P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Scotopic Electroretinogram (ERG) Analysis in Mice
2.3. Spectral-Domain Optical Coherence Tomography (SD-OCT) Imaging in Mice
2.4. Histology Analysis
2.5. Measurement of Cytokine Concentrations by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Cell Culture
2.7. Flow Cytometry Analysis
2.8. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.9. Immunoblotting
2.10. Determination of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Level
2.11. Statistical Analysis
3. Results
3.1. NaIO3-Induced Retinopathy Is Alleviated in P2X7 Knockout Mice
3.2. P2X7 Knockout Reduces NLRP3, IL-1β and IL-6 Gene Expression in Retinal Tissues after NaIO3 Injection
3.3. P2X7 Knockout Reduces Executional Caspases Expression in Retinal Tissues after NaIO3 Injection without Affecting NADP/NADPH
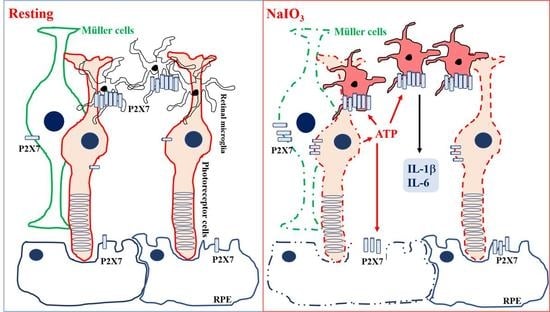
3.4. P2X7 Is Differentially Expressed in Retinal Cells and P2X7 Activation Increases NaIO3-Induced Cytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnstock, G. Historical review: ATP as a neurotransmitter. Trends Pharmacol. Sci. 2006, 27, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Seeland, S.; Kettiger, H.; Murphy, M.; Treiber, A.; Giller, J.; Kiss, A.; Sube, R.; Krahenbuhl, S.; Hafner, M.; Huwyler, J. ATP-induced cellular stress and mitochondrial toxicity in cells expressing purinergic P2X7 receptor. Pharmacol. Res. Perspect. 2015, 3, e00123. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar] [CrossRef]
- Hwang, S.M.; Koo, N.Y.; Choi, S.Y.; Chun, G.S.; Kim, J.S.; Park, K. P2X7 receptor-mediated membrane blebbing in salivary epithelial cells. Korean J. Physiol. Pharmacol. 2009, 13, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Sekar, P.; Huang, D.Y.; Hsieh, S.L.; Chang, S.F.; Lin, W.W. AMPK-dependent and independent actions of P2X7 in regulation of mitochondrial and lysosomal functions in microglia. Cell Commun. Signal. 2018, 16, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekar, P.; Huang, D.Y.; Chang, S.F.; Lin, W.W. Coordinate effects of P2X7 and extracellular acidification in microglial cells. Oncotarget 2018, 9, 12718–12731. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.P.; Herzlich, A.A.; Sauer, T.; Chan, C.C. Retinal anatomy and pathology. Dev. Ophthalmol. 2016, 55, 7–17. [Google Scholar] [CrossRef]
- Bok, D. The retinal pigment epithelium: A versatile partner in vision. J. Cell Sci. Suppl. 1993, 17, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Silverman, S.M.; Wong, W.T. Microglia in the retina: Roles in development, maturity, and disease. Annu. Rev. Vis. Sci. 2018, 4, 45–77. [Google Scholar] [CrossRef]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.A.; Kaur, C. Retinal microglia—A key player in healthy and diseased retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Role of microglial cells in photoreceptor degeneration. Neural Regen. Res. 2019, 14, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Wooff, Y.; Fernando, N.; Wong, J.H.C.; Dietrich, C.; Aggio-Bruce, R.; Chu-Tan, J.A.; Robertson, A.A.B.; Doyle, S.L.; Man, S.M.; Natoli, R. Caspase-1-dependent inflammasomes mediate photoreceptor cell death in photo-oxidative damage-induced retinal degeneration. Sci. Rep. 2020, 10, 2263. [Google Scholar] [CrossRef] [Green Version]
