang, H.N.; Gu, Z.H.; Yang, S.Y.; Ye, X.P.; Pan, C.M.; Zhao, S.X.; Xue, L.Q.; Xie, H.J.; Yu, S.S.; et al. Identification of bach2 as a susceptibility gene for graves’ disease in the chinese han population based on a three-stage genome-wide association study. Hum. Genet. 2014, 133, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Roderique, J.D.; Josef, C.S.; Feldman, M.J.; Spiess, B.D. A modern literature review of carbon monoxide poisoning theories, therapies, and potential targets for therapy advancement. Toxicology 2015, 334, 45–58. [Google Scholar] [CrossRef]
- Varma, D.R.; Mulay, S.; Chemtob, S. Chapter 20—Carbon monoxide: From public health risk to painless killer. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 271–292. [Google Scholar]
- Ginsberg, M.D. Delayed neurological deterioration following hypoxia. Adv. Neurol. 1979, 26, 21–44. [Google Scholar]
- Sun, Q.; Cai, J.; Zhou, J.; Tao, H.; Zhang, J.H.; Zhang, W.; Sun, X.J. Hydrogen-rich saline reduces delayed neurologic sequelae in experimental carbon monoxide toxicity. Crit. Care Med. 2011, 39, 765–769. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Zhang, J.; Demchenko, I.T. Production of hydroxyl radical in the hippocampus after co hypoxia or hypoxic hypoxia in the rat. Free Radic. Biol. Med. 1997, 22, 725–732. [Google Scholar] [CrossRef]
- Hara, S.; Kobayashi, M.; Kuriiwa, F.; Mukai, T.; Mizukami, H. Different mechanisms of hydroxyl radical production susceptible to purine p2 receptor antagonists between carbon monoxide poisoning and exogenous atp in rat striatum. Free Radic. Res. 2014, 48, 1322–1333. [Google Scholar] [CrossRef]
- Hara, S.; Kobayash, M.; Kuriiwa, F.; Kurosaki, K.; Mizukami, H. Gene expression in rat striatum following carbon monoxide poisoning. Genom Data 2017, 12, 74–75. [Google Scholar] [CrossRef]
- Thom, S.R.; Ohnishi, S.T.; Ischiropoulos, H. Nitric oxide released by platelets inhibits neutrophil b2 integrin function following acute carbon monoxide poisoning. Toxicol. Appl. Pharmacol. 1994, 128, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Katoh, A.; Ishimaru, H.; Yoneda, Y.; Ogita, K.; Murase, K.; Ohtsuka, H.; Inari, K.; Fukuta, T.; Kameyama, T. Carbon monoxide-induced delayed amnesia, delayed neuronal death and change in acetylcholine concentration in mice. J. Pharmacol. Exp. Ther. 1991, 256, 378–384. [Google Scholar] [PubMed]
- Fujita, K.; Seike, T.; Yutsudo, N.; Ohno, M.; Yamada, H.; Yamaguchi, H.; Sakumi, K.; Yamakawa, Y.; Kido, M.A.; Takaki, A.; et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of parkinson’s disease. PLoS ONE 2009, 4, e7247. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, L.; Holopainen, S.; Pessa-Morikawa, T.; Lappalainen, A.K.; Hytonen, M.K.; Lohi, H.; Iivanainen, A. Genetic dissection of canine hip dysplasia phenotypes and osteoarthritis reveals three novel loci. BMC Genom. 2019, 20, 1027. [Google Scholar] [CrossRef] [PubMed]
- Skurkova, L.; Hluchy, M.; Lackova, M.; Mihalova, M.; Ledecky, V. Relation of the norberg angle and position of the femoral head centre to the dorsal acetabular edge in evaluation of canine hip dysplasia. Vet. Comp. Orthop. Traumatol. 2010, 23, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, B.J.G.; Vezzoni, A.; Bogaerts, E.; Bertal, M.; Bosmans, T.; Stock, E.; Deforce, D.; Peelman, L.; Saunders, J.H. Comparison of three methods to quantify laxity in the canine hip joint. Vet. Comp. Orthop. Traumatol. V.C.O.T 2018, 31, 23–29. [Google Scholar] [PubMed]
- Sanchez-Molano, E.; Woolliams, J.A.; Pong-Wong, R.; Clements, D.N.; Blott, S.C.; Wiener, P. Quantitative trait loci mapping for canine hip dysplasia and its related traits in uk labrador retrievers. BMC Genom. 2014, 15, 833. [Google Scholar] [CrossRef]
