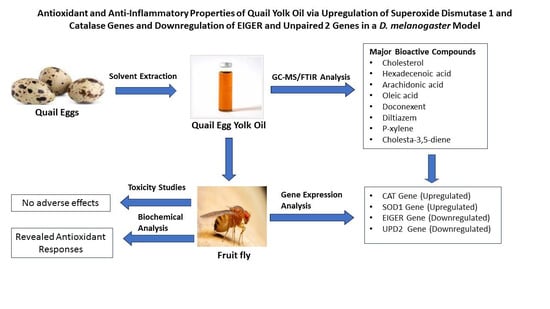
Antioxidant and Anti-Inflammatory Properties of Quail Yolk Oil via Upregulation of Superoxide Dismutase 1 and Catalase Genes and Downregulation of EIGER and Unpaired 2 Genes in a D. melanogaster Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Extraction of Oil from Liquid Quail Yolk
2.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.3. Gas Chromatography/Mass Spectrometry (GC–MS) of QEYO Bioactives
2.4. Fly Culture
2.5. Experimental Design
2.6. Toxicity Studies
2.7. The Negative Geotaxis Assay
2.8. The Biochemical Assay
2.9. The Superoxide Dismutase (SOD) Assay
2.10. The Total Antioxidant Capacity (TAOC) Assay
2.11. The Malondialdehyde (MDA) Assay
2.12. The Catalase (CAT) Assay
2.13. Gene Expression Study
2.13.1. RNA Extraction
2.13.2. Primer Design
2.13.3. Quantitative Real-Time PCR Analysis
2.14. Statistical Analysis
3. Results
3.1. Quail Egg Oil (QEYO) Yield
3.2. FTIR Analysis
3.2.1. Ethanol/Chloroform QEYO Extract
3.2.2. 2-Propanol/Hexane Quail Egg Yolk Oil Extract
3.3. GC–MS Analysis
3.3.1. Ethanol/Chloroform Quail Egg Yolk Oil Extract
3.3.2. 2-Propanol/Hexane Quail Egg Yolk Oil Extract
3.4. Toxicity Study
3.4.1. Acute Toxicity
3.4.2. Sub-Chronic Toxicity
3.4.3. Chronic Toxicity
3.4.4. The Negative Geotaxis Assay
3.5. Biochemical Assays
3.5.1. Superoxide Dismutase (SOD) Activity
3.5.2. Catalase Activity
3.5.3. Total Antioxidant Capacity
3.5.4. Malondialdehyde Levels
3.6. Gene Expression Study
4. Discussion
| Peak | RT (min) | Area | Compound Name | Molecular Formula | Pharmacological Use of the Most Important Compounds |
|---|---|---|---|---|---|
| 20 | 35.082 | 10.58 | Hexadecanoic acid, methyl ester | C17H34O2 | Antibacterial [36], antifungal [37], anti-inflammation, antifibrosis, and peripheral vasodilation effects [38] |
| 23 | 35.717 | 0.68 | n-Hexadecanoic acid | C16H32O2 | Anti-inflammatory [39], antibacterial, and antioxidant activities [40] |
| 24 | 36.335 | 0.32 | 1-Tetracosene | C24H48 | Cytotoxic effect [41] |
| 25 | 37.926 | 0.09 | Gamolenic acid | C18H30O2 | Anti-inflammatory, antithrombotic, antiproliferative, and lipid-lowering effects [42] |
| 26 | 38.223 | 5.67 | Methyl 10-trans,12-cis-octadecadienoate | C19H34O2 | Antifungal and antioxidant [43] |
| 27 | 38.349 | 15.01 | cis-13-Octadecenoic acid, methyl ester | C19H36O2 | Anti-inflammatory [44] |
| 29 | 38.796 | 7.42 | Methyl stearate | C19H38O2 | Antibacterial, antioxidant, antifungal [45], antidiarrheal, cytotoxic, and anti-inflammatory [46] |
| 30 | 38.979 | 0.93 | Oleic Acid | C18H34O2 | Decreases myocardial infarction rate, platelet aggregation and secretion of TXA2, reduces systolic blood pressure, and improves immunity [47] |
| 37 | 41.090 | 2.51 | Arachidonic acid | C20H32O2 | An integral constituent of the biological cell membrane that is necessary for the function of all cells, especially in the nervous system, skeletal muscle, and immune system. It modulates the function of ion channels, several receptors, and enzymes via activation as well as inhibition [48] |
| 45 | 44.226 | 0.82 | Doconexent | C22H32O2 | DHA acts as a ligand at PPARs that has an anti-inflammatory effect and regulates inflammatory gene expression and NFκB activation [35] |
