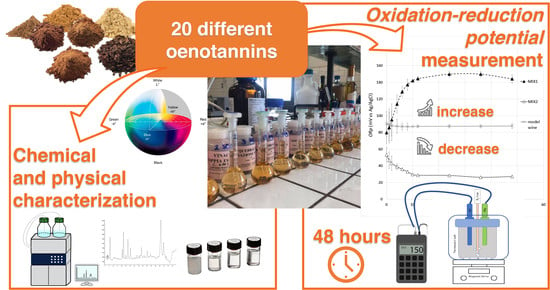
Characterization of 20 Oenological Tannins from Different Botanical Origins for Formulation of Blends with Redox Potential Tuning Ability in Model Wine Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Tannins
- nine belonged to the condensed tannins (i.e., from SDS to THD codes in Table 1): two from grape seeds (proanthocyanidins including procyanidins types), two from quebracho (profisetinidins type), one from mimosa (prorobinetidins type), two from tea (catechin/epicatechin gallates types), one from prunus wood (procyanidins type) and one from citrus wood;
- eleven belonged to the hydrolysable tannins (i.e., from GAS to OEE codes in Table 1). They were gallotannins, two from nut gall and one from tara, but also ellagitannins, one from mirtacee, one from chestnut and six from oak.
2.1.3. Model Wine Solution with Tannins (1 g/L Oenotannin Solutions)
2.2. Methods
2.2.1. Coloring Properties of the Oenotannin Solutions
2.2.2. CIELab Coordinates of the Oenotannin Solutions
2.2.3. Precipitation Assay of the Oenotannin Solutions
2.2.4. Total Phenols Estimation
2.2.5. Phenolic Characterization by HPLC-DAD-MS Analysis
2.2.6. Antioxidant Property by DPPH• Assay
2.2.7. Redox Potential (ORp)
2.3. Data Processing
3. Results and Discussion
3.1. Basic Characteristics of the Oenotannin Solutions
3.2. Colorimetric Characteristics of the Oenotannin Solutions
3.3. Phenolic Characterization by HPLC-DAD-MS Analysis
3.4. The Redox Potential (ORp)
3.5. Blending Formulations of Oenotannins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vignault, A.; Gonzalez-Centeno, M.R.; Pascual, O.; Gombau, J.; Jourdes, M.; Moine, V.; Iturmendi, N.; Canals, J.M.; Zamora, F.; Teissedre, P.L. Chemical characterization, antioxidant properties and oxygen consumption rate of 36 commercial oenological tannins in a model wine solution. Food Chem. 2018, 268, 210–219. [Google Scholar] [CrossRef]
- Herderich, M.J.; Smith, P.A. Analysis of grape and wine tannins: Methods, applications and challenges. Aust. J. Grape Wine Res. 2005, 11, 205–214. [Google Scholar] [CrossRef]
- Versari, A.; du Toit, W.; Parpinello, G.P. Oenological tannins: A review. Aust. J. Grape Wine Res. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed. Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Souquet, J.-M.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric proanthocyanidins from grape skins. Phytochemistry 1996, 43, 509–512. [Google Scholar] [CrossRef]
- Canuti, V.; Cecchi, L.; Khatib, M.; Guerrini, L.; Mulinacci, N.; Zanoni, B. A new extract from pomegranate (Punica granatum L.) by-products as a potential oenological tannin: Preliminary characterization and comparison with existing commercial products. Molecules 2020, 25, 4460. [Google Scholar] [CrossRef]
- Jourdes, M.; Pouysegu, L.; Deffieux, D.; Teissedre, P.-L.; Quideau, S. Hydrolyzable tannins: Gallotannins and ellagitannins. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1795–2010. [Google Scholar] [CrossRef]
- Balli, D.; Cecchi, L.; Khatib, M.; Bellumori, M.; Cairone, F.; Carradori, S.; Zengin, G.; Cesa, S.; Innocenti, M.; Mulinacci, N. Characterization of Arils Juice and Peel Decoction of Fifteen Varieties of Punica granatum L.; A Focus on Anthocyanins, Ellagitannins and Polysaccharides. Antioxidants 2020, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MS(n). Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Classification of oligomeric hydrolysable tannins and specificity of their occurrence in plants. Phytochemistry 1993, 32, 507–521. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Correlation of oxidative transformation of hydrolysable tannins and plant evolution. Phytochemistry 2000, 55, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Wakamori, S.; Hirokane, T.; Ikeuchi, K.; Matsumoto, S. Structural revisions in natural ellagitannins. Molecules 2018, 23, 1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Update of the General Monograph on Tannins—Resolution OIV-OENO 624-2022. Available online: https://www.oiv.int/node/2881 (accessed on 11 April 2023).
