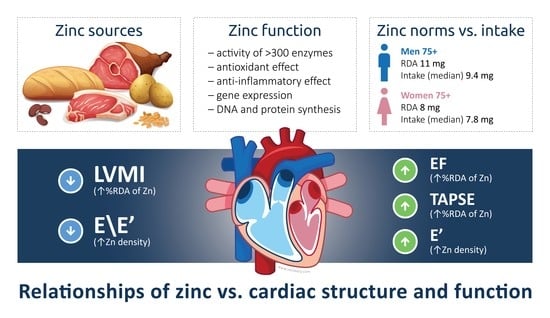
Dietary Zinc Is Associated with Cardiac Function in the Older Adult Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Zinc Intake
2.3. Echocardiography
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Baseline Characteristics of the Studied Population
4.2. Dietary Zinc Intake in the Elderly
4.3. Characteristics of the Cardiac Structure and Function of the Studied Population
4.4. Zinc and Cardiac Function
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- van Riet, E.E.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Maruyama, K.; Umesawa, M.; Tamakoshi, A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J. Nutr. Biochem. 2018, 56, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Romeo, J.; Malavolta, M.; Costarelli, L.; Giacconi, R.; Diaz, L.E.; Marcos, A. Zinc: Dietary intake and impact of supplementation on immune function in elderly. Age 2013, 35, 839–860. [Google Scholar] [CrossRef] [Green Version]
- Gać, P.; Czerwińska, K.; Macek, P.; Jaremków, A.; Mazur, G.; Pawlas, K.; Poręba, R. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ. Toxicol. Pharmacol. 2021, 82, 103553. [Google Scholar] [CrossRef] [PubMed]
- Darroudi, S.; Saberi-Karimian, M.; Tayefi, M.; Tayefi, B.; Khashyarmanesh, Z.; Fereydouni, N.; Haghighi, H.M.; Mahmoudi, A.A.; Kharazmi-Khorassani, J.; Gonoodi, K.; et al. Association Between Hypertension in Healthy Participants and Zinc and Copper Status: A Population-Based Study. Biol. Trace. Elem. Res. 2019, 190, 38–44. [Google Scholar] [CrossRef]
- Nakatani, S.; Mori, K.; Shoji, T.; Emoto, M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.F.; Polegato, B.F.; Fernandes, A.A.; Ishikawa, L.L.; Okoshi, K.; Bazan, S.G.Z.; Minicucci, M.F.; Azevedo, P.S.; Ikoma, M.R.; Penitenti, M.; et al. Zinc Supplementation Attenuates Cardiac Remodeling After Experimental Myocardial Infarction. Cell Physiol Biochem. 2018, 50, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.; Wessler, J.D.; Gupta, A.; Maurer, M.S.; Bikdeli, B. Zinc Deficiency and Heart Failure: A Systematic Review of the Current Literature. J. Card Fail. 2020, 26, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Alexanian, I.; Parissis, J.; Farmakis, D.; Athanaselis, S.; Pappas, L.; Gavrielatos, G.; Mihas, C.; Paraskevaidis, I.; Sideris, A.; Kremastinos, D.; et al. Clinical and echocardiographic correlates of serum copper and zinc in acute and chronic heart failure. Clin. Res. Cardiol. 2014, 103, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Sabbioni, E.; Fortaner, S.; Farina, M.; del Torchio, R.; Tafani, M.; Morgante, E.; Ciriolo, M.R.; Russo, M.A.; Chimenti, C. Selenium- and zinc-deficient cardiomyopathy in human intestinal malabsorption: Preliminary results of selenium/zinc infusion. Eur. J. Heart Fail. 2012, 14, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblum, H.; Bikdeli, B.; Wessler, J.; Gupta, A.; Jacoby, D.L. Zinc Deficiency as a Reversible Cause of Heart Failure. Tex. Heart Inst. J. 2020, 47, 152–154. [Google Scholar] [CrossRef]
