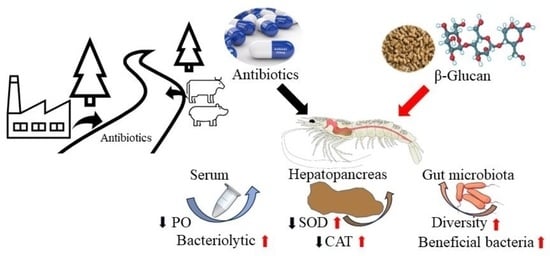
Dietary β-Glucan Alleviates Antibiotic-Associated Side Effects by Increasing the Levels of Antioxidant Enzyme Activities and Modifying Intestinal Microbiota in Pacific White Shrimp (Litopenaeus vannamei)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dietary Design
2.2. Experimental Shrimp and Design
2.3. Sample Collection and Calculation
2.4. Immune and Antioxidant Enzyme Activities
2.5. Immune and Antioxidant Enzyme Activities
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Antioxidant and Immune Capacity
3.3. Composition and Diversity of the Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broom, L.J. The Sub-Inhibitory Theory for Antibiotic Growth Promoters. Poult. Sci. 2017, 96, 3104–3108. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, P.J.G.; Rico, A.; Troell, M.; Klinger, D.H.; Buschmann, A.H.; Saksida, S.; Chadag, M.V.; Zhang, W. Unpacking Factors Influencing Antimicrobial Use in Global Aquaculture and Their Implication for Management: A Review from a Systems Perspective. Sustain. Sci. 2018, 13, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Occurrence of Antibiotics and Antibiotic Resistance Genes in Soils from Wastewater Irrigation Areas in the Pearl River Delta Region, Southern China. Sci. Total Environ. 2018, 624, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the Aquatic Environments: A Review of the European Scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, C.; Fan, L.; Qiu, L.; Wu, W.; Meng, S.; Hu, G.; Kamira, B.; Chen, J. Occurrence of Antibiotics and Their Impacts to Primary Productivity in Fishponds around Tai Lake, China. Chemosphere 2016, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, Q.; Wang, X.; Torres, O.L.; Tang, S.; Zhang, S.; Guo, R.; Chen, J. Correlation between Antibiotic-Induced Feeding Depression and Body Size Reduction in Zooplankton (Rotifer, Brachionus calyciflorus): Neural Response and Digestive Enzyme Inhibition. Chemosphere 2019, 218, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Bray, W.A.; Williams, R.R.; Lightner, D.V.; Lawrence, A.L. Growth, Survival and Histological Responses of the Marine Shrimp, Litopenaeus vannamei, to Three Dosage Levels of Oxytetracycline. Aquaculture 2006, 258, 97–108. [Google Scholar] [CrossRef]
- Lundén, T.; Lilius, E.-M.; Bylund, G. Respiratory Burst Activity of Rainbow Trout (Oncorhynchus mykiss) Phagocytes Is Modulated by Antimicrobial Drugs. Aquaculture 2002, 207, 203–212. [Google Scholar] [CrossRef]
- Limbu, S.M.; Zhou, L.; Sun, S.-X.; Zhang, M.-L.; Du, Z.-Y. Chronic Exposure to Low Environmental Concentrations and Legal Aquaculture Doses of Antibiotics Cause Systemic Adverse Effects in Nile Tilapia and Provoke Differential Human Health Risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef]
- Tapia-Paniagua, S.T.; Vidal, S.; Lobo, C.; García de la Banda, I.; Esteban, M.A.; Balebona, M.C.; Moriñigo, M.A. Dietary Administration of the Probiotic SpPdp11: Effects on the Intestinal Microbiota and Immune-Related Gene Expression of Farmed Solea Senegalensis Treated with Oxytetracycline. Fish Shellfish Immunol. 2015, 46, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C. Heavy Use of Prophylactic Antibiotics in Aquaculture: A Growing Problem for Human and Animal Health and for the Environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, H.; Lukezic, T.; Šušković, J. Biosynthesis of Oxytetracycline by Streptomyces rimosus: Past, Present and Future Directions in the Development of Tetracycline Antibiotics. Food Technol. Biotechnol. 