- Ventura, A.L.M.; Dos Santos-Rodrigues, A.; Mitchell, C.H.; Faillace, M.P. Purinergic signaling in the retina: From development to disease. Brain Res. Bull. 2019, 151, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, D. Targeting the P2X7 receptor in age-related macular degeneration. Vision 2017, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, E.L.; Wang, A.Y.; Jobling, A.I.; Rutar, M.V.; Greferath, U.; Gu, B.; Vessey, K.A. Targeting P2X7 receptors as a means for treating retinal disease. Drug Discov. Today 2019, 24, 1598–1605. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Filippo Drago, F.; Bucolo, C. The P2X7 receptor as a new pharmacological target for retinal diseases. Biochem. Pharmacol. 2022, 198, 114942. [Google Scholar] [CrossRef]
- Notomi, S.; Hisatomi, T.; Murakami, Y.; Terasaki, H.; Sonoda, S.; Asato, R.; Takeda, A.; Ikeda, Y.; Enaida, H.; Sakamoto, T.; et al. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS ONE 2013, 8, e53338. [Google Scholar] [CrossRef] [Green Version]
- Pavlou, S.; Augustine, J.; Cunning, R.; Harkin, K.; Stitt, A.W.; Xu, H.; Chen, M. Attenuating diabetic vascular and neuronal defects by targeting P2rx7. Int. J. Mol. Sci. 2019, 20, 2101. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; Krautloher, A.; et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. elife 2018, 7, e36217. [Google Scholar] [CrossRef] [PubMed]
- Clapp, C.; Diaz-Lezama, N.; Adan-Castro, E.; Ramirez-Hernandez, G.; Moreno-Carranza, B.; Sarti, A.C.; Falzoni, S.; Solini, A.; Di Virgilio, F. Pharmacological blockade of the P2X7 receptor reverses retinal damage in a rat model of type 1 diabetes. Acta Diabetol. 2019, 56, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Perez de Lara, M.J.; Aviles-Trigueros, M.; Guzman-Aranguez, A.; Valiente-Soriano, F.J.; de la Villa, P.; Vidal-Sanz, M.; Pintor, J. Potential role of P2X7 receptor in neurodegenerative processes in a murine model of glaucoma. Brain Res. Bull. 2019, 150, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Morigiwa, K.; Quan, M.; Murakami, M.; Yamashita, M.M.; Fukuda, Y. P2 purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci. Lett. 2000, 282, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Pannicke, T.; Fischer, W.; Biedermann, B.; Schadlich, H.; Grosche, J.; Faude, F.; Wiedemann, P.; Allgaier, C.; Illes, P.; Burnstock, G.; et al. P2X7 receptors in Muller glial cells from the human retina. J. Neurosci. 2000, 20, 5965–5972. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Iacovelli, J.; Spencer, C.; Saint-Geniez, M. Direct effect of sodium iodate on neurosensory retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef] [Green Version]
- Chowers, G.; Cohen, M.; Marks-Ohana, D.; Stika, S.; Eijzenberg, A.; Banin, E.; Obolensky, A. Course of sodium iodate-induced retinal degeneration in albino and pigmented mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2239–2249. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Xie, J.; Hu, J.; Huang, X.; Ren, F.; Li, L. Protective effect of hydrogen on sodium iodate-induced age-related macular degeneration in mice. Front. Aging Neurosci. 2018, 10, 389. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Correction to: Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 66. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Yang, F.; Ding, X.Q. Inhibition of thyroid hormone signaling protects retinal pigment epithelium and photoreceptors from cell death in a mouse model of age-related macular degeneration. Cell Death Dis. 2020, 11, 24. [Google Scholar] [CrossRef]
- Lin, F.L.; Lin, C.H.; Ho, J.D.; Yen, J.L.; Chang, H.M.; Chiou, G.C.; Cheng, Y.W.; Hsiao, G. The natural retinoprotectant chrysophanol attenuated photoreceptor cell apoptosis in an N-methyl-N-nitrosourea-induced mouse model of retinal degenaration. Sci. Rep. 2017, 7, 41086. [Google Scholar] [CrossRef] [Green Version]