- Canuti, V.; Puccioni, S.; Giovani, G.; Salmi, M.; Rosi, I.; Bertuccioli, M. Effect of oenotannin addition on the composition of sangiovese wines from grapes with different characteristics. Am. J. Enol. Vitic. 2012, 63, 220–231. [Google Scholar] [CrossRef]
- Jeremic, J.; Vongluanngam, I.; Ricci, A.; Parpinello, G.P.; Versari, A. The oxygen consumption kinetics of commercial oenological tannins in model wine solution and Chianti red wine. Molecules 2020, 25, 1215. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Ortin, A.B.; Martinez-Cautillas, A.; Ros-Garcia, J.M.; Lopez-Roca, J.M.; Gomez-Plaza, E. Improving color extraction and stability in red wines: The use of maceration enzymes and enological tannins. Int. J. Food Sci. Technol. 2005, 40, 867–878. [Google Scholar] [CrossRef]
- Pascual, O.; Vignault, A.; Gombau, J.; Navarro, M.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Teissedre, P.-L.; Zamora, F. Oxygen consumption rates by different oenological tannins in a model wine solution. Food Chem. 2017, 234, 26–32. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B. Handbook of Enology, 2nd ed.; John Wiley: Hoboken, NJ, USA; West Sussex, UK, 2006. [Google Scholar]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [Green Version]
- Vignault, A.; Gombau, J.; Jourdes, M.; Moine, V.; Canals, J.M.; Fermaud, M.; Roudet, J.; Zamora, F.; Teissedre, P.L. Oenological tannins to prevent Botrytis cinerea damage in grapes and musts: Kinetics and electrophoresis characterization of laccase. Food Chem. 2020, 316, 126334. [Google Scholar] [CrossRef]
- Caillé, S.; Samson, A.; Wirth, J.; Diéval, J.B.; Vidal, S.; Cheynier, V. Sensory characteristics changes of red Grenache wines submitted to different oxygen exposures pre and post bottling. Anal. Chim. Acta 2010, 660, 35–42. [Google Scholar] [CrossRef]
- Ferreira, V.; Carrascon, V.; Bueno, M.; Ugliano, M.; Fernandez-Zurbano, P. Oxygen consumption by red wines. Part I: Consumption rates, relationship with chemical composition and role of SO2. J. Agric. Food Chem. 2015, 63, 10928–10937. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Bocharova, O.V. Electrochemical Basis for Re-evaluating Antioxidant Effect of Redox Substances in Foodstuffs. ACS Food Sci. Technol. 2022, 2, 738–750. [Google Scholar] [CrossRef]
- Killeen, D.J.; Boulton, R.; Knoesen, A. Advanced monitoring and control of redox potential in wine fermentation. Am. J. Enol. Vitic. 2018, 69, 394–399. [Google Scholar] [CrossRef]