2017, 55, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Mog, M.; Pandey, P.K.; Khatei, A.; Parhi, J.; Barman, A.S.; Acharya, A.; Choudhury, T.G. Pathophysiological Response and IL-1β Gene Expression of Labeo rohita (Hamilton, 1822) Fingerlings Fed with Oxytetracycline Based Pharmaceutical Diet against Aeromonas hydrophila Infection. Aquaculture 2021, 540, 736716. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Zou, X.; Fu, G.; Li, C.; Fan, P.; Fang, W. Pharmacokinetics of Oxytetracycline in Pacific White Shrimp, Penaeus vannamei, after Oral Administration of a Single-Dose and Multiple-Doses. Aquaculture 2019, 512, 734348. [Google Scholar] [CrossRef]
- Wilson, W.R. Tetracyclines, Chloramphenicol, Erythromycin, and Clindamycin. Mayo Clin. Proc. 1987, 62, 10. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Cerezuela, R.; Meseguer, J.; Esteban, M.A. Modulation of the Immune Parameters and Expression of Genes of Gilthead Seabream (Sparus aurata L.) by Dietary Administration of Oxytetracycline. Aquaculture 2012, 334–337, 51–57. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Cabello, F.; Good, C.M.; Lunestad, B.T. Veterinary Drug Use in United States Net Pen Salmon Aquaculture: Implications for Drug Use Policy. Aquaculture 2020, 518, 734820. [Google Scholar] [CrossRef]
- Leal, J.F.; Santos, E.B.H.; Esteves, V.I. Oxytetracycline in Intensive Aquaculture: Water Quality during and after Its Administration, Environmental Fate, Toxicity and Bacterial Resistance. Rev. Aquac. 2019, 11, 1176–1194. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Teunissen, A.G.; Van Oosterom, R.; Van Muiswinkel, W.B. The Immune System of Cyprinid Fish. The Immunosuppressive Effect of the Antibiotic Oxytetracycline in Carp (Cyprinus carpio L.). Aquaculture 1980, 19, 177–189. [Google Scholar] [CrossRef]
- Enis Yonar, M.; Mişe Yonar, S.; Silici, S. Protective Effect of Propolis against Oxidative Stress and Immunosuppression Induced by Oxytetracycline in Rainbow Trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol. 2011, 31, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histological Alterations in Gills and Liver of Rainbow Trout (Oncorhynchus mykiss) after Exposure to the Antibiotic Oxytetracycline. Environ. Toxicol. Pharmacol. 2017, 53, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.; Tacão, M.; Machado, A.L.; Golovko, O.; Zlabek, V.; Domingues, I.; Henriques, I. Long-Term Effects of Oxytetracycline Exposure in Zebrafish: A Multi-Level Perspective. Chemosphere 2019, 222, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Fagutao, F.F.; Yasuike, M.; Santos, M.D.; Ruangpan, L.; Sangrunggruang, K.; Tassanakajon, A.; Takahashi, Y.; Ueno, R.; Kondo, H.; Hirono, I.; et al. Differential Gene Expression in Black Tiger Shrimp, Penaeus monodon, Following Administration of Oxytetracycline and Oxolinic Acid. Dev. Comp. Immunol. 2009, 33, 1088–1092. [Google Scholar] [CrossRef]
- Brugman, S.; Liu, K.; Lindenbergh–Kortleve, D.; Samsom, J.N.; Furuta, G.T.; Renshaw, S.A.; Willemsen, R.; Nieuwenhuis, E.E.S. Oxazolone-Induced Enterocolitis in Zebrafish Depends on the Composition of the Intestinal Microbiota. Gastroenterology 2009, 137, 1757–1767.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.G.; Chen, L. Gut Microbiota and Its Modulation for Healthy Farming of Pacific White Shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Huyben, D.; Sun, L.; Moccia, R.; Kiessling, A.; Dicksved, J.; Lundh, T. Dietary Live Yeast and Increased Water Temperature Influence the Gut Microbiota of Rainbow Trout. J. Appl. Microbiol. 2018, 124, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ni, J.; Li, J.; Wang, C.; Li, X.; Wu, S.; Zhang, T.; Yu, Y.; Yan, Q. Comparative Study on Gastrointestinal Microbiota of Eight Fish Species with Different Feeding Habits. J. Appl. Microbiol. 2014, 117, 1750–1760. [Google Scholar] [CrossRef]