- Lavrijsen, I.C.; Leegwater, P.A.; Martin, A.J.; Harris, S.J.; Tryfonidou, M.A.; Heuven, H.C.; Hazewinkel, H.A. Genome wide analysis indicates genes for basement membrane and cartilage matrix proteins as candidates for hip dysplasia in labrador retrievers. PLoS ONE 2014, 9, e87735. [Google Scholar] [CrossRef]
- Fels, L.; Distl, O. Identification and validation of quantitative trait loci (qtl) for canine hip dysplasia (chd) in german shepherd dogs. PLoS ONE 2014, 9, e96618. [Google Scholar] [CrossRef]
- Pfahler, S.; Distl, O. Identification of quantitative trait loci (qtl) for canine hip dysplasia and canine elbow dysplasia in bernese mountain dogs. PLoS ONE 2012, 7, e49782. [Google Scholar] [CrossRef]
- Zhou, Z.; Sheng, X.; Zhang, Z.; Zhao, K.; Zhu, L.; Guo, G.; Friedenberg, S.G.; Hunter, L.S.; Vandenberg-Foels, W.S.; Hornbuckle, W.E.; et al. Differential genetic regulation of canine hip dysplasia and osteoarthritis. PLoS ONE 2010, 5, e13219. [Google Scholar] [CrossRef]
- Carlstrom, K.E.; Ewing, E.; Granqvist, M.; Gyllenberg, A.; Aeinehband, S.; Enoksson, S.L.; Checa, A.; Badam, T.V.S.; Huang, J.; Gomez-Cabrero, D.; et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ros pathway in monocytes. Nat. Commun. 2019, 10, 3081. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Mikulskis, A.; Gold, R.; Fox, R.J.; Dawson, K.T.; Amaravadi, L. Evidence of activation of the nrf2 pathway in multiple sclerosis patients treated with delayed-release dimethyl fumarate in the phase 3 define and confirm studies. Mult. Scler. 2017, 23, 1875–1883. [Google Scholar] [CrossRef]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-controlled phase 3 study of oral bg-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef]
- Kraaij, M.D.; Savage, N.D.; van der Kooij, S.W.; Koekkoek, K.; Wang, J.; van den Berg, J.M.; Ottenhoff, T.H.; Kuijpers, T.W.; Holmdahl, R.; van Kooten, C.; et al. Induction of regulatory t cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2010, 107, 17686–17691. [Google Scholar] [CrossRef]
- George-Chandy, A.; Nordstrom, I.; Nygren, E.; Jonsson, I.M.; Postigo, J.; Collins, L.V.; Eriksson, K. Th17 development and autoimmune arthritis in the absence of reactive oxygen species. Eur. J. Immunol. 2008, 38, 1118–1126. [Google Scholar] [CrossRef]
- Mossberg, N.; Movitz, C.; Hellstrand, K.; Bergstrom, T.; Nilsson, S.; Andersen, O. Oxygen radical production in leukocytes and disease severity in multiple sclerosis. J. Neuroimmunol. 2009, 213, 131–134. [Google Scholar] [CrossRef]
- Mix, E.; Stefan, K.; Hoppner, J.; Klauer, T.; Zettl, U.K.; Strauss, U.; Meyer-Rienecker, H.J.; Rolfs, A. Lymphocyte subpopulations, oxidative burst and apoptosis in peripheral blood cells of patients with multiple sclerosis-effect of interferon-beta. Autoimmunity 2003, 36, 291–305. [Google Scholar] [CrossRef]
- Becanovic, K.; Jagodic, M.; Sheng, J.R.; Dahlman, I.; Aboul-Enein, F.; Wallstrom, E.; Olofsson, P.; Holmdahl, R.; Lassmann, H.; Olsson, T. Advanced intercross line mapping of eae5 reveals ncf-1 and cldn4 as candidate genes for experimental autoimmune encephalomyelitis. J. Immunol. 2006, 176, 6055–6064. [Google Scholar] [CrossRef]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the nrf2 antioxidant pathway. Brain A J. Neurol. 2011, 134, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sheng, Q.; Zeng, H.; Li, W.; Wang, Q.; Ma, G.; Xu, X.; Qiu, M.; Zhang, W.; Shan, C. Specific genetic aberrations of parathyroid in chinese patients with tertiary hyperparathyroidism using whole-exome sequencing. Front. Endocrinol. 2023, 14, 1221060. [Google Scholar] [CrossRef] [PubMed]