- Walker, G.A.; Nelson, J.; Halligan, T.; Lima, M.M.; Knoesen, A.; Runnebaum, R.C. Monitoring site-specific fermentation outcomes via oxidation reduction potential and uv-vis spectroscopy to characterize “hidden” parameters of pinot noir wine fermentations. Molecules 2021, 26, 4748. [Google Scholar] [CrossRef] [PubMed]
- Makhotkina, O.; Kilmartin, P.A. The use of cyclic voltammetry for wine analysis: Determination of polyphenols and free sulfur dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C.; Tunbridge, P.; Kilmartin, P.A. Wine reduction potentials: Are these measured values really reduction potentials? J. Agric. Food Chem. 2019, 67, 4145–4153. [Google Scholar] [CrossRef]
- Vivas, N. Les tannins et les extraicts vegetaux riches en polyphenols. In Produits de Traitement et Auxiliaires D’élaboration des Moûts et des Vins, 2nd ed.; Bertrand, A., Canal-Llauberes, R.-M., Feuillat, M., Hardy, G., Lamadon, F., Lonvaud-Funel, A., Pellerin, P., Vivas, N., Eds.; Editions Feret: Bordeaux, France, 2000. [Google Scholar]
- Petrovic, S.C. Correlation of perceived wine astringency to cyclic voltammetric response. Am. J. Enol. Vitic. 2009, 60, 373–378. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Teslić, N.; Kilmartin, P.A.; Versari, A. Suitability of the cyclic voltammetry measurements and DPPH• spectrophotometric assay to determine the antioxidant capacity of food-grade oenological tannins. Molecules 2019, 24, 2925. [Google Scholar] [CrossRef] [Green Version]
- Ugliano, M.; Slaghenaufi, D.; Picariello, L.; Olivieri, G. Oxygen and SO2 consumption of different enological tannins in relationship to their chemical and electrochemical characteristics. J. Agric. Food Chem. 2020, 68, 13418–13425. [Google Scholar] [CrossRef]
- Determination of the Chromatic Characteristics of Wine According to CIELab-Resolution OIV—Oeno 1-2006. Available online: https://www.oiv.int/public/medias/849/oeno-01-2006-en.pdf (accessed on 11 April 2023).
- Del Alaya, F.; Echavarru, J.F.; Negueruela, A.I. A new simplified method for measuring the color of wines. I. Red and Rosé Wines. Am. J. Enol. Vitic. 1997, 48, 357–363. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Rice, M.E.; Ritchard, N.T. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin16 (4→8) catechin (procyanidin). J. Agric. Food Chem. 1998, 46, 2590–2595. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P. Le dosage des composés phénoliques totaux dans les vins rouges. Chim. Anal. 1970, 52, 627–631. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate the antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Measurement of the Oxidation-Reduction Potential in Wines—Resolution Oeno 3/2000. Method OIV-MA-AS2-06. Available online: http://188.165.107.123/public/medias/2474/oiv-ma-as2-06.pdf (accessed on 11 April 2023).