- Zhang, N.; Liang, T.; Jin, Q.; Shen, C.; Zhang, Y.; Jing, P. Chinese Yam (Dioscorea opposita Thunb.) Alleviates Antibiotic-Associated Diarrhea, Modifies Intestinal Microbiota, and Increases the Level of Short-Chain Fatty Acids in Mice. Food Res. Int. 2019, 122, 191–198. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, J.; Kiron, V. Antibiotic-Induced Perturbations Are Manifested in the Dominant Intestinal Bacterial Phyla of Atlantic Salmon. Microorganisms 2019, 7, 233. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The Effects of Antibiotics on the Microbiome throughout Development and Alternative Approaches for Therapeutic Modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y. A Global Analysis on the Systemic Effects of Antibiotics in Cultured Fish and Their Potential Human Health Risk: A Review. Rev. Aquac. 2021, 13, 1015–1059. [Google Scholar] [CrossRef]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased Microglial Activation through Gut-Brain Axis by Prebiotics, Probiotics, or Synbiotics Effectively Restored Cognitive Function in Obese-Insulin Resistant Rats. J. Neuroinflammation 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Qamar, T.R.; Syed, F.; Nasir, M.; Rehman, H.; Zahid, M.N.; Liu, R.H.; Iqbal, S. Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats. Nutrients 2016, 8, 465. [Google Scholar] [CrossRef]
- Zhong, Y.; Cai, D.; Cai, W.; Geng, S.; Chen, L.; Han, T. Protective Effect of Galactooligosaccharide-Supplemented Enteral Nutrition on Intestinal Barrier Function in Rats with Severe Acute Pancreatitis. Clin. Nutr. Edinb. Scotl. 2009, 28, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.I.; Park, B.J.; Kim, H.-L.; Lee, M.H.; Kim, J.; Yang, Y.-I.; Kim, J.K.; Tsubaki, K.; Han, D.-W.; Park, J.-C. The Biological Activities of (1,3)-(1,6)-β-d-Glucan and Porous Electrospun PLGA Membranes Containing β-Glucan in Human Dermal Fibroblasts and Adipose Tissue-Derived Stem Cells. Biomed. Mater. 2010, 5, 044109. [Google Scholar] [CrossRef]
- Przybylska-Diaz, D.A.; Schmidt, J.G.; Vera-Jiménez, N.I.; Steinhagen, D.; Nielsen, M.E. β-Glucan Enriched Bath Directly Stimulates the Wound Healing Process in Common Carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2013, 35, 998–1006. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abdo, S.E.; Gewaily, M.S.; Moustafa, E.M.; SaadAllah, M.S.; AbdEl-kader, M.F.; Hamouda, A.H.; Omar, A.A.; Alwakeel, R.A. The Influence of Dietary β-Glucan on Immune, Transcriptomic, Inflammatory and Histopathology Disorders Caused by Deltamethrin Toxicity in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 98, 301–311. [Google Scholar] [CrossRef]