| 46 | 44.306 | 0.46 | Diltiazem | C22H26N2O4S | A calcium channel blocker that is clinically used as an antihypertensive, anti-arrhythmic, and anti-anginal agent for the management of cardiovascular conditions, such as hypertension, chronic stable angina, atrial fibrillation, and atrial flutter [49] |
| 55 | 47.751 | 1.22 | 9-Octadecenoic acid (Z)-, 2-hydroxyethyl ester | C20H38O3 | Antimicrobial and antifungal [50] |
| 60 | 53.639 | 41.64 | Cholesterol | C27H46O | Helps build new tissue and repairs damage to existing tissue, produces steroid hormones, including estrogen, helps create bile in the liver, and aids in the production of vitamin D [51] |
| 61 | 54.125 | 1.04 | Desmosterol | C27H44O | Suppresses macrophage inflammasome activation and protects against vascular inflammation and atherosclerosis [33] |
| Peak | RT | Area | Compound | Molecular Formula | Pharmacological Use |
|---|---|---|---|---|---|
| 6 | 7.216 | 3.24 | p-xylene | C8H10 | Antipsoriatic, antimicrobial, antioxidant, and antifungal activity [52,53] |
| 8 | 7.994 | 2.80 | Benzene, 1,3-dimethyl | C8H10 | Anti-tumor [54] |
| 38 | 14.975 | 0.77 | Undecane | C11H24 | Antiallergic and anti-inflammatory [55] |
| 87 | 43.791 | 0.47 | Oleic acid, 3-hydroxypropyl ester | C21H34O3 | Protection against cardiovascular diseases [56] |
| 88 | 44.186 | 0.12 | Octadecanoic acid, 2,3-dihydroxypropyl ester | C21H42O4 | Antimicrobial and anticancer effects [57] |
| 91 | 49.679 | 0.30 | Squalene | C30H50 | In animals, supplementation of the diet with squalene can reduce cholesterol and triglyceride levels. In humans, it potentiates the effects of some cholesterol-lowering drugs. It functions in the skin as a quencher of singlet oxygen, protecting the skin from lipid peroxidation due to exposure to UV and other radiations. Its primary therapeutic use is as an adjunctive therapy in a variety of cancers [58] |
| 94 | 50.366 | 2.94 | Cholesta-4,6-dien-3-ol, (3.beta.) | C27H44O | Anticancer effect [59] |
| 95 | 50.646 | 7.03 | Cholesta-3,5-diene | C27H44 | Promotes wound healing [60] and exhibits a cytotoxic effect [52] |
| 96 | 52.557 | 0.27 | Silicic acid, diethyl bis (trimethylsilyl) ester | C10H28O4Si3 | Anti-inflammatory, antibacterial, and antioxidant effects [61] |
| 97 | 53.559 | 50.32 | Cholesterol | C27H46O | Helps build new tissue and repairs damage to existing tissue, produces steroid hormones, including estrogen, helps create bile in the liver, and aids in the production of vitamin D [51] |
| 98 | 54.091 | 0.90 | 4-Dehydroxy-N-(4,5-methylenedioxy-2-nitrobenzylidene) tyramine | C16H14N2O4 | Antibacterial, antidepressant, antidiarrheal, and anti-inflammatory effects [62] |
| 99 | 55.453 | 1.86 | Cholest-4-en-3-one | C27H44O | A novel drug candidate for amyotrophic lateral sclerosis [63]. An oxime with neuroprotective and antinociceptive activity [64]. It also has an anti-obesity effect [65] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tunsaringkarn, T.; Tungjaroenchai, W.; Siriwong, W. Nutrient benefits of quail (Coturnix coturnix japonica) eggs. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Truffier, J. Approche therapeutique de la maladie allergique par ingestion d’oeufs de caille. La Clin. 1978, 22, 2–4. [Google Scholar]