- Vivas, N.; Zamora, F.; Glories, Y. Étude des phénomenes d’oxydoréduction dans les vins. Mise au point d’une méthode rapide de mesure du potential d’oxydoréduction. J. Int. Sci. Vigne Vin. 1992, 26, 271–285. [Google Scholar]
- Vivas, N.; Glories, Y.; Bertrand, A.; Zamora, F. Principe et méthode de mesure du potentiel d’oxydoréduction dans les vins. Bull. OIV 1996, 69, 617–633. [Google Scholar]
- Bates, R.G. Determination of pH, Theory and Practice, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1973; pp. 333–336. [Google Scholar]
- Romanini, E.; McRae, J.M.; Colangelo, D.; Lambri, M. First trials to assess the feasibility of grape seed powder (GSP) as a novel and sustainable bentonite alternative. Food Chem. 2020, 305, 125484. [Google Scholar] [CrossRef]
- Romanini, E.; McRae, J.M.; Bilogrevic, E.; Colangelo, D.; Gabrielli, M.; Lambri, M. Use of grape seeds to reduce haze formation in white wines. Food Chem. 2021, 341, 128250. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Yoshimura, M.; Amakura, Y.; Tokuhara, M.; Yoshida, T. Polyphenolic compounds isolated from the leaves of Myrtus communis. J. Nat. Med. 2008, 62, 366–368. [Google Scholar] [CrossRef]
- Malacarne, M.; Nardin, T.; Bertoldi, D.; Nicolini, G.; Larcher, R. Verifying the botanical authenticity of commercial tannins through sugars and simple phenols profiles. Food Chem. 2016, 206, 274–283. [Google Scholar] [CrossRef]
- Ferreira, V.; Franco-Luesma, E.; Vela, E.; López, R.; Hernández-Orte, P. Elusive chemistry of hydrogen sulfide and mercaptans in wine. J. Agric. Food Chem. 2017, 66, 2237–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivas, N.; Glories, Y. Vinification et e’ le ‘vage des vins. Potential d’oxydore ‘duction en oenologie. Rev. Oenologues Tech. Vitivinic. Oenologiques 1995, 21, 10–14. [Google Scholar]
- Moreno, J.; Peinado, R. Chapter 17—Redox Phenomena in Must and Wine. In Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Danilewicz, J.C. Review of oxidative processes in wine and value of reduction potentials in enology. Am. J. Enol. Vitic. 2012, 63, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Siebert, T.E.; Solomon, M.R.; Pollnitz, A.P.; Jeffery, D.W. Selective determination of volatile sulfur compounds in wine by gas chromatography with sulfur chemiluminescence detection. J. Agric. Food Chem. 2010, 58, 9454–9462. [Google Scholar] [CrossRef]




| Tannin Code | Botanical Origin | Geographical Origin | Structure of Tannin | Extraction Cycles | Treatment |
|---|---|---|---|---|---|
| SDS | Grape Seed (Vitis vinifera L.) | French | Condensed | 1 | - |