- Meena, D.K.; Das, P.; Kumar, S.; Mandal, S.C.; Prusty, A.K.; Singh, S.K.; Akhtar, M.S.; Behera, B.K.; Kumar, K.; Pal, A.K.; et al. Beta-Glucan: An Ideal Immunostimulant in Aquaculture (a Review). Fish Physiol. Biochem. 2013, 39, 431–457. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, G.; Lei, W.; Sagada, G.; Zhang, J.; Shao, Q.J. The Influence of Dietary Β-1,3-glucan on Growth Performance, Feed Utilization, Antioxidative and Immune Status of Pacific White Shrimp, Litopenaeus vannamei. Aquac. Nutr. 2021, 27, 1590–1601. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhou, L.; Qu, Y.; Lu, K.; Han, F.; Li, E. Effects of Different Dietary β-Glucan Levels on Antioxidant Capacity and Immunity, Gut Microbiota and Transcriptome Responses of White Shrimp (Litopenaeus vannamei) under Low Salinity. Antioxidants 2022, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef] [PubMed]
- Metian, M.; Troell, M.; Christensen, V.; Steenbeek, J.; Pouil, S. Mapping Diversity of Species in Global Aquaculture. Rev. Aquac. 2020, 12, 1090–1100. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Lee, P.-T.; Wu, Y.-S.; Chen, H.-Y.; Liao, Z.-H.; Huang, H.-T.; Chen, Y.-C.; Nan, F.-H. Positive and Synergistic Effects of Oxytetracycline and β-Glucan on Non-Specific Immune Responses in the White Shrimp, Litopenaeus vannamei (Boone, 1931) (Dendrobranchiata, Penaeidae). Crustaceana 2020, 93, 633–651. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Artington, VA, USA, 2000. [Google Scholar]
- Xu, Z.; Wang, Y.; Gul, Y.; Li, Q.; Song, J.; Hu, M. Effects of Copper Supplement on the Immune Function and Blood-Chemistry in Adult Chinese Horseshoe Crab Tachypleus tridentatus. Aquaculture 2020, 515, 734576. [Google Scholar] [CrossRef]
- Yu, Q.; Fu, Z.; Huang, M.; Xu, C.; Wang, X.; Qin, J.G.; Chen, L.; Han, F.; Li, E. Growth, Physiological, Biochemical, and Molecular Responses of Pacific White Shrimp Litopenaeus vannamei Fed Different Levels of Dietary Selenium. Aquaculture 2021, 535, 736393. [Google Scholar] [CrossRef]
- Hernández-López, J.; Gollas-Galván, T.; Vargas-Albores, F. Activation of the Prophenoloxidase System of the Brown Shrimp (Penaeus californiensis Holmes). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 113, 61–66. [Google Scholar] [CrossRef]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect Immunity. Purification and Properties of Three Inducible Bactericidal Proteins from Hemolymph of Immunized Pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef]
- Adams, A. Progress, Challenges and Opportunities in Fish Vaccine Development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.-C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredía, S.; de Verdal, H.; Gozlan, R.E. Aquaculture at the Crossroads of Global Warming and Antimicrobial Resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating Antimicrobial Resistance in the Global Shrimp Industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, P.; Matsumoto, T.; Husson, M. Intraphagocytic Penetration of Antibiotics. J. Antimicrob. Chemother. 1988, 22, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hand, W.L.; Hand, D.L.; King-Thompson, N.L. Antibiotic Inhibition of the Respiratory Burst Response in Human Polymorphonuclear Leukocytes. Antimicrob. Agents Chemother. 1990, 34, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Trushenski, J.T.; Aardsma, M.P.; Barry, K.J.; Bowker, J.D.; Jackson, C.J.; Jakaitis, M.; McClure, R.L.; Rombenso, A.N. Oxytetracycline Does Not Cause Growth Promotion in Finfish. J. Anim. Sci. 2018, 96, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, P.; Wang, F. β-Glucans as Potential Immunoadjuvants: A Review on the Adjuvanticity, Structure-Activity Relationship and Receptor Recognition Properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Vogt, G. Life-Cycle and Functional Cytology of the Hepatopancreatic Cells of Astacus astacus (Crustacea, Decapoda). Zoomorphology 1994, 114, 83–101. [Google Scholar] [CrossRef]