- Jeke, A.; Phiri, C.; Chitindingu, K.; Taru, P. Ethnomedicinal use and pharmacological potential of Japanese quail (Coturnix coturnix japonica) birdsmeat and eggs, and its potential implications on wild quail conservation in Zimbabwe: A review. Cogent Food Agric. 2018, 4, 1507305. [Google Scholar] [CrossRef]
- Chang, G.B.; Chang, H.; Liu, X.P.; Xu, W.; Wang, H.Y.; Zhao, W.M.; Olowofeso, O. Developmental research on the origin and phylogeny of quails. World’s Poult. Sci. J. 2005, 61, 105–112. [Google Scholar] [CrossRef]
- Genchev, A. Quality, and composition of Japanese quail eggs (Coturnix japonica). Trakia J. Sci. 2012, 10, 91–101. [Google Scholar]
- Perennou, C. European Union-Management Plan 2009–2011. Common Quail, Coturnix Coturnix; Office for Official Publications of the European Communities: Luxembourg, 2009. [Google Scholar]
- Chepkemoi, M.; Sila, D.; Oyier, P.; Malaki, P.; Ndiema, E.; Agwanda, B.; Obanda, V.; Ngeiywa, K.J.; Lichoti, J.; Ommeh, S. Nutritional diversity of meat and eggs of five poultry species in Kenya. In Proceedings of the Scientific Conference Proceedings, Bursa, Turkey, 4 May 2016. [Google Scholar]
- Song, K.T.; Choi, S.H.; Oh, H.R. A comparison of egg quality of pheasant, chukar, quail and guinea fowl. Asian-Australas. J. Anim. Sci. 2000, 13, 986–990. [Google Scholar] [CrossRef]
- Pisseri, F.; Bertoli, A.; Pistelli, L. Essential oils in medicine: Principles of therapy. Parassitologia 2008, 50, 89–91. [Google Scholar]
- Shen, Q.; Riedl, K.M.; Cole, R.M.; Lehman, C.; Xu, L.; Alder, H.; Belury, M.A.; Schwartz, S.J.; Ziouzenkova, O. Egg yolks inhibit activation of NF-κB and expression of its target genes in adipocytes after partial delipidation. J. Agric. Food Chem. 2015, 63, 2013–2025. [Google Scholar] [CrossRef]
- Rastegar, F.; Azarpira, N.E.; Amiri, M.; Azarpira, A. The effect of egg yolk oil in the healing of third degree burn wound in rats. Iran. Red Crescent Med. J. 2011, 13, 739. [Google Scholar]
- Mahmoudi, M.; Ebrahimzadeh, M.A.; Pourmorad, F.; Rezaie, N.; Mahmoudi, M.A. Anti-inflammatory and analgesic effects of egg yolk: A comparison between organic and machine made. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 472–476. [Google Scholar]
- Ganley, O.H.; Graessle, O.E.; Robinson, H.J. Anti-inflammatory activity of compounds obtained from egg yolk, peanut oil, and soybean lecithin. J. Lab. Clin. Med. 1958, 51, 709–714. [Google Scholar] [PubMed]
- Xiao, N.; Zhao, Y.; He, W.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Tu, Y. Egg yolk oils exert anti-inflammatory effect via regulating Nrf2/NF-κB pathway. J. Ethnopharmacol. 2021, 274, 114070. [Google Scholar] [CrossRef] [PubMed]
- nee Kricsfalussy, M.N.; nee Szabo, A.Z.; Rakoczi, J.; Halmos, J. Quail Egg Based Stabilized Foam Compositions for Cosmetic Purposes. U.S. Patent 4,661,340, 28 April 1987. [Google Scholar]