- Huang, J.C.; Huang, Y.C.; Wu, P.Y.; Lee, W.H.; Tsai, Y.C.; Chen, Y.P.; Chen, S.C.; Su, H.M.; Chiu, Y.W.; Chang, J.M. Association between Reduced Serum Zinc and Diastolic Dysfunction in Maintenance Hemodialysis Patients. Nutrients 2021, 13, 2077. [Google Scholar] [CrossRef]
- Malekahmadi, M.; Firouzi, S.; Rezayi, M.; Ghazizadeh, H.; Ranjbar, G.; Ferns, G.A.; Mobarhan, M.G. Association of Zinc and Copper Status with Cardiovascular Diseases and their Assessment Methods: A Review Study. Mini. Rev. Med. Chem. 2020, 20, 2067–2078. [Google Scholar] [CrossRef]
- Yao, J.; Hu, P.; Zhang, D. Associations Between Copper and Zinc and Risk of Hypertension in US Adults. Biol. Trace. Elem. Res. 2018, 186, 346–353. [Google Scholar] [CrossRef]
- Yao, B.; Wang, Y.; Xu, L.; Lu, X.; Qu, H.; Zhou, H. Associations Between Copper and Zinc and High Blood Pressure in Children and Adolescents Aged 8-17 Years: An Exposure-Response Analysis of NHANES 2007-2016. Biol. Trace. Elem. Res. 2020, 198, 423–429. [Google Scholar] [CrossRef]
- Sauer, A.C.; Alish, C.J.; Strausbaugh, K.; West, K.; Quatrara, B. Nurses needed: Identifying malnutrition in hospitalized older adults. NursingPlus Open 2016, 2, 21–25. [Google Scholar] [CrossRef]
- Bartali, B.; Salvini, S.; Turrini, A.; Lauretani, F.; Russo, C.R.; Corsi, A.M.; Bandinelli, S.; D’Amicis, A.; Palli, D.; Guralnik, J.M.; et al. Age and disability affect dietary intake. J. Nutr. 2003, 133, 2868–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bzikowska-Jura, A.; Sobieraj, P.; Raciborski, F. Low Comparability of Nutrition-Related Mobile Apps against the Polish Reference Method-A Validity Study. Nutrients 2021, 13, 2868. [Google Scholar] [CrossRef] [PubMed]
- Kunachowicz, H.; Przygoda, B.; Iwanow, K.; Nadolna, I. Tabele Wartości Odżywczej Produktów Spożywczych I Potraw [Tables of the Composition and Nutritional Value Of Food]; PZWL: Warszawa, Poland, 2017. [Google Scholar]
- Jarosz, M.; Rychlik, E.; Stos, K.; Charzewska, J. [Nutrition Standards for the Polish Population and Their Application]; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2020.
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistics Poland. Information on the Situation of the Elderly in Poland in 2019, 2020th ed.; Statistics Poland: Warszawa, Poland, 2020; p. 585. Available online: https://das.mpips.gov.pl/source/2020/Informacja%20za%202019%20r.%2027.10.2020%20r..pdf (accessed on 2 January 2023).