- Vogt, G.; Stöcker, W.; Storch, V.; Zwilling, R. Biosynthesis of Astacus Protease, a Digestive Enzyme from Crayfish. Histochemistry 1989, 91, 373–381. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Wang, Q.; Li, B. The Influence of Three Antibiotics on the Growth, Intestinal Enzyme Activities, and Immune Response of the Juvenile Sea Cucumber Apostichopus japonicus Selenka. Fish Shellfish Immunol. 2019, 84, 434–440. [Google Scholar] [CrossRef]
- Ji, L.; Sun, G.; Li, J.; Wang, Y.; Du, Y.; Li, X.; Liu, Y. Effect of Dietary β-Glucan on Growth, Survival and Regulation of Immune Processes in Rainbow Trout (Oncorhynchus mykiss) Infected by Aeromonas salmonicida. Fish Shellfish Immunol. 2017, 64, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, C.; Zhou, L.; Dong, Y.; Su, Y.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Beneficial Effects of Dietary β-Glucan on Growth and Health Status of Pacific White Shrimp Litopenaeus vannamei at Low Salinity. Fish Shellfish Immunol. 2019, 91, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lu, G.; Ye, Q.; Liu, J. Long-Term Effects of Antibiotics, Norfloxacin, and Sulfamethoxazole, in a Partial Life-Cycle Study with Zebrafish (Danio rerio): Effects on Growth, Development, and Reproduction. Environ. Sci. Pollut. Res. 2016, 23, 18222–18228. [Google Scholar] [CrossRef] [PubMed]
- Avunje, S.; Patil, P.K.; Ezaz, W.; Praveena, E.; Ray, A.; Viswanathan, B.; Alavandi, S.V.; Puthiyedathu, S.K.; Vijayan, K.K. Effect of Oxytetracycline on the Biosafety, Gut Microbial Diversity, Immune Gene Expression and Withdrawal Period in Pacific Whiteleg Shrimp, Penaeus vannamei. Aquaculture 2021, 543, 736957. [Google Scholar] [CrossRef]
- Xu, N.; Li, M.; Fu, Y.; Zhang, X.; Ai, X.; Lin, Z. Tissue Residue Depletion Kinetics and Withdrawal Time Estimation of Doxycycline in Grass Carp, Ctenopharyngodon idella, Following Multiple Oral Administrations. Food Chem. Toxicol. 2019, 131, 110592. [Google Scholar] [CrossRef]
- Röszer, T. The Invertebrate Midintestinal Gland (“hepatopancreas”) Is an Evolutionary Forerunner in the Integration of Immunity and Metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef]
- Abele, D.; Puntarulo, S. Formation of Reactive Species and Induction of Antioxidant Defence Systems in Polar and Temperate Marine Invertebrates and Fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 405–415. [Google Scholar] [CrossRef]
- Pani, G.; Bedogni, B.; Anzevino, R.; Colavitti, R.; Palazzotti, B.; Borrello, S.; Galeotti, T. Deregulated Manganese Superoxide Dismutase Expression and Resistance to Oxidative Injury in P53-Deficient Cells1. Cancer Res. 2000, 60, 4654–4660. [Google Scholar]
- Fridovich, I. Superoxide Dismutases: An Adaptation to a Paramagnetic Gas. J. Biol. Chem. 1989, 264, 7761–7764. [Google Scholar] [CrossRef]
- Heck, D.E.; Shakarjian, M.; Kim, H.D.; Laskin, J.D.; Vetrano, A.M. Mechanisms of Oxidant Generation by Catalase. Ann. N. Y. Acad. Sci. 2010, 1203, 120–125. [Google Scholar] [CrossRef]
- Luu, Q.H.; Nguyen, T.B.T.; Nguyen, T.L.A.; Do, T.T.T.; Dao, T.H.T.; Padungtod, P. Antibiotics Use in Fish and Shrimp Farms in Vietnam. Aquac. Rep. 2021, 20, 100711. [Google Scholar] [CrossRef]
- Rigos, G.; Katharios, P.; Papandroulakis, N. Single Intramuscular Administration of Long-Acting Oxytetracycline in Grouper (Epinephelus marginatus). Turk. J. Vet. Anim. Sci. 2010, 34, 441–445. [Google Scholar] [CrossRef]