- Balmer, J.; Zulliger, R.; Roberti, S.; Enzmann, V. Retinal cell death caused by sodium iodate involves multiple caspase-dependent and caspase-independent cell death pathways. Int. J. Mol. Sci. 2015, 16, 15086–15103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, G.L.; Amato, R.; Lazzara, F.; Porciatti, V.; Chou, T.H.; Drago, F.; Bucolo, C. P2X7 receptor antagonism preserves retinal ganglion cells in glaucomatous mice. Biochem. Pharmacol. 2020, 180, 114199. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.S.; Tang, Y.; Song, J.T. ATP and adenosine in the retina and retinal diseases. Front. Pharmacol. 2021, 12, 654445. [Google Scholar] [CrossRef] [PubMed]
- Vessey, K.A.; Fletcher, E.L. Rod and cone pathway signalling is altered in the P2X7 receptor knock out mouse. PLoS ONE 2012, 7, e29990. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, T.A.; Canning, P.; Tipping, N.; Archer, D.B.; Stitt, A.W. Abnormal glycogen storage by retinal neurons in diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8008–8018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756. [Google Scholar] [CrossRef]
- Thawkar, B.S.; Kaur, G. Inhibitors of NF-kappaB and P2X7/NLRP3/caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 2019, 326, 62–74. [Google Scholar] [CrossRef]
- Ferrari, D.; Los, M.; Bauer, M.K.; Vandenabeele, P.; Wesselborg, S.; Schulze-Osthoff, K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999, 447, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Mishra, A.; Krishnamurthy, S. Purinergic antagonism prevents mitochondrial dysfunction and behavioral deficits associated with dopaminergic toxicity induced by 6-OHDA in rats. Neurochem. Res. 2017, 42, 3414–3430. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Moll, V.; Milenkovic, I.; Faude, F.; Enzmann, V.; Wolf, S.; Reichenbach, A. Upregulation of P2X(7) receptor currents in Muller glial cells during proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2001, 42, 860–867. [Google Scholar]
- Jabs, R.; Guenther, E.; Marquordt, K.; Wheeler-Schilling, T.H. Evidence for P2X3, P2X4, P2X5 but not for P2X7 containing purinergic receptors in Muller cells of the rat retina. Brain Res. Mol. Brain Res. 2000, 76, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Trueblood, K.E.; Mohr, S.; Dubyak, G.R. Purinergic regulation of high-glucose-induced caspase-1 activation in the rat retinal Muller cell line rMC-1. Am. J. Physiol. Cell Physiol. 2011, 301, C1213–C1223. [Google Scholar] [CrossRef] [PubMed]
- Notomi, S.; Hisatomi, T.; Kanemaru, T.; Takeda, A.; Ikeda, Y.; Enaida, H.; Kroemer, G.; Ishibashi, T. Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am. J. Pathol. 2011, 179, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Corso, L.; Cavallero, A.; Baroni, D.; Garbati, P.; Prestipino, G.; Bisti, S.; Nobile, M.; Picco, C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016, 12, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Resta, V.; Novelli, E.; Di Virgilio, F.; Galli-Resta, L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development 2005, 132, 2873–2882. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Lu, W.; Zhang, M.; Zhang, X.; Argall, A.J.; Patel, S.; Lee, G.E.; Kim, Y.C.; Jacobson, K.A.; Laties, A.M.; et al. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp. Eye Res. 2010, 91, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J.D.; Broadway, D.C.; Sanderson, J. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2163–2170. [Google Scholar] [CrossRef] [Green Version]