- Gori, M.; Lam, C.S.; Gupta, D.K.; Santos, A.B.; Cheng, S.; Shah, A.M.; Claggett, B.; Zile, M.R.; Kraigher-Krainer, E.; Pieske, B.; et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 535–542. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868c. [Google Scholar] [CrossRef]
- Tromp, J.; Paniagua, S.M.A.; Lau, E.S.; Allen, N.B.; Blaha, M.J.; Gansevoort, R.T.; Hillege, H.L.; Lee, D.E.; Levy, D.; Vasan, R.S.; et al. Age dependent associations of risk factors with heart failure: Pooled population based cohort study. Bmj 2021, 372, n461. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace. Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Briefel, R.R.; Bialostosky, K.; Kennedy-Stephenson, J.; McDowell, M.A.; Ervin, R.B.; Wright, J.D. Zinc intake of the U.S. population: Findings from the third National Health and Nutrition Examination Survey, 1988-1994. J. Nutr. 2000, 130, 1367s–1373s. [Google Scholar] [CrossRef] [Green Version]
- Life Sciences Research Office; Federation of American Societies for Experimental Biology; Interagency Board for Nutrition Monitoring. Third Report on Nutrition Monitoring in the United States; Interagency Board for Nutrition Monitoring: Hyattsville, MD, USA, 1995; Volume 1. [Google Scholar]
- Tay, E.; Barnett, D.; Leilua, E.; Kerse, N.; Rowland, M.; Rolleston, A.; Waters, D.L.; Edlin, R.; Connolly, M.; Hale, L.; et al. The Diet Quality and Nutrition Inadequacy of Pre-Frail Older Adults in New Zealand. Nutrients 2021, 13, 2384. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Armah, S.M. Fractional Zinc Absorption for Men, Women, and Adolescents Is Overestimated in the Current Dietary Reference Intakes. J. Nutr. 2016, 146, 1276–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Xanthakis, V.; Sullivan, L.M.; Lieb, W.; Massaro, J.; Aragam, J.; Benjamin, E.J.; Vasan, R.S. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: Longitudinal observations from the Framingham Heart Study. Circulation 2010, 122, 570–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, F.F.; Coller, J.M.; McGrady, M.; Boffa, U.; Shiel, L.; Liew, D.; Stewart, S.; Owen, A.J.; Krum, H.; Reid, C.M.; et al. Age-related longitudinal change in cardiac structure and function in adults at increased cardiovascular risk. ESC Heart Fail. 2020, 7, 1344–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhard, C.; Stähli, B.E.; Gebhard, C.E.; Tasnady, H.; Zihler, D.; Wischnewsky, M.B.; Jenni, R.; Tanner, F.C. Age- and gender-dependent left ventricular remodeling. Echocardiography 2013, 30, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.L.; Gonçalves, A.; Shah, A.M.; Cheng, S.; Kitzman, D.; Solomon, S.D. Age- and Sex-Related Influences on Left Ventricular Mechanics in Elderly Individuals Free of Prevalent Heart Failure: The ARIC Study (Atherosclerosis Risk in Communities). Circ. Cardiovasc. Imaging 2017, 10, e004510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merz, A.A.; Cheng, S. Sex differences in cardiovascular ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef]