- Sani, I.M.; Balogun, S.O.; Khalid, M.; Agaie, B.M.; Sani, A.A.; Salisu, B. Physicochemical characteristics and antioxidant activities of Japanese quail (Coturnix coturnix japonica) egg yolk oil (Qeyo) extracted using two different methods. Pol. J. Nat. Sci. 2019, 34, 515–529. [Google Scholar]
- Zhao, Y.; Zhang, Y.; Liu, X.; Kong, H.; Wang, Y.; Qin, G.; Cao, P.; Song, X.; Yan, X.; Wang, Q.; et al. Novel carbon quantum dots from egg yolk oil and their hemostatic effects. Sci. Rep. 2017, 7, 4452. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Pan, Y.; Yan, J.; Huang, D.; Li, S. Assessment of egg yolk oil extraction methods of for ShiZhenKang oil by pharmacodynamic index evaluation. Molecules 2016, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Kovalcuks, A.; Duma, M. Solvent extraction of egg oil from liquid egg yolk. In Proceedings of the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being, Jelgava, Latvia, 8–9 May 2014; p. 253. [Google Scholar]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential oil quality and purity evaluation via ft-ir spectroscopy and pattern recognition techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Ng, F.; Basri, N.; Wu, W.; Thong, A.; Thong, G.; Chew, W.; Dharmawan, J. Characterization of volatile compounds in Ylang-Ylang essential oils from Comoros and Madagascar by gas chromatography and principal component analysis. Flavour Fragr. J. 2021, 36, 159–166. [Google Scholar] [CrossRef]
- Semaniuk, U.V.; Gospodaryov, D.V.; Feden’Ko, K.M.; Yurkevych, I.S.; Vaiserman, A.M.; Storey, K.B.; Simpson, S.J.; Lushchak, O. Insulin-like peptides regulate feeding preference and metabolism in Drosophila. Front. Physiol. 2018, 9, 1083. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M. Stabilisation of edible oils with natural antioxidants. Eur. J. Lipid Sci. Technol. 2001, 103, 752–767. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Ori-Jesu, M. The use of antioxidants in vegetable oils—A review. Afr. J. Biotechnol. 2008, 7, 4836–4842. [Google Scholar]
- Lindley, M. The impact of food processing on antioxidants in vegetable oils, fruits and vegetables. Trends Food Sci. Technol. 1998, 9, 336–340. [Google Scholar] [CrossRef]
- Horrobin, D.F. Medical uses of essential fatty acids (EFAs). Vet. Dermatol. 1993, 4, 161–166. [Google Scholar] [CrossRef]
- Hansen, A.E. Serum lipid changes and therapeutic effects of various oils in infantile eczema. Proc. Soc. Exp. Biol. Med. 1933, 31, 160–161. [Google Scholar] [CrossRef]
- Hasen, A.E.I. Role of Unsaturated Dietary Fat in Infant Nutrition. Am. J. Public Health Nations Health 1957, 47 Pt 1, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Ham, Y.M.; Heo, S.J.; Yoon, S.A.; Cho, S.H.; Kwon, S.H.; Jeong, M.S.; Jeon, Y.J.; Sanjeewa, K.K.; Yoon, W.J.; et al. Anti-inflammation effects of 8-oxo-9-octadecenoic acid isolated from Undaria peterseniana in lipopolysaccharide-stimulated macrophage cells. EXCLI J. 2018, 17, 775. [Google Scholar] [PubMed]
- Kawashima, H. Intake of arachidonic acid-containing lipids in adult humans: Dietary surveys and clinical trials. Lipids Health Dis. 2019, 18, 101. [Google Scholar] [CrossRef]
