Comparative Study on the Oxidative Stability of Encapsulated Fish Oil by Monoaxial or Coaxial Electrospraying and Spray-Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
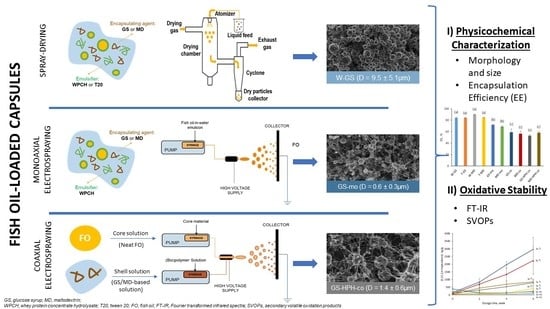
2.2. Production of the Spray-Dried Capsules
2.3. Production of the Electrosprayed Capsules
2.3.1. Monoaxially Electrosprayed Capsules
2.3.2. Coaxially Electrosprayed Capsules
2.4. Characterization of the Capsules
2.4.1. Morphology and Particle Size Distribution
2.4.2. Encapsulation Efficiency
2.5. Oxidative Stability
2.5.1. Fourier Transform Infrared Spectra (FT-IR) Analysis
2.5.2. Secondary Volatile Oxidation Products–Dynamic Headspace GC-MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Capsules
3.1.1. Morphology and Particle Size Distribution
3.1.2. Encapsulation Efficiency (EE)
3.2. Oxidative Stability of the Capsules
3.2.1. FT-IR
3.2.2. Secondary Volatile Oxidation Products (SVOPs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Ghelichi, S.; Hajfathalian, M.; García-Moreno, P.J.; Yesiltas, B.; Moltke-Sørensen, A.-D.; Jacobsen, C. Food Enrichment with Omega-3 Polyunsaturated Fatty Acid. In Omega-3 Delivery Systems; García-Moreno, P.J., Jacobsen, C., Sørensen, A.-D.M., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; ISBN 9780128213919. [Google Scholar]
- Rahmani-Manglano, N.E.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Pérez-Gálvez, A.R.; Guadix-Escobar, E.M. The Role of Antioxidants and Encapsulation Processes in Omega-3 Stabilization. In Emulsion-Based Encapsulation of Antioxidants. Food Bioactive Ingredients.; Aboudzadeh, M.A., Ed.; Springer: Cham, Swtzerland, 2020; pp. 339–386. ISBN 9783030620523. [Google Scholar]
- Turchiuli, C.; Jimenez Munguia, M.T.; Hernandez Sanchez, M.; Cortes Ferre, H.; Dumoulin, E. Use of Different Supports for Oil Encapsulation in Powder by Spray Drying. Powder Technol. 2014, 255, 103–108. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Guo, Q.; Nasef, N.; Singh, H. Aspects of Food Structure in Digestion and Bioavailability of LCn-3PUFA-Rich Lipids. In Omega-3 Delivery Systems; García-Moreno, P.J., Jacobsen, C., Sørensen, A.-D.M., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 427–448. ISBN 9780128213919. [Google Scholar]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of Bioactive Compounds through Electrospinning/Electrospraying and Spray Drying: A Comparative Assessment of Food-Related Applications. Dry. Technol. 2017, 35, 139–162. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schwarz, K. Chemical Stabilisation of Oils Rich in Long-Chain Polyunsaturated Fatty Acids during Homogenisation, Microencapsulation and Storage. Food Chem. 2009, 113, 1106–1112. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Rahmani-Manglano, N.E.; Chronakis, I.S.; Guadix, E.M.; Yesiltas, B.; Sørensen, A.-D.M.; Jacobsen, C. Omega-3 Nano-Microencapsulates Produced by Electrohydrodynamic Processing. In Omega-3 Delivery Systems. Production, Physical Characterization and Oxidative Stability; García-Moreno, P.J., Jacobsen, C., Sørensen, A.-D.M., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 345–370. ISBN 9780128213919. [Google Scholar]
- Jaworek, A.; Sobczyk, A.T. Electrospraying Route to Nanotechnology: An Overview. J. Electrostat. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Özdemir, N.; Stephansen, K.; Mateiu, R.V.; Echegoyen, Y.; Lagaron, J.M.; Chronakis, I.S.; Jacobsen, C. Development of Carbohydrate-Based Nano-Microstructures Loaded with Fish Oil by Using Electrohydrodynamic Processing. Food Hydrocoll. 2017, 69, 273–285. [Google Scholar] [CrossRef] [Green Version]