- Cauwenberghs, N.; Knez, J.; D’Hooge, J.; Thijs, L.; Yang, W.Y.; Wei, F.F.; Zhang, Z.Y.; Staessen, J.A.; Kuznetsova, T. Longitudinal Changes in LV Structure and Diastolic Function in Relation to Arterial Properties in General Population. JACC Cardiovasc Imaging 2017, 10, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Lindahl, B.; Venge, P.; Lind, L. Predictors of 10-year changes in levels of N-terminal pro B-type natriuretic peptide and cardiac troponin I in the elderly. Int. J. Cardiol. 2018, 257, 300–305. [Google Scholar] [CrossRef]
- González, A.; Ravassa, S.; López, B.; Moreno, M.U.; Beaumont, J.; San José, G.; Querejeta, R.; Bayés-Genís, A.; Díez, J. Myocardial Remodeling in Hypertension. Hypertension 2018, 72, 549–558. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.D.; Conte, J.V. The pathophysiology of heart failure. Cardiovasc Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008, 43, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Tuncay, E. Impact of Labile Zinc on Heart Function: From Physiology to Pathophysiology. Int. J. Mol. Sci. 2017, 18, 2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen-Redpath, K.; Ou, O.; Beattie, J.H.; Kwun, I.S.; Feldmann, J.; Nixon, G.F. Marginal dietary zinc deficiency in vivo induces vascular smooth muscle cell apoptosis in large arteries. Cardiovasc Res. 2013, 99, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, K.K.; Nikitin, N.P.; Parker, A.C.; von Haehling, S.; Volk, H.D.; Anker, S.D.; Clark, A.L.; Cleland, J.G. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur. Heart J. 2005, 26, 2238–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeejeebhoy, F.; Keith, M.; Freeman, M.; Barr, A.; McCall, M.; Kurian, R.; Mazer, D.; Errett, L. Nutritional supplementation with MyoVive repletes essential cardiac myocyte nutrients and reduces left ventricular size in patients with left ventricular dysfunction. Am. Heart J. 2002, 143, 1092–1100. [Google Scholar] [CrossRef]
- Huang, L.; Teng, T.; Bian, B.; Yao, W.; Yu, X.; Wang, Z.; Xu, Z.; Sun, Y. Zinc Levels in Left Ventricular Hypertrophy. Biol. Trace. Elem. Res. 2017, 176, 48–55. [Google Scholar] [CrossRef]
- Gorter, T.M.; van Veldhuisen, D.J.; Bauersachs, J.; Borlaug, B.A.; Celutkiene, J.; Coats, A.J.S.; Crespo-Leiro, M.G.; Guazzi, M.; Harjola, V.P.; Heymans, S.; et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: Mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 16–37. [Google Scholar] [CrossRef]
- Ferrara, F.; Rudski, L.G.; Vriz, O.; Gargani, L.; Afilalo, J.; D’Andrea, A.; D’Alto, M.; Marra, A.M.; Acri, E.; Stanziola, A.A.; et al. Physiologic correlates of tricuspid annular plane systolic excursion in 1168 healthy subjects. Int. J. Cardiol. 2016, 223, 736–743. [Google Scholar] [CrossRef]


| Variable | All (n = 251) | Male (n = 71) | Female (n = 180) | p (Male vs Female) |
|---|---|---|---|---|
| Age [years] | 80 (78–83) | 80 (78–84) | 80 (78–83) | ns |