- Messa, P.; Alfieri, C.M. Secondary and tertiary hyperparathyroidism. Front. Horm. Res. 2019, 51, 91–108. [Google Scholar] [PubMed]
- Alfieri, C.; Mattinzoli, D.; Messa, P. Tertiary and postrenal transplantation hyperparathyroidism. Endocrinol. Metab. Clin. North Am. 2021, 50, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Dream, S.; Chen, H.; Lindeman, B. Tertiary hyperparathyroidism: Why the delay? Ann. Surg. 2021, 273, e120–e122. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Mukherjea, D.; Jajoo, S.; Kaur, T.; Ramkumar, V. Sirna-mediated knock-down of nox3: Therapy for hearing loss? Cell. Mol. Life Sci. 2012, 69, 2429–2434. [Google Scholar] [CrossRef] [PubMed]
- Southall, M.D.; Li, T.; Gharibova, L.S.; Pei, Y.; Nicol, G.D.; Travers, J.B. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J. Pharmacol. Exp. Ther. 2003, 304, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Puntambekar, P.; Mukherjea, D.; Jajoo, S.; Ramkumar, V. Essential role of rac1/NADPH oxidase in nerve growth factor induction of trpv1 expression. J. Neurochem. 2005, 95, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, Y.; Hamilton, T.A. Requirement for stat1 in lps-induced gene expression in macrophages. J. Leukoc. Biol. 2001, 69, 598–604. [Google Scholar] [CrossRef]
- Sugawara, I.; Yamada, H.; Mizuno, S. Stat1 knockout mice are highly susceptible to pulmonary mycobacterial infection. Tohoku J. Exp. Med. 2004, 202, 41–50. [Google Scholar] [CrossRef]
- Yoshimura, A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006, 97, 439–447. [Google Scholar] [CrossRef]
- Porta, C.; Hadj-Slimane, R.; Nejmeddine, M.; Pampin, M.; Tovey, M.G.; Espert, L.; Alvarez, S.; Chelbi-Alix, M.K. Interferons alpha and gamma induce p53-dependent and p53-independent apoptosis, respectively. Oncogene 2005, 24, 605–615. [Google Scholar] [CrossRef]
- Al Aameri, R.F.H.; Alanisi, E.M.A.; Oluwatosin, A.; Al Sallami, D.; Sheth, S.; Alberts, I.; Patel, S.; Rybak, L.P.; Ramkumar, V. Targeting cxcl1 chemokine signaling for treating cisplatin ototoxicity. Front. Immunol. 2023, 14, 1125948. [Google Scholar] [CrossRef]
- Dai, M.; Yang, Y.; Omelchenko, I.; Nuttall, A.L.; Kachelmeier, A.; Xiu, R.; Shi, X. Bone marrow cell recruitment mediated by inducible nitric oxide synthase/stromal cell-derived factor-1alpha signaling repairs the acoustically damaged cochlear blood-labyrinth barrier. Am. J. Pathol. 2010, 177, 3089–3099. [Google Scholar] [CrossRef]
- Tan, W.J.; Thorne, P.R.; Vlajkovic, S.M. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem. Cell Biol. 2016, 146, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.B.; Zuo, J. The contribution of immune infiltrates to ototoxicity and cochlear hair cell loss. Front. Cell. Neurosci. 2017, 11, 106. [Google Scholar] [CrossRef]
- Ou, H.C.; Raible, D.W.; Rubel, E.W. Cisplatin-induced hair cell loss in zebrafish (danio rerio) lateral line. Hear. Res. 2007, 233, 46–53. [Google Scholar] [CrossRef]
- Pang, J.; Xiong, H.; Zhan, T.; Cheng, G.; Jia, H.; Ye, Y.; Su, Z.; Chen, H.; Lin, H.; Lai, L.; et al. Sirtuin 1 and autophagy attenuate cisplatin-induced hair cell death in the mouse cochlea and zebrafish lateral line. Front. Cell. Neurosci. 2018, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Coffin, A.B.; Ou, H.; Owens, K.N.; Santos, F.; Simon, J.A.; Rubel, E.W.; Raible, D.W. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish 2010, 7, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Pyun, J.H.; Kang, S.U.; Hwang, H.S.; Oh, Y.T.; Kang, S.H.; Lim, Y.A.; Choo, O.S.; Kim, C.H. Epicatechin inhibits radiation-induced auditory cell death by suppression of reactive oxygen species generation. Neuroscience 2011, 199, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Ucur, E.; Herzer, K.; Okouoyo, S.; Ridder, R.; Krammer, P.H.; von Knebel Doeberitz, M.; Debatin, K.M. Glucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res. 2003, 63, 3112–3120. [Google Scholar] [PubMed]