| SDD | Grape Seed (Vitis vinifera L.) | French | Condensed | 2 | - |
| QBS | Quebracho | Argentina | Condensed | 1 | - |
| QBD | Quebracho | Argentina | Condensed | 2 | - |
| MIM | Mimosa wood | Brazil | Condensed | 1 | - |
| PRU | Prunus wood | Unknown | Condensed | 1 | - |
| CIT | Citrus wood | Unknown | Condensed | 1 | - |
| THS | Tea (Camelia sinensis L.) | Unknown | Condensed | 1 | - |
| THD | Tea (Camelia sinensis L.) | Unknown | Condensed | 2 | - |
| GAS | Nut gall (Quercus) | Turkey | Gallic hydrolysable | 1 | - |
| GAD | Nut gall (Quercus) | Turkey | Gallic hydrolysable | 1 | - |
| TAR | Tara (Caesalpinia spinosa L.) | Peru | Gallic hydrolysable | 1 | - |
| MIR | Mirtacee (Myrtus communis L.) | Spain | Ellagic hydrolysable | 1 | - |
| CHS | Chestnut wood (Castanea sativa) | Unknown | Ellagic hydrolysable | 1 | - |
| OFN | Oak wood (Quercus petraea L.) | French | Ellagic hydrolysable | 1 | - |
| OFT | Oak wood (Quercus petraea L.) | French | Ellagic hydrolysable | 1 | Toasted |
| OAN | Oak wood (Quercus alba L.) | U.S.A. | Ellagic hydrolysable | 1 | - |
| OAT | Oak wood (Quercus alba L.) | U.S.A. | Ellagic hydrolysable | 1 | Toasted |
| OFE | Oak wood (Quercus petraea L.) | French | Ellagic hydrolysable | 1 | Dried |
| OEE | Oak wood (Quercus petraea L.) | Croatia | Ellagic hydrolysable | 1 | Dried |
| Tannin Code | Total Phenols Estimation (w/w %) 1 | Turbidity (ΔNTU1) 2 | Turbidity (ΔNTU2) 2 | AP % | A420 | A520 | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| SDS | 37.55 ± 0.01 | 2525 ± 6 | 2605 ± 4 | 93.11 ± 0.13 | 0.158 ± 0.001 | 0.071 ± 0.001 | 99.08 ± 0.05 | 0.28 ± 0.06 | 2.33 ± 0.22 | 2.42 |
| SDD | 35.18 ± 0.01 | 210 ± 1 | 149 ± 6 | 93.29 ± 0.01 | 0.102 ± 0.001 | 0.042 ± 0.000 | 99.93 ± 0.12 | 0.09 ± 0.14 | 1.05 ± 0.53 | 0.88 |
| QBS | 31.80 ± 0.02 | 574 ± 3 | 451 ± 3 | 90.99 ± 0.68 | 0.177 ± 0.001 | 0.086 ± 0.000 | 98.63 ± 0.04 | 0.32 ± 0.11 | 3.26 ± 0.34 | 3.45 |
| QBD | 41.07 ± 0.02 | 596 ± 3 | 505 ± 4 | 92.61 ± 0.14 | 0.136 ± 0.000 | 0.079 ± 0.001 | 99.28 ± 0.06 | 0.18 ± 0.05 | 2.25 ± 0.26 | 2.24 |
| MIM | 19.72 ± 0.01 | 512 ± 4 | 583 ± 4 | 94.24 ± 0.24 | 0.077 ± 0.002 | 0.028 ± 0.001 | 99.02 ± 0.05 | 0.04 ± 0.02 | 2.22 ± 0.11 | 2.36 |
| PRU | 34.71 ± 0.01 | 566 ± 3 | 609 ± 5 | 93.89 ± 0.39 | 0.130 ± 0.001 | 0.072 ± 0.002 | 99.22 ± 0.04 | 0.01 ± 0.03 | 2.55 ± 0.12 | 3.01 |
| CIT | 41.06 ± 0.01 | 995 ± 5 | 915 ± 8 | 90.10 ± 0.11 | 0.178 ± 0.001 | 0.086 ± 0.001 | 99.36 ± 0.02 | −0.28 ± 0.03 | 2.23 ± 0.14 | 3.72 |
| THS | 27.42 ± 0.01 | 36 ± 1 | 27 ± 62 | 94.73 ± 0.01 | 0.320 ± 0.000 | 0.104 ± 0.000 | 99.88 ± 0.04 | −0.20 ± 0.06 | 1.53 ± 0.22 | 2.24 |