- Higgins, A.J.; Lees, P. The acute inflammatory process, arachidonic acid metabolism and the mode of action of anti-inflammatory drugs. Equine Vet. J. 1984, 16, 163–175. [Google Scholar] [CrossRef]
- Zhang, X.; McDonald, J.G.; Aryal, B.; Canfrán-Duque, A.; Goldberg, E.L.; Araldi, E.; Ding, W.; Fan, Y.; Thompson, B.M.; Singh, A.K.; et al. Desmosterol suppresses macrophage inflammasome activation and protects against vascular inflammation and atherosclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2107682118. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.; Kim, D.P. From p-Xylene to Ibuprofen in Flow: Three-Step Synthesis by a Unified Sequence of Chemoselective C–H Metalations. Chem. Eur. J. 2019, 25, 11641–11645. [Google Scholar] [CrossRef]
- Cederholm, T.; Salem, N., Jr.; Palmblad, J. omega-3 fatty acids in the prevention of cognitive decline in humans. Adv. Nutr. 2013, 4, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Abubacker, M.N.; Deepalakshmi, T. In vitro antifungal potential of bioactive compound methyl ester of hexadecanoic acid isolated from Annona muricata linn (annonaceae) leaves. Biosci. Biotechnol. Res. Asia 2013, 10, 879–884. [Google Scholar] [CrossRef]
- Wang, N.; Kuczmanski, A.; Dubrovska, G.; Gollasch, M. Palmitic acid methyl ester and its relation to control of tone of human visceral arteries and rat aortas by perivascular adipose tissue. Front. Physiol. 2018, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, T.; Subban, M.; Christopher Leslee, D.B.; Kuppannan, S.B.; Seedevi, P. Structural characterization of n-hexadecanoic acid from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activities. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Paudel, M.R.; Chand, M.B.; Pant, B.; Pant, B. Assessment of antioxidant and cytotoxic activities of extracts of Dendrobium crepidatum. Biomolecules 2019, 12, 478. [Google Scholar] [CrossRef]
- Dobryniewski, J.; Szajda, S.D.; Waszkiewicz, N.; Zwierz, K. Kwas gamma-linolenowy (GLA)—znaczenie terapeutyczne [The gamma-linolenic acid (GLA)—the therapeutic value]. Przegl. Lek. 2007, 64, 100–102. (In Polish) [Google Scholar]
- Adekoyeni, O.O.; Ajayi, F.; Adegoke, A. GC-MS analysis and identification of pharmacological Components of doum palm nuts. Niger. J. Sci. Res. 2019, 18, 571–578. [Google Scholar]
- Diab, T.A.; Donia, T.; Saad-Allah, K.M. Characterization, antioxidant, and cytotoxic effects of some Egyptian wild plant extracts. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 13. [Google Scholar] [CrossRef]
- Pinto, M.E.; Araujo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Ciências 2017, 89, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Basa’ar, O.; Fatema, S.; Alrabie, A.; Mohsin, M.; Farooqui, M. Supercritical carbon dioxide extraction of Triognella foenum graecum Linn seeds: Determination of bioactive compounds and pharmacological analysis. Asian Pac. J. Trop. Biomed. 2017, 7, 1085–1091. [Google Scholar] [CrossRef]
- Karacor, K.; Cam, M. Effects of oleic acid. Med. Sci. Discov. 2015, 2, 125–132. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits–a review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Talreja, O.; Cassagnol, M. Diltiazem; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar] [PubMed]