- Bas, E.; Gupta, C.; Van De Water, T.R. A novel organ of corti explant model for the study of cochlear implantation trauma. Anat. Rec. 2012, 295, 1944–1956. [Google Scholar] [CrossRef]
- Dinh, C.T.; Haake, S.; Chen, S.; Hoang, K.; Nong, E.; Eshraghi, A.A.; Balkany, T.J.; Van De Water, T.R. Dexamethasone protects organ of corti explants against tumor necrosis factor-alpha-induced loss of auditory hair cells and alters the expression levels of apoptosis-related genes. Neuroscience 2008, 157, 405–413. [Google Scholar] [CrossRef]
- Calli, C.; Pinar, E.; Oncel, S.; Alper Bagriyanik, H.; Umut Sakarya, E. Recovery of hearing in cisplatin-induced ototoxicity in the guinea pig with intratympanic dexamethasone. Indian J. Otolaryngol. Head Neck Surg. 2012, 64, 46–50. [Google Scholar] [CrossRef]
- Shafik, A.G.; Elkabarity, R.H.; Thabet, M.T.; Soliman, N.B.; Kalleny, N.K. Effect of intratympanic dexamethasone administration on cisplatin-induced ototoxicity in adult guinea pigs. Auris Nasus Larynx 2013, 40, 51–60. [Google Scholar] [CrossRef]
- Murphy, D.; Daniel, S.J. Intratympanic dexamethasone to prevent cisplatin ototoxicity: A guinea pig model. Otolaryngol. Head Neck Surg. 2011, 145, 452–457. [Google Scholar] [CrossRef]
- Paksoy, M.; Ayduran, E.; Sanli, A.; Eken, M.; Aydin, S.; Oktay, Z.A. The protective effects of intratympanic dexamethasone and vitamin e on cisplatin-induced ototoxicity are demonstrated in rats. Med. Oncol. 2011, 28, 615–621. [Google Scholar] [CrossRef]
- Daldal, A.; Odabasi, O.; Serbetcioglu, B. The protective effect of intratympanic dexamethasone on cisplatin-induced ototoxicity in guinea pigs. Otolaryngol. Head Neck Surg. 2007, 137, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.W.; Morest, D.K.; Parham, K. Cisplatin-induced ototoxicity: Effect of intratympanic dexamethasone injections. Otol. Neurotol. 2008, 29, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Marshak, T.; Steiner, M.; Kaminer, M.; Levy, L.; Shupak, A. Prevention of cisplatin-induced hearing loss by intratympanic dexamethasone: A randomized controlled study. Otolaryngol. Head Neck Surg. 2014, 150, 983–990. [Google Scholar] [CrossRef]
- Dinh, C.T.; Chen, S.; Bas, E.; Dinh, J.; Goncalves, S.; Telischi, F.; Angeli, S.; Eshraghi, A.A.; Van De Water, T. Dexamethasone protects against apoptotic cell death of cisplatin-exposed auditory hair cells in vitro. Otol. Neurotol. 2015, 36, 1566–1571. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, C.; Lee, J.N.; Park, R. Protective roles of fenofibrate against cisplatin-induced ototoxicity by the rescue of peroxisomal and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2018, 353, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M.; Croom, K.F. Fenofibrate: A review of its use in primary dyslipidaemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs 2007, 67, 121–153. [Google Scholar] [CrossRef]
- Park, C.; Ji, H.M.; Kim, S.J.; Kil, S.H.; Lee, J.N.; Kwak, S.; Choe, S.K.; Park, R. Fenofibrate exerts protective effects against gentamicin-induced toxicity in cochlear hair cells by activating antioxidant enzymes. Int. J. Mol. Med. 2017, 39, 960–968. [Google Scholar] [CrossRef]