| Education [years] | 16 (12–18) | 16 (12–18) | 16 (12–18) | ns |
| BMI [kg/m2] | 27.5 (25.2–30.1) | 27.6 (25.9–29.9) | 27.4 (24.6–30.2) | ns |
| WHtR | 0.59 (0.54–0.63) | 0.59 (0.56–0.63) | 0.59 (0.54–0.63) | ns |
| Systolic blood pressure [mmHg] | 135 (122–153) | 132 (118–156) | 136 (124–152) | ns |
| Diastolic blood pressure [mmHg] | 74 (67–81) | 73 (65–81) | 75 (68–82) | ns |
| Heart rate [beats/min] | 75 (68–80) | 75 (65–85) | 75 (68–80) | ns |
| Zinc [mg] | 8.22 (6.31–10.2) | 9.42 (7.55–11.7) | 7.82 (6.17–9.63) | p < 0.001 |
| Zinc [% RDA] | 94.8 (74.6–118) | 85.7 (68.7–106) | 97.7 (77.1–120.4) | 0.003 |
| Zinc density [mg/1000 kcal] | 5.71 (4.69–6.83) | 5.59 (4.68–7.04) | 5.74 (4.7–6.78) | ns |
| Zinc intake ≥ RDA, n (%) | 109 (43) | 25 (35) | 84 (47) | ns |
| Previous stroke; n (%) | 11 (4.4) | 3 (4.2) | 8 (4.4) | ns |
| Previous MI; n (%) | 13 (5.2) | 9 (12.7) | 4 (2.2) | p < 0.001 |
| Coronary artery disease; n (%) | 54 (21.5) | 26 (36.6) | 28 (15.6) | p < 0.001 |
| Hypertension; n (%) | 198 (78.9) | 56 (78.9) | 142 (79) | ns |
| Diabetes; n (%) | 43 (17.1) | 13 (18.3) | 30 (16.7) | ns |
| Heart failure; n (%) | 70 (27.9) | 13 (18.3) | 57 (31.7) | 0.034 |
| COPD; n (%) | 16 (6.4) | 5 (7) | 11 (6.1) | ns |
| Medications; n (%): | ||||
| ACEI/ARB | 158 (63) | 47 (66.2) | 111 (61.7) | ns |
| Beta-blockers | 154 (61.4) | 42 (59.2) | 112 (62.2) | ns |
| Calcium channel blockers | 87 (34.7) | 22 (31) | 65 (36.1) | ns |
| Diuretics | 89 (35.5) | 18 (25.4) | 71 (39.4) | 0.036 |
| MRA | 41 (16.3) | 14 (19.7) | 27 (15) | ns |
| Statins | 147 (58.6) | 43 (60.6) | 104 (57.8) | ns |
| Variable | All (n = 251) Median (Quartiles) | Male (n = 71) Median (Quartiles) | Female (n = 180) Median (Quartiles) | p (Male vs Female) |
|---|---|---|---|---|
| LA [cm] | 3.7 (3.4–4) | 4 (3.7–4.2) | 3.5 (3.3–3.9) | p < 0.001 |
| RVOT [cm] | 2.5 (2.3–2.6) | 2.7 (2.5–2.8) | 2.4 (2.3–2.5) | p < 0.001 |
| LAVI [mL/m2] | 27.08 (22.4–32.5 | 30.4 (24.4–35.6) | 25.8 (21.4–31.6) | p < 0.001 |
| LVEDV [mL] | 66 (53–80) | 83 (73–102) | 60 (48–71) | p < 0.001 |
| LVEDVI [mL/m2] | 38.7 (32.6–45.2) | 45.3 (40.4–54.1) | 35.6 (30.3–42.1) | p < 0.001 |
| LVESV [mL] | 26 (21–32) | 33 (30–40.5) | 23 (19–28) | p < 0.001 |
| LVESVI [mL/m2] | 15.3 (12.7–18.1) | 18 (15.8–21.9) | 14.1 (11.6–16.6) | p < 0.001 |
| LVEF [%] | 60 (58–62) | 60 (58–62) | 60 (58–62) | ns |
| RWT | 0.44 (0.41–0.47) | 0.44 (0.40–0.47) | 0.44 (0.41–0.48) | ns |
| LV mass [g] | 193 (160–227) | 237 (203–267) | 178 (155–208) | p < 0.001 |
| LVMI [g/m2] | 112 (99–128) | 125 (108–142) | 108 (95–121) | p < 0.001 |
| E [cm/s] | 59 (50–71) | 59 (50–70) | 59.5 (50–72) | ns |
| E/A ratio | 0.7 (0.58–0.81) | 0.68 (0.57–0.81) | 0.71 (0.58–0.81) | ns |
| E’ [cm/s] | 7 (6–8) | 7 (6–8) | 7 (6–8) | ns |
| E/E’ ratio | 8.62 (7.28–10.2) | 8.40 (7.0–10) | 8.64 (7.47–10.3) | ns |
| S’ [cm/s] | 9 (8–9.5) | 9 (8–10) | 8.5 (8–9) | 0.005 |
| TAPSE [mm] | 25 (23–27) | 26 (24–28) | 25 (23–27) | 0.007 |