| THD | 46.85 ± 0.02 | 20 ± 1 | 7 ± 0 | 95.50 ± 0.07 | 0.380 ± 0.001 | 0.134 ± 0.001 | 99.26 ± 0.03 | −0.26 ± 0.04 | 2.42 ± 0.18 | 0.64 |
| GAS | 94.79 ± 0.02 | 1526 ± 6 | 1008 ± 7 | 95.81 ± 0.06 | 0.308 ± 0.001 | 0.110 ± 0.001 | 99.81 ± 0.02 | −0.27 ± 0.06 | 1.6 ± 0.16 | 1.91 |
| GAD | 106.66 ± 0.05 | 1430 ± 7 | 1108 ± 7 | 95.75 ± 0.10 | 0.221 ± 0.001 | 0.106 ± 0.000 | 99.87 ± 0.02 | 0.04 ± 0.08 | 0.24 ± 0.19 | 0.83 |
| TAR | 106.29 ± 0.03 | 3328 ± 11 | 1823 ± 9 | 95.96 ± 0.38 | 0.001 ± 0.000 | 0.001 ± 0.001 | 100.46 ± 0.05 | 0.21 ± 0.04 | −1.49 ± 0.14 | 0.61 |
| MIR | 41.36 ± 0.04 | 571 ± 3 | 404 ± 6 | 92.57 ± 0.09 | 0.015 ± 0.001 | 0.004 ± 0.001 | 100.14 ± 0.45 | −0.25 ± 0.53 | −0.30 ± 1.28 | 2.52 |
| CHS | 41.28 ± 0.01 | 699 ± 3 | 697 ± 7 | 94.91 ± 0.33 | 0.012 ± 0.000 | 0.007 ± 0.001 | 100.10 ± 0.05 | −0.10 ± 0.05 | −0.00 ± 0.17 | 0.67 |
| OFN | 25.96 ± 0.02 | 285 ± 2 | 261 ± 3 | 93.90 ± 0.01 | 0.202 ± 0.000 | 0.114 ± 0.001 | 99.30 ± 0.04 | −0.20 ± 0.05 | 2.28 ± 0.19 | 2.16 |
| OFT | 34.20 ± 0.01 | 181 ± 2 | 204 ± 2 | 94.42 ± 0.13 | 0.011 ± 0.001 | 0.001 ± 0.000 | 100.10 ± 0.03 | −0.12 ± 0.04 | 0.83 ± 0.11 | 1.31 |
| OAN | 28.86 ± 0.01 | 215 ± 6 | 259 ±3 | 93.86 ± 0.86 | 0.212 ± 0.001 | 0.060 ± 0.001 | 100.06 ± 0.05 | −0.39 ± 0.06 | 3.25 ± 0.17 | 2.40 |
| OAT | 30.74 ± 0.02 | 119 ± 3 | 106 ± 2 | 94.48 ± 0.13 | 0.243 ± 0.002 | 0.080 ± 0.003 | 98.05 ± 0.03 | −0.43 ± 0.02 | 3.15 ± 0.04 | 1.42 |
| OFE | 16.69 ± 0.01 | 528 ± 4 | 588 ± 7 | 94.36 ± 0.01 | 0.264 ± 0.001 | 0.098 ± 0.003 | 99.05 ± 0.02 | −0.26 ± 0.02 | 2.63 ± 0.05 | 2.69 |
| OEE | 22.52 ± 0.01 | 1010 ± 5 | 930 ± 9 | 94.82 ± 0.01 | 0.162 ± 0.001 | 0.045 ± 0.002 | 99.41 ± 0.02 | −0.28 ± 0.01 | 1.84 ± 0.02 | 1.84 |
| Model wine | - | - | - | - | - | - | 100.52 ± 0.05 | −0.02 ± 0.03 | 0.40 ± 0.01 | - |
| Tannin Code | Gallic Acid 1 | Castalagin 1 | (+)-Catechin 2 | (−)-Epicatechin 2 | Ellagic Acid 1 | Epicatechin Gallate 2 | Procyanidin 2 | Dimer Procyanidin 2 | Trimer Procianidin 2 | Polymers 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| SDS | 1.77 ± 0.03 a | nd | 45.68 ± 3.16 c | 41.79 ± 2.43 c | nd | 3.28 ± 0.14 a | 27.50 ± 0.47 b | 75.96 ± 0.40 b | 8.12 ± 0.40 a | 48.85 ± 1.06 h |
| SDD | 13.06 ± 0.20 f | nd | 175.70 ± 12.16 d | 130.89 ± 7.60 e | nd | 62.91 ± 2.74 c | 61.35 ± 1.04 c | 215.70 ± 0.65 c | 13.15 ± 0.65 c | 37.34 ± 0.81 g |
| QBS | 7.98 ± 0.12 c,d | nd | 10.24 ± 0.71 a,b | 6.01 ± 0.35 a | nd | nd | nd | nd | nd | 69.22 ± 1.50 j |
| QBD | 13.63 ± 0.20 f | nd | 3.39 ± 0.23 a | 1.79 ± 0.10 a | nd | nd | nd | nd | nd | 93.52 ± 2.03 k |
| MIM | 1.41 ± 0.02 a | nd | 4.30 ± 0.30 a | 1.79 ± 0.10 a | nd | nd | nd | nd | 11.58 ± 0.58 b | 19.51 ± 0.42 d |