- Thongnuanjan, P.; Soodvilai, S.; Chatsudthipong, V.; Soodvilai, S. Fenofibrate reduces cisplatin-induced apoptosis of renal proximal tubular cells via inhibition of jnk and p38 pathways. J. Toxicol. Sci. 2016, 41, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Daynes, R.A.; Jones, D.C. Emerging roles of ppars in inflammation and immunity. Nat. Rev. Immunol. 2002, 2, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Portilla, D.; Dai, G.; McClure, T.; Bates, L.; Kurten, R.; Megyesi, J.; Price, P.; Li, S. Alterations of pparalpha and its coactivator pgc-1 in cisplatin-induced acute renal failure. Kidney Int. 2002, 62, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Morizono, T. Calcium transport mechanism in the endolymph of the chinchilla. Hear. Res. 1988, 34, 307–311. [Google Scholar] [CrossRef]
- Fridberger, A.; Flock, A.; Ulfendahl, M.; Flock, B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc. Natl. Acad. Sci. USA 1998, 95, 7127–7132. [Google Scholar] [CrossRef]
- Chan, D.K.; Rouse, S.L. Sound-induced intracellular Ca2+ dynamics in the adult hearing cochlea. PLoS ONE 2016, 11, e0167850. [Google Scholar] [CrossRef]
- Li, W.; Zhao, L.; Jiang, S.; Gu, R. Effects of high intensity impulse noise on ionic concentrations in cochlear endolymph of the guinea pig. Chin. Med. J. 1997, 110, 883–886. [Google Scholar]
- Hirose, K.; Discolo, C.M.; Keasler, J.R.; Ransohoff, R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005, 489, 180–194. [Google Scholar] [CrossRef]
- So, H.; Kim, H.; Lee, J.H.; Park, C.; Kim, Y.; Kim, E.; Kim, J.K.; Yun, K.J.; Lee, K.M.; Lee, H.Y.; et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of erk and nf-kappab. J. Assoc. Res. Otolaryngol. JARO 2007, 8, 338–355. [Google Scholar] [CrossRef]
- Bas, E.; Martinez-Soriano, F.; Lainez, J.M.; Marco, J. An experimental comparative study of dexamethasone, melatonin and tacrolimus in noise-induced hearing loss. Acta Oto-Laryngol. 2009, 129, 385–389. [Google Scholar] [CrossRef]
- Arslan, H.H.; Satar, B.; Serdar, M.; Yilmaz, E. Changes in proinflammatory cytokines in the cochlea in relation to hearing thresholds in noise-exposed rats. J. Int. Adv. Otol. 2017, 13, 308–312. [Google Scholar] [CrossRef]
- Infante, E.B.; Channer, G.A.; Telischi, F.F.; Gupta, C.; Dinh, J.T.; Vu, L.; Eshraghi, A.A.; Van De Water, T.R. Mannitol protects hair cells against tumor necrosis factor alpha-induced loss. Otol. Neurotol. 2012, 33, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of trpv1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- Mohler, K.M.; Torrance, D.S.; Smith, C.A.; Goodwin, R.G.; Stremler, K.E.; Fung, V.P.; Madani, H.; Widmer, M.B. Soluble tumor necrosis factor (tnf) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both tnf carriers and tnf antagonists. J. Immunol. 1993, 151, 1548–1561. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Chen, F.Q.; Zheng, H.W.; Sha, S.H. Intra-tympanic delivery of short interfering rna into the adult mouse cochlea. Hear. Res. 2013, 296, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: Microrna biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Hutvagner, G.; Du, T.; Xu, Z.; Aronin, N.; Zamore, P.D. Asymmetry in the assembly of the rnai enzyme complex. Cell 2003, 115, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Chandradoss, S.D.; Schirle, N.T.; Szczepaniak, M.; MacRae, I.J.; Joo, C. A dynamic search process underlies microrna targeting. Cell 2015, 162, 96–107. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.; Yeom, K.H.; Nam, J.W.; Heo, I.; Rhee, J.K.; Sohn, S.Y.; Cho, Y.; Zhang, B.T.; Kim, V.N. Molecular basis for the recognition of primary micrornas by the drosha-dgcr8 complex. Cell 2006, 125, 887–901. [Google Scholar] [CrossRef]
- Sullenger, B.A.; Nair, S. From the rna world to the clinic. Science 2016, 352, 1417–1420. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.S. Therapeutic mirna and sirna: Moving from bench to clinic as next generation medicine. Mol. Ther. Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef]