- Rajasekar, G.; Ebenezar, E.; Thiruvudainambi, S.; Vanniarajan, C.; Vellaikumar, M.S.S. Exploitation and identification of antifungal compounds of botanicals through gas chromatography-mass spectrometry (GC-MS) against Bipolaris oryzae in rice. J. Entomol. Zool. Stud. 2020, 8, 1509–1515. [Google Scholar]
- Zampelas, A.; Magriplis, E. New insights into cholesterol functions: A friend or an enemy? Nutrients 2019, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Naqvi, S.F.; Khan, I.H. Ethyl acetate extract of Chenopodium murale root, a source of bioactive compounds. Pak. J. Weed Sci. Res. 2021, 27, 93. [Google Scholar] [CrossRef]
- Tiwari, S.; Mishra, S.; Misra, D.R.; Upadhyay, R. Identification of new bioactive compounds from fruit of Abutilon indicum through GCMS analysis. Biol. Forum–Int. J. 2016, 8, 548–554. [Google Scholar]
- Caruso, F.; Monti, E.; Matthews, J.; Rossi, M.; Gariboldi, M.B.; Pettinari, C.; Pettinari, R.; Marchetti, F. Synthesis, characterization, and antitumor activity of water-soluble (arene) ruthenium (II) derivatives of 1, 3-Dimethyl-4-acylpyrazolon-5-ato ligands. First example of Ru (arene)(ligand) antitumor species involving simultaneous Ru–N7 (guanine) bonding and ligand intercalation to DNA. Inorg. Chem. 2014, 53, 3668–3677. [Google Scholar]
- Choi, D.; Kang, W.; Park, T. Anti-allergic and anti-inflammatory effects of undecane on mast cells and keratinocytes. Molecules 2020, 25, 1554. [Google Scholar] [CrossRef]
- Damayanti, A.; Hakim, A.R.; Saputri, R. GC-MS Analysis of Metabolite Composition in Edible Bird’s Nest From Jenamas Central Kalimantan. Int. J. Pharm. Appl. Health Sci. 2023, 1, 6–9. [Google Scholar]
- Arora, S.; Kumar, G. Phytochemical screening of root, stem and leaves of Cenchrus biflorus Roxb. J. Pharmacogn. Phytochem. 2018, 7, 1445–1450. [Google Scholar]
- Kelly, G.S. Squalene and its potential clinical uses. Altern. Med. Rev. J. Clin. Ther. 1999, 4, 29–36. [Google Scholar]
- Shen, Y.; Sun, Z.; Shi, P.; Wang, G.; Wu, Y.; Li, S.; Zheng, Y.; Huang, L.; Lin, L.; Lin, X.; et al. Anticancer effect of petroleum ether extract from Bidens pilosa L and its constituent’s analysis by GC-MS. J. Ethnopharmacol. 2018, 217, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassan, J.M.; Hinek, A.; Renno, W.M.; Wang, Y.; Liu, Y.F.; Guan, R.; Wen, X.Y.; Litvack, M.L.; Lindenmaier, A.; Afzal, M.; et al. Potential mechanism of dermal wound treatment with preparations from the skin gel of Arabian Gulf catfish: A unique furan fatty acid (F6) and cholesta-3, 5-diene (S5) Recruit neutrophils and fibroblasts to promote wound healing. Front. Pharmacol. 2020, 11, 899. [Google Scholar] [CrossRef]
- Kumar, S.V.; Rajeshkumar, S. Anti-inflammatory, Antioxidant, Antibacterial effect and Phytochemical Analysis of Mucuna pruriens seed extract. Int. J. ChemTech Res. 2017, 10, 273–283. [Google Scholar]
- Chatterjee, A.; Acherjee, M.; Das, B.K.; Chakraborty, S.; Pal, H. Multi-target Inhibitory Potency of Active Metabolites Dictates the Antimicrobial Activity of Indigenous Medicinal Plant Leucas biflora: GC-MS Analysis, Biological Evaluations, and Molecular Docking Studies. J. Herbs Spices Med. Plants 2023, 29, 134–144. [Google Scholar] [CrossRef]