| PRU | 27.39 ± 0.41 i | 12.42 ± 0.65 b | 15.74 ± 1.09 b | 5.50 ± 0.32 a | 2.02 ± 0.15 a,b | 3.53 ± 0.15 a | nd | nd | nd | 31.36 ± 0.68 f |
| CIT | 29.85 ± 0.45 j | 16.09 ± 0.84 d | nd | nd | 1.67 ± 0.13 a | 2.15 ± 0.99 a | nd | nd | nd | 25.70 ± 0.56 e |
| THS | 4.41 ± 0.07 b | nd | 9.47 ± 0.66 a,b | 22.19 ± 1.29 b | nd | 22.37 ± 0.97 b | nd | nd | nd | 4.59 ± 0.10 a |
| THD | 9.24 ± 0.14 e | nd | 13.48 ± 0.93 b | 96.25 ± 5.59 d | nd | 353.01 ± 15.36 d | 10.49 ± 0.18 a | 2.81 ± 0.16 a | nd | 55.20 ± 1.20 i |
| GAS | 34.32 ± 0.52 k | nd | nd | nd | nd | nd | nd | nd | nd | 180.30 ± 3.91 n |
| GAD | 83.45 ± 1.25 o | nd | nd | nd | 15.41 ± 1.18 e | nd | nd | nd | nd | 110.90 ± 2.41 m |
| TAR | 65.75 ± 0.99 n | nd | nd | nd | nd | nd | nd | nd | nd | 265.97 ± 5.77 o |
| MIR | 14.62 ± 0.22 g | nd | nd | nd | nd | nd | nd | nd | nd | 97.22 ± 2.11 l |
| CHS | 36.36 ± 0.55 l | 24.90 ± 1.30 e | nd | nd | 1.97 ± 0.15 a,b | nd | nd | nd | nd | 10.97 ± 0.24 b |
| OFN | 20.11 ± 0.30 h | 16.68 ± 0.87 d | nd | nd | 2.35 ± 0.18 a,b | nd | nd | nd | nd | 14.43 ± 0.31 c |
| OFT | 7.36 ± 0.11 c | 16.37 ± 0.85 d | nd | nd | 2.49 ± 0.19 b | nd | nd | nd | nd | 26.85 ± 0.58 e |
| OAN | 7.79 ± 0.12 c,d | 8.35 ± 0.44 a | nd | nd | 4.68 ± 0.36 c | nd | nd | nd | nd | 19.37 ± 0.42 d |
| OAT | 8.14 ± 0.12 d | 8.39 ± 0.44 a | nd | nd | 6.24 ± 0.48 d | nd | nd | nd | nd | 20.98 ± 0.46 d |
| OFE | 14.80 ± 0.22 g | 14.12 ± 0.74 c | nd | nd | 2.17 ± 0.17 a,b | 2.91 ± 0.13 a | nd | nd | nd | 21.05 ± 0.46 d |
| OEE | 37.92 ± 0.57 m | 25.03 ± 1.31 e | nd | nd | 2.01 ± 0.15 a,b | 0.55 ± 0.02 a | nd | nd | nd | 14.10 ± 0.31 c |
| Mean values of condensed tannins | 12.08 | 14.25 | 34.75 | 38.27 | 1.84 | 74.54 | 33.11 | 98.15 | 10.95 | 42.81 |
| Mean values of gallic hydrolizable tannins | 61.17 | nd | nd | nd | 15.41 | nd | nd | nd | nd | 185.72 |
| Mean values of ellagic hydrolizable tannins | 18.39 | 16.26 | nd | nd | 3.13 | 1.73 | nd | nd | nd | 28.12 |
| Tannin Code | ORpt48 (mV vs. Ag/AgCl) | ORpt48 (mV vs. SHE) | ΔORpt48 (mV) |
|---|---|---|---|
| SDS | 108.4 ± 3.8 | 316.4 ± 11.2 | 20.7 |
| SDD | 78.8 ± 2.8 | 286.8 ± 10.2 | −9.0 |
| QBS | 75.6 ± 2.7 | 283.6 ± 10.1 | −12.2 |
| QBD | 72.5 ± 2.6 | 280.5 ± 10.0 | −15.3 |
| MIM | 135.8 ± 4.8 | 343.8 ± 12.2 | 48.1 |
| PRU | 62.6 ± 2.2 | 270.6 ± 9.6 | −25.2 |
| CIT | 61.3 ± 2.2 | 269.3 ± 9.6 | −26.5 |
| THS | 130.5 ± 4.6 | 338.5 ± 12.0 | 42.8 |
| THD | 131.5 ± 4.7 | 339.5 ± 12.0 | 43.8 |
| GAS | 210.3 ± 7.5 | 418.3 ± 14.8 | 122.6 |
| GAD | 139.0 ± 5.1 | 347.0 ± 12.5 | 55.7 |
| TAR | 81.1 ± 3.0 | 289.1 ± 10.3 | −4.6 |
| MIR | 86.6 ± 3.1 | 294.6 ± 10.4 | −1.4 |