- Aiuti, A.; Biasco, L.; Scaramuzza, S.; Ferrua, F.; Cicalese, M.P.; Baricordi, C.; Dionisio, F.; Calabria, A.; Giannelli, S.; Castiello, M.C.; et al. Lentiviral hematopoietic stem cell gene therapy in patients with wiskott-aldrich syndrome. Science 2013, 341, 1233151. [Google Scholar] [CrossRef]
- Musacchio, T.; Torchilin, V.P. Sirna delivery: From basics to therapeutic applications. Front. Biosci. 2013, 18, 58–79. [Google Scholar]
- Boudreau, R.L.; Monteys, A.M.; Davidson, B.L. Minimizing variables among hairpin-based rnai vectors reveals the potency of shrnas. RNA 2008, 14, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, R.L.; Martins, I.; Davidson, B.L. Artificial micrornas as sirna shuttles: Improved safety as compared to shrnas in vitro and in vivo. Mol. Ther. J. Am. Soc. Gene Ther. 2009, 17, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Glover, S.J.; Burgess, P.I.; Cohen, D.B.; Harding, S.P.; Hofland, H.W.; Zijlstra, E.E.; Allain, T.J. Prevalence of diabetic retinopathy, cataract and visual impairment in patients with diabetes in sub-saharan africa. Br. J. Ophthalmol. 2012, 96, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Min, H.; Kim, S.J.; Oh, S.; Kim, K.; Yu, H.G.; Park, T.; Kim, Y. Development of diagnostic biomarkers for detecting diabetic retinopathy at early stages using quantitative proteomics. J. Diabetes Res. 2016, 2016, 6571976. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, T.; Mackey, G.; Lusk, J.; Kyle, U.G.; Fontenot, T.; Marshall, P.; Shekerdemian, L.S.; Coss-Bu, J.A.; Nishigaki, A.; Yatabe, T.; et al. Esicm lives 2016: Part three. Intensive Care Med. Exp. 2016, 4, 28. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, N.; Chen, L.; Cai, N. Survivin contributes to the progression of diabetic retinopathy through hif-1alpha pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 9161–9167. [Google Scholar]
- Dorrell, M.; Uusitalo-Jarvinen, H.; Aguilar, E.; Friedlander, M. Ocular neovascularization: Basic mechanisms and therapeutic advances. Surv. Ophthalmol. 2007, 52 (Suppl. S1), S3–S19. [Google Scholar] [CrossRef]
- Wan, T.T.; Li, X.F.; Sun, Y.M.; Li, Y.B.; Su, Y. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed. Pharmacother. 2015, 74, 145–147. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chuang, L.M. The role of oxidative stress in the pathogenesis of type 2 diabetes: From molecular mechanism to clinical implication. Am. J. Transl. Res. 2010, 2, 316–331. [Google Scholar]
- Naudi, A.; Jove, M.; Ayala, V.; Cassanye, A.; Serrano, J.; Gonzalo, H.; Boada, J.; Prat, J.; Portero-Otin, M.; Pamplona, R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp. Diabetes Res. 2012, 2012, 696215. [Google Scholar] [CrossRef] [PubMed]
- Malendowicz, L.K.; Macchi, C.; Nussdorfer, G.G.; Nowak, K.W.; Zyterska, A.; Ziolkowska, A. Effects of prolonged exendin-4 administration on entero-insular axis of normal and streptozotocin-induced diabetic rats. Int. J. Mol. Med. 2003, 11, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Kleine, N.; Orskov, C.; Holst, J.J.; Willms, B.; Creutzfeldt, W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993, 36, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm. Metab. Res. 2008, 40, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Oeseburg, H.; de Boer, R.A.; Buikema, H.; van der Harst, P.; van Gilst, W.H.; Sillje, H.H. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase a. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Wu, H.; Deng, M.; Peng, J.; Li, F.; Yang, Y. Brevilin a induces apoptosis and autophagy of colon adenocarcinoma cell ct26 via mitochondrial pathway and pi3k/akt/mtor inactivation. Biomed. Pharmacother. 2018, 98, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, Y.; Nan, J.; Zhang, X.; Qin, X.; Wang, Y.; Hou, J.; Wang, Q.; Yang, J. Brevilin a, a novel natural product, inhibits janus kinase activity and blocks stat3 signaling in cancer cells. PLoS ONE 2013, 8, e63697. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Cui, X.; Lv, D.; Jin, L.; Khan, M.; Ma, T. Brevilin a promotes oxidative stress and induces mitochondrial apoptosis in u87 glioblastoma cells. OncoTargets Ther. 2018, 11, 7031–7040. [Google Scholar] [CrossRef]