- García-Moreno, P.J.; Pelayo, A.; Yu, S.; Busolo, M.; Lagaron, J.M.; Chronakis, I.S.; Jacobsen, C. Physicochemical Characterization and Oxidative Stability of Fish-oil-loaded Electrosprayed Capsules: Combined Use of Whey Protein and Carbohydrates as Wall Materials. J. Food Eng. 2018, 231, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Prieto, C.; Lagaron, J.M. Nanodroplets of Docosahexaenoic Acid-Enriched Algae Oil Encapsulated within Microparticles of Hydrocolloids by Emulsion Electrospraying Assisted by Pressurized Gas. Nanomaterials 2020, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Gañán-Calvo, A.M. Micro/Nano Encapsulation via Electrified Coaxial Liquid Jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Tordera, F.; Fabra, M.J.; Martínez-Sanz, M.; Lopez-Rubio, A. Coaxial Electrospraying of Biopolymers as a Strategy to Improve Protection of Bioactive Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 2–11. [Google Scholar] [CrossRef]
- Padial-Domínguez, M.; Espejo-Carpio, F.J.; García-Moreno, P.J.; Jacobsen, C.; Guadix, E.M. Protein Derived Emulsifiers with Antioxidant Activity for Stabilization of Omega-3 Emulsions. Food Chem. 2020, 329, 127148. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Manglano, N.E.; González-Sánchez, I.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Jacobsen, C.; Guadix, E.M. Development of Fish-oil-loaded Microcapsules Containing Whey Protein Hydrolysate as Film-Forming Material for Fortification of Low-Fat Mayonnaise. Foods 2020, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Manglano, N.E.; Tirado-Delgado, M.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Influence of Emulsifier Type and Encapsulating Agent on the in Vitro Digestion of Fish-oil-loaded Microcapsules Produced by Spray-Drying. Food Chem. 2022, 392, 133257. [Google Scholar] [CrossRef] [PubMed]
- Stijnman, A.C.; Bodnar, I.; Hans Tromp, R. Electrospinning of Food-Grade Polysaccharides. Food Hydrocoll. 2011, 25, 1393–1398. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Damberg, C.; Chronakis, I.S.; Jacobsen, C. Oxidative Stability of Pullulan Electrospun Fibers Containing Fish Oil: Effect of Oil Content and Natural Antioxidants Addition. Eur. J. Lipid Sci. Technol. 2017, 119, 1600305. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, C.; García-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of Electrohydrodynamic Processing for Encapsulation of Sensitive Bioactive Compounds and Applications in Food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef]
- Siemons, I.; Politiek, R.G.A.; Boom, R.M.; van der Sman, R.G.M.; Schutyser, M.A.I. Dextrose Equivalence of Maltodextrins Determines Particle Morphology Development during Single Sessile Droplet Drying. Food Res. Int. 2020, 131, 108988. [Google Scholar] [CrossRef]
- Paximada, P.; Howarth, M.; Dubey, B. Electrosprayed Particles Derived from Nano-Emulsions as Carriers of Fish Oil. In Proceedings of the TechConnect World Innovation Conference & Expo, Anaheim, CA, USA, 13–1 May 2018; 3, pp. 1–4. [Google Scholar]
- Ramakrishnan, S.; Ferrando, M.; Aceña-Muñoz, L.; Mestres, M.; de Lamo-Castellví, S.; Güell, C. Influence of Emulsification Technique and Wall Composition on Physicochemical Properties and Oxidative Stability of Fish Oil Microcapsules Produced by Spray Drying. Food Bioproc. Tech. 2014, 7, 1959–1972. [Google Scholar] [CrossRef]
- Hogan, S.A.; McNamee, B.F.; O’Riordan, E.D.; O’Sullivan, M. Emulsification and Microencapsulation Properties of Sodium Caseinate/Carbohydrate Blends. Int. Dairy, J. 2001, 11, 137–144. [Google Scholar] [CrossRef]
- Drusch, S.; Berg, S. Extractable Oil in Microcapsules Prepared by Spray-Drying: Localisation, Determination and Impact on Oxidative Stability. Food Chem. 2008, 109, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Masiá, R.; Lagaron, J.M.; Lopez-Rubio, A. Morphology and Stability of Edible Lycopene-Containing Micro- and Nanocapsules Produced Through Electrospraying and Spray Drying. Food Bioproc. Tech. 2015, 8, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Mascaraque, L.G.; López-Rubio, A. Protein-Based Emulsion Electrosprayed Micro- and Submicroparticles for the Encapsulation and Stabilization of Thermosensitive Hydrophobic Bioactives. J. Colloid Interface Sci. 2016, 465, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillén, M.D.; Cabo, N. Usefulness of the Frequency Data of the Fourier Transform Infrared Spectra to Evaluate the Degree of Oxidation of Edible Oils. J. Agric. Food Chem. 1999, 47, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Guillen, M.D.; Cabo, N. Some of the Most Significant Changes in the Fourier Transform Infrared Spectra of Edible Oils under Oxidative Conditions. J. Sci. Food Agric. 2000, 80, 2028–2036. [Google Scholar] [CrossRef]