- Bordet, T.; Buisson, B.; Michaud, M.; Drouot, C.; Galéa, P.; Delaage, P.; Akentieva, N.P.; Evers, A.S.; Covey, D.F.; Ostuni, M.A.; et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 2007, 322, 709–720. [Google Scholar] [CrossRef]
- Xiao, W.H.; Zheng, F.Y.; Bennett, G.J.; Bordet, T.; Pruss, R.M. Olesoxime (cholest-4-en-3-one, oxime): Analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. PAIN® 2009, 147, 202–209. [Google Scholar] [CrossRef]
- Nagao, K.; Inoue, N.; Suzuki, K.; Shimizu, T.; Yanagita, T. The cholesterol metabolite cholest-5-en-3-one alleviates hyperglycemia and hyperinsulinemia in obese (db/db) mice. Metabolites 2021, 12, 26. [Google Scholar] [CrossRef]
- Chaffman, M.; Brogden, R.N. Diltiazem: A review of its pharmacological properties and therapeutic efficacy. Drugs 1985, 29, 387–454. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and other health properties of olive pomace oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Yusufzai, S.K.; Kaun, L.P.; Shah, M.D.; Idris, R. Chemical composition and antioxidant activity of essential oil of leaves and flowers of Alternanthera sessilis red from Sabah. J. Appl. Pharm. Sci. 2016, 6, 157–161. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, H.Y.; Pyo, J.S.; Yokozawa, T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol. Pharm. Bull. 2006, 29, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, K.; Senthil-Nathan, S.; Stanley-Raja, V.; Vasantha-Srinivasan, P. Physiological and biochemical alterations in Vigna rdiate L. triggered by sesame derived elicitors as defense mechanism against Rhizoctonia and Macrophomina infestation. Sci. Rep. 2023, 13, 13884. [Google Scholar] [CrossRef]
- Nasrin, S.; Islam, M.N.; Tayab, M.A.; Nasrin, M.S.; Siddique, M.A.; Emran, T.B.; Reza, A.A. Chemical profiles and pharmacological insights of Anisomeles indica Kuntze: An experimental chemico-biological interaction. Biomed. Pharmacother. 2022, 149, 112842. [Google Scholar] [CrossRef]
- Magriplis, E.; Mitsopoulou, A.; Karageorgou, D.; Bakogianni, I.; Dimakopoulos, I.; Micha, R.; Michas, G.; Chourdakis, M.; Chrousos, G.; Roma, E.; et al. Frequency and Quantity of Egg Intake Is Not Associated with Dyslipidemia: The Hellenic National Nutrition and Health Survey (HNNHS). Nutrients 2019, 11, 1105. [Google Scholar] [CrossRef]
- Yin, W.; Li, Z.; Zhang, W. Modulation of bone and marrow niche by cholesterol. Nutrients 2019, 11, 1394. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Kratky, D.; Rendina-Ruedy, E. Influence of Cholesterol on the Regulation of Osteoblast Function. Metabolites 2023, 13, 578. [Google Scholar] [CrossRef]
- Suzuki, A.; Minamide, M.; Iwaya, C.; Ogata, K.; Iwata, J. Role of metabolism in bone development and homeostasis. Int. J. Mol. Sci. 2020, 21, 8992. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, A.F. Two forms of sexual dimorphism in gene expression in Drosophila melanogaster: Their coincidence and evolutionary genetics. Mol. Biol. Evol. 2023, 40, msad091. [Google Scholar] [CrossRef] [PubMed]