- Choi, J.H.; Oh, J.; Lee, M.J.; Bae, H.; Ko, S.G.; Nah, S.Y.; Cho, I.H. Inhibition of lysophosphatidic acid receptor 1–3 deteriorates experimental autoimmune encephalomyelitis by inducing oxidative stress. J. Neuroinflamm. 2021, 18, 240. [Google Scholar] [CrossRef]
- Zarzuelo Romero, M.J.; Perez Ramirez, C.; Carrasco Campos, M.I.; Sanchez Martin, A.; Calleja Hernandez, M.A.; Ramirez Tortosa, M.C.; Jimenez Morales, A. Therapeutic value of single nucleotide polymorphisms on the efficacy of new therapies in patients with multiple sclerosis. J. Pers. Med. 2021, 11, 335. [Google Scholar] [CrossRef]
- Adamczyk, B.; Adamczyk-Sowa, M. New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxidative Med. Cell. Longev. 2016, 2016, 1973834. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Han, J.; Song, S.Y.; Kim, W.S.; Shin, S.; Kim, J.H.; Ahn, H.; Jeong, J.H.; Hwang, S.J.; Sung, J.H. Lysophosphatidic acid increases the proliferation and migration of adipose-derived stem cells via the generation of reactive oxygen species. Mol. Med. Rep. 2015, 12, 5203–5210. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, C.E.; Lin, Y.C.; Ju, T.K.; Huang, Y.L.; Lee, M.S.; Chen, J.H.; Lee, H. Lysophosphatidic acid induces reactive oxygen species generation by activating protein kinase c in pc-3 human prostate cancer cells. Biochem. Biophys. Res. Commun. 2013, 440, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.A.; Rogers, L.C.; Klomsiri, C.; Poole, L.B.; Daniel, L.W. Reactive oxygen species mediate lysophosphatidic acid induced signaling in ovarian cancer cells. Free Radic. Biol. Med. 2010, 49, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J. Lysophosphatidic acid signaling in the nervous system. Neuron 2015, 85, 669–682. [Google Scholar] [CrossRef]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. Lpa receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef]
- Geraldo, L.H.M.; Spohr, T.; Amaral, R.F.D.; Fonseca, A.; Garcia, C.; Mendes, F.A.; Freitas, C.; dos Santos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef]
- Schmitz, K.; Brunkhorst, R.; de Bruin, N.; Mayer, C.A.; Haussler, A.; Ferreiros, N.; Schiffmann, S.; Parnham, M.J.; Tunaru, S.; Chun, J.; et al. Dysregulation of lysophosphatidic acids in multiple sclerosis and autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2017, 5, 42. [Google Scholar] [CrossRef]
- Dittel, B.N. Cd4 t cells: Balancing the coming and going of autoimmune-mediated inflammation in the cns. Brain Behav. Immun. 2008, 22, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moises, F.P.; Macias-Islas, M.A.; Flores-Alvarado, L.J.; Mireles-Ramirez, M.A.; Gonzalez-Renovato, E.D.; Hernandez-Navarro, V.E.; Sanchez-Lopez, A.L.; Alatorre-Jimenez, M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herb, M. NADPH Oxidase 3: Beyond the Inner Ear. Antioxidants 2024, 13, 219. https://doi.org/10.3390/antiox13020219
Herb M. NADPH Oxidase 3: Beyond the Inner Ear. Antioxidants. 2024; 13(2):219. https://doi.org/10.3390/antiox13020219
Chicago/Turabian StyleHerb, Marc. 2024. "NADPH Oxidase 3: Beyond the Inner Ear" Antioxidants 13, no. 2: 219. https://doi.org/10.3390/antiox13020219