| S’ RV [cm/s] | 15 (13–16) | 15 (13–16) | 15 (13–16) | ns |
| TR Vmax [m/s] | 2.55 (2.3–2.77) | 2.6 (2.3–2.77) | 2.55 (2.3–2.77) | ns |
| Variable | Zinc [%RDA] | Zinc [mg] /Zinc [%RDA] | Zinc Density [mg/1000 kcal] | Age [Years] | |||||
|---|---|---|---|---|---|---|---|---|---|
| All | Female | Male | All | Female | Male | All | Female | Male | |
| LA [cm] | 0.000 | 0.121 | 0.006 | −0.066 | −0.136 | 0.007 | 0.091 | 0.048 | 0.159 |
| RVOT [cm] | 0.021 | 0.133 | 0.120 | 0.038 | 0.046 | −0.068 | 0.057 | 0.001 | 0.135 |
| LAVI [mL/m2] | −0.024 | 0.056 | −0.091 | −0.028 | −0.032 | −0.072 | 0.169 ** | 0.203 ** | 0.012 |
| LVEDV [mL] | −0.036 | 0.079 | 0.134 | 0.021 | 0.018 | −0.031 | 0.003 | −0.027 | −0.105 |
| LVEDVI [mL/m2] | −0.038 | 0.035 | 0.124 | 0.016 | 0.004 | −0.013 | 0.029 | 0.035 | −0.119 |
| LVESV [mL] | −0.063 | 0.055 | 0.076 | 0.020 | 0.015 | −0.013 | 0.029 | 0.005 | −0.049 |
| LVESVI [mL/m2] | −0.072 | 0.004 | 0.050 | 0.015 | 0.005 | −0.010 | 0.055 | 0.064 | −0.077 |
| LVEF [%] | 0.196 * | 0.184 * | 0.178 | 0.069 | 0.047 | 0.115 | −0.147 * | −0.137 | −0.156 |
| RWT | −0.072 | −0.046 | −0.153 | −0.028 | −0.026 | −0.021 | 0.085 | 0.058 | 0.159 |
| LV mass [g] | −0.087 | 0.000 | 0.074 | 0.011 | −0.029 | −0.003 | 0.087 | 0.036 | 0.184 |
| LVMI [g/m2] | −0.137 * | −0.086 | −0.010 | −0.047 | −0.066 | −0.038 | 0.129 * | 0.105 | 0.181 |
| E [cm/s] | 0.089 | 0.086 | 0.062 | 0.029 | 0.054 | −0.012 | 0.063 | 0.055 | 0.109 |
| E/A ratio | 0.086 | 0.044 | 0.138 | 0.006 | 0.006 | 0.018 | −0.048 | −0.051 | −0.044 |
| E’ [cm/s] | 0.083 | 0.033 | 0.216 | 0.122 | 0.040 | 0.324 ** | −0.114 | −0.145 | −0.034 |
| E/E’ ratio | −0.074 | −0.049 | −0.189 | −0.127 * | −0.052 | −0.281 * | 0.191 ** | 0.230 ** | 0.106 |
| S’ [cm/s] | 0.079 | 0.133 | 0.073 | 0.095 | 0.048 | 0.205 | −0.041 | −0.028 | −0.098 |
| TAPSE [mm] | 0.153 * | 0.139 | 0.353 * | 0.021 | 0.033 | −0.027 | −0.005 | −0.062 | 0.113 |
| S’ RV [cm/s] | 0.046 | 0.032 | 0.096 | 0.078 | −0.015 | 0.289 * | 0.089 | 0.063 | 0.139 |
| TR Vmax [m/s] | −0.075 | −0.059 | −0.175 | −0.117 | −0.051 | −0.431 | 0.139 | 0.136 | 0.115 |
| Age [years] | −0.115 | −0.120 | −0.075 | −0.140 * | −0.200 ** | 0.005 | x | x | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szadkowska, I.; Kostka, T.; Wlazeł, R.N.; Kroc, Ł.; Jegier, A.; Guligowska, A. Dietary Zinc Is Associated with Cardiac Function in the Older Adult Population. Antioxidants 2023, 12, 265. https://doi.org/10.3390/antiox12020265
Szadkowska I, Kostka T, Wlazeł RN, Kroc Ł, Jegier A, Guligowska A. Dietary Zinc Is Associated with Cardiac Function in the Older Adult Population. Antioxidants. 2023; 12(2):265. https://doi.org/10.3390/antiox12020265
Chicago/Turabian StyleSzadkowska, Iwona, Tomasz Kostka, Rafał Nikodem Wlazeł, Łukasz Kroc, Anna Jegier, and Agnieszka Guligowska. 2023. "Dietary Zinc Is Associated with Cardiac Function in the Older Adult Population" Antioxidants 12, no. 2: 265. https://doi.org/10.3390/antiox12020265