- Dobšíková, R.; Blahová, J.; Mikulíková, I.; Modrá, H.; Prášková, E.; Svobodová, Z.; Škorič, M.; Jarkovský, J.; Siwicki, A.-K. The Effect of Oyster Mushroom β-1.3/1.6-D-Glucan and Oxytetracycline Antibiotic on Biometrical, Haematological, Biochemical, and Immunological Indices, and Histopathological Changes in Common Carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2013, 35, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Korheina, D.K.A.; Fu, H.; Ge, X. Chronic Exposure to Dietary Antibiotics Affects Intestinal Health and Antibiotic Resistance Gene Abundance in Oriental River Prawn (Macrobrachium nipponense), and Provokes Human Health Risk. Sci. Total Environ. 2020, 720, 137478. [Google Scholar] [CrossRef] [PubMed]
- Gopalakannan, A.; Arul, V. Enhancement of the Innate Immune System and Disease-Resistant Activity in Cyprinus carpio by Oral Administration of β-Glucan and Whole Cell Yeast. Aquac. Res. 2010, 41, 884–892. [Google Scholar] [CrossRef]
- Little, T.J.; Hultmark, D.; Read, A.F. Invertebrate Immunity and the Limits of Mechanistic Immunology. Nat. Immunol. 2005, 6, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salgado, J.L.; Pereyra, M.A.; Alpuche-Osorno, J.J.; Zenteno, E. Pattern Recognition Receptors in the Crustacean Immune Response against Bacterial Infections. Aquaculture 2021, 532, 735998. [Google Scholar] [CrossRef]
- Söderhäll, K.; Cerenius, L. Role of the Prophenoloxidase-Activating System in Invertebrate Immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-System: Pros and Cons for Its Role in Invertebrate Immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef]
- Kulkarni, A.; Krishnan, S.; Anand, D.; Kokkattunivarthil Uthaman, S.; Otta, S.K.; Karunasagar, I.; Kooloth Valappil, R. Immune Responses and Immunoprotection in Crustaceans with Special Reference to Shrimp. Rev. Aquac. 2021, 13, 431–459. [Google Scholar] [CrossRef]
- Huang, H.-T.; Chang, J.-J.; Lin, Y.-R.; Chen, Y.-Y.; Chang, Y.-H.; Chen, B.-Y.; Nan, F.-H. Synergistic Effects of Dietary Oxolinic Acid Combined with Oxytetracycline on Nonspecific Immune Responses and Resistance against Vibrio parahaemolyticus Infection of White Shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2022, 127, 740–747. [Google Scholar] [CrossRef]
- Lee, P.-T.; Liao, Z.-H.; Huang, H.-T.; Chuang, C.-Y.; Nan, F.-H. β-Glucan Alleviates the Immunosuppressive Effects of Oxytetracycline on the Non-Specific Immune Responses and Resistance against Vibrio alginolyticus Infection in Epinephelus fuscoguttatus × Epinephelus lanceolatus Hybrids. Fish Shellfish Immunol. 2020, 100, 467–475. [Google Scholar] [CrossRef]
- Reda, R.M.; Ibrahim, R.E.; Ahmed, E.-N.G.; El-Bouhy, Z.M. Effect of Oxytetracycline and Florfenicol as Growth Promoters on the Health Status of Cultured Oreochromis niloticus. Egypt. J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef]
- Evariste, L.; Barret, M.; Mottier, A.; Mouchet, F.; Gauthier, L.; Pinelli, E. Gut Microbiota of Aquatic Organisms: A Key Endpoint for Ecotoxicological Studies. Environ. Pollut. 2019, 248, 989–999. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Zhang, Y.; Zheng, K.; Xiang, Q.; Chen, N.; Chen, Z.; Zhang, N.; Zhu, J.; He, Q. Antibiotic-Induced Disruption of Gut Microbiota Alters Local Metabolomes and Immune Responses. Front. Cell. Infect. Microbiol. 2019, 9, 99. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics Modulated Gut Microbiota Suppresses Hepatocellular Carcinoma Growth in Mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef]