- Binsi, P.K.; Nayak, N.; Sarkar, P.C.; Jeyakumari, A.; Muhamed Ashraf, P.; Ninan, G.; Ravishankar, C.N. Structural and Oxidative Stabilization of Spray Dried Fish Oil Microencapsulates with Gum Arabic and Sage Polyphenols: Characterization and Release Kinetics. Food Chem. 2017, 219, 158–168. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Puthenveetil Kizhakkethil, B.; Annamalai, J.; Ninan, G.; Aliyamveetil Abubacker, Z.; Chandragiri Nagarajarao, R. Tuna Red Meat Hydrolysate as Core and Wall Polymer for Fish Oil Encapsulation: A Comparative Analysis. J. Food Sci. Technol. 2019, 56, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Headspace Volatile Aldehydes as Indicators of Lipid Oxidation in Foods. In Headspace Analysis of Food and Flavors: Theory and Practice; Plenum Publishers: New York, NY, USA, 2001; Volume 488, pp. 113–123. [Google Scholar]
- Boerekamp, D.M.W.; Andersen, M.L.; Jacobsen, C.; Chronakis, I.S.; García-Moreno, P.J. Oxygen Permeability and Oxidative Stability of Fish-oil-loaded Electrosprayed Capsules Measured by Electron Spin Resonance: Effect of Dextran and Glucose Syrup as Main Encapsulating Materials. Food Chem. 2019, 287, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakry, A.M.; Fang, Z.; Ni, Y.; Cheng, H.; Chen, Y.Q.; Liang, L. Stability of Tuna Oil and Tuna Oil/Peppermint Oil Blend Microencapsulated Using Whey Protein Isolate in Combination with Carboxymethyl Cellulose or Pullulan. Food Hydrocoll. 2016, 60, 559–571. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring Oxidative Stability and Changes in Key Volatile Compounds in Edible Oils during Ambient Storage through HS-SPME/GC–MS. Int. J. Food Prop. 2018, 20, S2926–S2938. [Google Scholar] [CrossRef] [Green Version]
- van Ruth, S.M.; Roozen, J.P. Release of Volatile Oxidation Products from Sunflower Oil and Its Oil-in-Water Emulsion in a Model Mouth System. ACS Symp. Ser. 2000, 763, 309–320. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, F.; Wang, B.; CHen, L.; Liu, W.; Tan, S. A Literature Review on Maillard Reaction Based on Milk Products: Advantages, Disadvantages and Avoidance Strategies. Foods 2021, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation Efficiency and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Different Combinations of Wall Materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Henna Lu, F.S.; Nielsen, N.S.; Jacobsen, C. Comparison of Two Methods for Extraction of Volatiles from Marine PL Emulsions. Eur. J. Lipid Sci. Technol. 2013, 115, 246–251. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani-Manglano, N.E.; Guadix, E.M.; Jacobsen, C.; García-Moreno, P.J. Comparative Study on the Oxidative Stability of Encapsulated Fish Oil by Monoaxial or Coaxial Electrospraying and Spray-Drying. Antioxidants 2023, 12, 266. https://doi.org/10.3390/antiox12020266
Rahmani-Manglano NE, Guadix EM, Jacobsen C, García-Moreno PJ. Comparative Study on the Oxidative Stability of Encapsulated Fish Oil by Monoaxial or Coaxial Electrospraying and Spray-Drying. Antioxidants. 2023; 12(2):266. https://doi.org/10.3390/antiox12020266
Chicago/Turabian StyleRahmani-Manglano, Nor E., Emilia M. Guadix, Charlotte Jacobsen, and Pedro J. García-Moreno. 2023. "Comparative Study on the Oxidative Stability of Encapsulated Fish Oil by Monoaxial or Coaxial Electrospraying and Spray-Drying" Antioxidants 12, no. 2: 266. https://doi.org/10.3390/antiox12020266