- Nanni, A.V.; Martinez, N.; Graze, R.; Morse, A.; Newman, J.R.; Jain, V.; Vlaho, S.; Signor, S.; Nuzhdin, S.V.; Renne, R.; et al. Sex-Biased Expression Is Associated With Chromatin State in Drosophila melanogaster and Drosophila simulans. Mol. Biol. Evol. 2023, 40, msad078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hambuch, T.M.; Parsch, J. Molecular evolution of sex-biased genes in Drosophila. Mol. Biol. Evol. 2004, 21, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Igaki, T.; Kanda, H.; Yamamoto-Goto, Y.; Kanuka, H.; Kuranaga, E.; Aigaki, T.; Miura, M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 2002, 21, 3009–3018. [Google Scholar] [CrossRef]
- Jo, J.; Im, S.H.; Babcock, D.T.; Iyer, S.C.; Gunawan, F.; Cox, D.N.; Galko, M.J. Drosophila caspase activity is required independently of apoptosis to produce active TNF/Eiger during nociceptive sensitization. Cell Death Dis. 2017, 8, e2786. [Google Scholar] [CrossRef]
- Evans, C.J.; Liu, T.; Girard, J.R.; Banerjee, U. Injury-induced inflammatory signaling and hematopoiesis in Drosophila. Proc. Natl. Acad. Sci. USA 2022, 119, e2119109119. [Google Scholar] [CrossRef]
- Azam, S.; Jakaria, M.; Kim, I.S.; Kim, J.; Haque, M.E.; Choi, D.K. Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: Focus on TLR4 signaling. Front. Immunol. 2019, 10, 1000. [Google Scholar] [CrossRef]










| Gene | Annotation Symbol | Fly Base ID | Accession | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|---|---|---|
| SOD1 | CG11793 | FBgn0003462 | NM_057387.5 | CGGTCACACCATAGAAGATACC | CAGACAGCTTTAACCACCATTTC |
| CAT | CG6871 | FBgn0000261 | NM_080483.3 | TGGTCGTCTGTTCTCCTACT | CCGCTGGAAGTTCTCAATCT |
| UPD2 | CG5988 | FBgn0030904 | NM_001370039.1 | TGAGGCAACTTCCAAAGAGAG | CGGATCTGGCTGAAAGAAGAG |
| EIGER | CG12919 | FBgn0033483 | NM_165735.4 | TTGACCATAAACGCCTCCTATC | GTGAAAGTTGAGACGCTCCT |
| RPL32 | CG7939 | FBgn0002626 | NM_170460.2 | GTCGTCGCTTTGTCATCT | GCAGGTTGTAGCCCTTCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismaila, M.S.; Sanusi, K.O.; Iliyasu, U.; Imam, M.U.; Georges, K.; Sundaram, V.; Jones, K.R. Antioxidant and Anti-Inflammatory Properties of Quail Yolk Oil via Upregulation of Superoxide Dismutase 1 and Catalase Genes and Downregulation of EIGER and Unpaired 2 Genes in a D. melanogaster Model. Antioxidants 2024, 13, 75. https://doi.org/10.3390/antiox13010075
Ismaila MS, Sanusi KO, Iliyasu U, Imam MU, Georges K, Sundaram V, Jones KR. Antioxidant and Anti-Inflammatory Properties of Quail Yolk Oil via Upregulation of Superoxide Dismutase 1 and Catalase Genes and Downregulation of EIGER and Unpaired 2 Genes in a D. melanogaster Model. Antioxidants. 2024; 13(1):75. https://doi.org/10.3390/antiox13010075
Chicago/Turabian StyleIsmaila, Muhammad Sani, Kamaldeen Olalekan Sanusi, Uwaisu Iliyasu, Mustapha Umar Imam, Karla Georges, Venkatesan Sundaram, and Kegan Romelle Jones. 2024. "Antioxidant and Anti-Inflammatory Properties of Quail Yolk Oil via Upregulation of Superoxide Dismutase 1 and Catalase Genes and Downregulation of EIGER and Unpaired 2 Genes in a D. melanogaster Model" Antioxidants 13, no. 1: 75. https://doi.org/10.3390/antiox13010075