- Stephens, W.Z.; Burns, A.R.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. The Composition of the Zebrafish Intestinal Microbial Community Varies across Development. ISME J. 2016, 10, 644–654. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-What Role Do Proteobacteria Play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- De Cock, H.E.V.; Marks, S.L.; Stacy, B.A.; Zabka, T.S.; Burkitt, J.; Lu, G.; Steffen, D.J.; Duhamel, G.E. Ileocolitis Associated with Anaerobiospirillum in Cats. J. Clin. Microbiol. 2004, 42, 2752–2758. [Google Scholar] [CrossRef]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of Probiotics in Aquaculture of China—A Review of the Past Decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef]
- Lam, M.Q.; Oates, N.C.; Thevarajoo, S.; Tokiman, L.; Goh, K.M.; McQueen-Mason, S.J.; Bruce, N.C.; Chong, C.S. Genomic Analysis of a Lignocellulose Degrading Strain from the Underexplored Genus Meridianimaribacter. Genomics 2020, 112, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Shibl, A. Role of Probiotics in Health Improvement, Infection Control and Disease Treatment and Management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef] [PubMed]





| Ingredients | Dietary β-(1,3)-Glucan Concentration | |||
|---|---|---|---|---|
| Control | β-Glucan | OTC | Mix | |
| Fish meal (g) | 26 | 26 | 26 | 26 |
| Soybean meal (g) | 28 | 28 | 28 | 28 |
| Corn starch (g) | 23 | 23 | 23 | 23 |
| Shrimp meal (g) | 4 | 4 | 4 | 4 |
| Calcium dihydrogen phosphate (g) | 1.5 | 1.5 | 1.5 | 1.5 |
| Vitamin premix 1 (g) | 2 | 2 | 2 | 2 |
| Mineral premix 2 (g) | 2 | 2 | 2 | 2 |
| Choline chloride (g) | 1 | 1 | 1 | 1 |
| Fish oil (g) | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean oil (g) | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean lecithin (g) | 1 | 1 | 1 | 1 |
| Cholesterol (g) | 0.5 | 0.5 | 0.5 | 0.5 |
| Carboxymethylcellulose (g) | 3 | 3 | 3 | 3 |
| Butylated hydroxytoluene (g) | 0.1 | 0.1 | 0.1 | 0.1 |
| Microcrystalline cellulose (g) | 2.9 | 2.8 | 2.895 | 2.795 |
| β-(1,3)-Glucan 3 (g) | 0 | 0.1 | 0 | 0.1 |
| Oxytetracycline 4 (g) | 0 | 0 | 0.005 | 0.005 |
| Total | 100 | 100 | 100 | 100 |
| Nutrient levels (%) | ||||
| Crude protein | 35.2 | 35.5 | 35.3 | 35.5 |
| Total lipid | 7.6 | 7.6 | 7.6 | 7.6 |
| Ash | 10.3 | 10.5 | 10.6 | 10.7 |
| Moisture | 9.2 | 9.2 | 9.2 | 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Han, F.; Peng, X.; Rombenso, A.; Li, E. Dietary β-Glucan Alleviates Antibiotic-Associated Side Effects by Increasing the Levels of Antioxidant Enzyme Activities and Modifying Intestinal Microbiota in Pacific White Shrimp (Litopenaeus vannamei). Antioxidants 2024, 13, 52. https://doi.org/10.3390/antiox13010052
Qiao Y, Han F, Peng X, Rombenso A, Li E. Dietary β-Glucan Alleviates Antibiotic-Associated Side Effects by Increasing the Levels of Antioxidant Enzyme Activities and Modifying Intestinal Microbiota in Pacific White Shrimp (Litopenaeus vannamei). Antioxidants. 2024; 13(1):52. https://doi.org/10.3390/antiox13010052
Chicago/Turabian StyleQiao, Yanbing, Fenglu Han, Xuhan Peng, Artur Rombenso, and Erchao Li. 2024. "Dietary β-Glucan Alleviates Antibiotic-Associated Side Effects by Increasing the Levels of Antioxidant Enzyme Activities and Modifying Intestinal Microbiota in Pacific White Shrimp (Litopenaeus vannamei)" Antioxidants 13, no. 1: 52. https://doi.org/10.3390/antiox13010052