| CHS | 114.6 ± 4.1 | 322.6 ± 11.4 | 26.9 |
| OFN | 74.1 ± 2.6 | 282.1 ± 10.0 | −13.7 |
| OFT | 70.2 ± 2.5 | 278.2 ± 9.9 | −17.6 |
| OAN | 58.6 ± 2.1 | 266.6 ± 9.5 | −29.2 |
| OAT | 53.7 ± 1.9 | 261.7 ± 9.3 | −34.1 |
| OFE | 51.1 ± 1.8 | 259.1 ± 9.2 | −36.7 |
| OEE | 210.0 ±7.5 | 418.0 ± 14.8 | 122.3 |
| Model wine | 87.8 ± 3.1 | 295.8 ± 10.5 | 0.0 |
| Tannin Code | Total Phenols Estimation (w/w %) 1 | Turbidity (ΔNTU1) 2 | Turbidity (ΔNTU2) 2 | AP% | A420 | A520 | L* | a* | b* | ΔE | ORpt48 (mV vs. Ag/AgCl) | ORpt48 (mV vs. SHE) | ΔORpt48 (mV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIX 1 | 37.90 ± 0.02 | 334 ± 2 | 380 ± 3 | 89.12 ± 0.03 | 0.453 ± 0.001 | 0.119 ± 0.001 | 99.00 ± 0.00 | −0.33 ± 0.03 | 3.13 ± 0.01 | 3.14 | 144.0 ± 5.1 | 352.0 ± 12.5 | 56.2 |
| MIX 2 | 20.90 ± 0.01 | 108 ± 1 | 92 ± 1 | 90.86 ± 0.02 | 0.104 ± 0.000 | 0.049 ± 0.000 | 99.40 ± 0.01 | −0.08 ± 0.02 | 1.08 ± 0.02 | 1.31 | 27.2 ± 1.0 | 235.2 ± 8.6 | −60.6 |
| Model wine | - | - | - | - | - | - | 100.52 ± 0.05 | −0.02 ± 0.03 | 0.40 ± 0.01 | 0.00 | 87.8 ± 3.1 | 295.8 ± 10.5 | 0.0 |
| Tannin Code | Gallic Acid 1 | Castalagin 1 | (+)-Catechin 2 | (−)-Epicatechin 2 | Ellagic Acid 1 | Epicatechin Gallate 2 | Procyanidin 2 | Dimer Procyanidin 2 | Trimer Procyanidin 2 | Polymers 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| MIX 1 | 12.94 ± 0.19 b | 14.36 ± 0.75 | 5.28 ± 0.37 a | 4.66 ± 0.27 a | 3.77 ± 0.29 | nd | 13.19 ± 0.22 b | 14.06 ± 0.81 a | 0.22 ± 0.01 a | 45.32 ± 0.98 b |
| MIX 2 | 5.07 ± 0.08 a | nd | 35.12 ± 2.43 b | 39.31 ± 2.28 b | nd | 60.77 ± 2.64 | 12.60 ± 0.21 a | 29.44 ± 1.69 b | 1.38 ± 0.07 b | 22.75 ± 0.49 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maioli, F.; Sanarica, L.; Cecchi, L.; Zanoni, B.; Mulinacci, N.; Canuti, V. Characterization of 20 Oenological Tannins from Different Botanical Origins for Formulation of Blends with Redox Potential Tuning Ability in Model Wine Solution. Antioxidants 2023, 12, 1399. https://doi.org/10.3390/antiox12071399
Maioli F, Sanarica L, Cecchi L, Zanoni B, Mulinacci N, Canuti V. Characterization of 20 Oenological Tannins from Different Botanical Origins for Formulation of Blends with Redox Potential Tuning Ability in Model Wine Solution. Antioxidants. 2023; 12(7):1399. https://doi.org/10.3390/antiox12071399
Chicago/Turabian StyleMaioli, Francesco, Luigi Sanarica, Lorenzo Cecchi, Bruno Zanoni, Nadia Mulinacci, and Valentina Canuti. 2023. "Characterization of 20 Oenological Tannins from Different Botanical Origins for Formulation of Blends with Redox Potential Tuning Ability in Model Wine Solution" Antioxidants 12, no. 7: 1399. https://doi.org/10.3390/antiox12071399