- Puthussery, T.; Fletcher, E. Extracellular ATP induces retinal photoreceptor apoptosis through activation of purinoceptors in rodents. J. Comp. Neurol. 2009, 513, 430–440. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Wang, W.; Alatantuya; Dongmei; Lu, Z.-J.; Li, L.-L.; Zhang, T.-Z. Down-regulated miR-187 promotes oxidative stress-induced retinal cell apoptosis through P2X7 receptor. Int. J. Biol. Macromol. 2018, 120, 801–810. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Wang, W.; Jiang, Y.; Li, L.L.; Lu, Z.J.; Chang, H.; Zhang, T.Z. GRGM-13 comprising 13 plant and animal products, inhibited oxidative stress induced apoptosis in retinal ganglion cells by inhibiting P2RX7/p38 MAPK signaling pathway. Biomed. Pharmacother. 2018, 101, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; Isaac, A.R.; Silva, T.M.; Diniz, G.O.F.; Dos Santos Dabdab, Y.; Bockmann, E.C.; Guimaraes, M.Z.P.; da Costa Calaza, K.; de Mello, F.G.; Ventura, A.L.M.; et al. Cannabinoids induce cell death and promote P2X7 receptor signaling in retinal glial progenitors in culture. Mol. Neurobiol. 2019, 56, 6472–6486. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Clark, A.J.; Hughes, B.A.; Petty, H.R.; Elner, V.M. Activation of P2X receptors induces apoptosis in human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Schmidt, S.; Larsen, P.P.; Meyer, J.H.; Roush, W.R.; Latz, E.; Holz, F.G.; Krohne, T.U. Efficacy of novel selective NLRP3 inhibitors in human and murine retinal pigment epithelial cells. J. Mol. Med. 2019, 97, 523–532. [Google Scholar] [CrossRef]
- Guha, S.; Baltazar, G.C.; Coffey, E.E.; Tu, L.A.; Lim, J.C.; Beckel, J.M.; Patel, S.; Eysteinsson, T.; Lu, W.; O’Brien-Jenkins, A.; et al. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J. 2013, 27, 4500–4509. [Google Scholar] [CrossRef] [Green Version]
- Nie, Q.; Gong, X.; Gong, L.; Zhang, L.; Tang, X.; Wang, L.; Liu, F.; Fu, J.L.; Xiang, J.W.; Xiao, Y.; et al. Sodium iodate-induced mouse model of age-related macular degeneration displayed altered expression patterns of sumoylation enzymes E1, E2 and E3. Curr. Mol. Med. 2018, 18, 550–555. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, H.; Qin, S.; Liu, C.; Chen, Y.; Yang, Y.; Xu, C. The P2X7 receptor involved in gp120-induced cell injury in BV2 microglia. Inflammation 2016, 39, 1814–1826. [Google Scholar] [CrossRef]
- Huang, C.; Yu, W.; Cui, H.; Wang, Y.; Zhang, L.; Han, F.; Huang, T. P2X7 blockade attenuates mouse liver fibrosis. Mol. Med. Rep. 2014, 9, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Xie, Y.; Xue, Y.; Hu, N.; Zhang, G.; Guan, H.; Ji, M. Involvement of P2X7 receptors in retinal ganglion cell apoptosis induced by activated Muller cells. Exp. Eye Res. 2016, 153, 42–50. [Google Scholar] [CrossRef]
- Portillo, J.C.; Lopez Corcino, Y.; Dubyak, G.R.; Kern, T.S.; Matsuyama, S.; Subauste, C.S. Ligation of CD40 in human Muller cells induces P2X7 receptor-dependent death of retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6278–6286. [Google Scholar] [CrossRef] [Green Version]
- Kakurai, K.; Sugiyama, T.; Kurimoto, T.; Oku, H.; Ikeda, T. Involvement of P2X7 receptors in retinal ganglion cell death after optic nerve crush injury in rats. Neurosci. Lett. 2013, 534, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Sun, Q.; Xue, S.; Guan, H.; Ji, M. Activation of P2X7R- NLRP3 pathway in retinal microglia contribute to retinal ganglion cells death in chronic ocular hypertension (COH). Exp. Eye Res. 2019, 188, 107771. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Calippe, B.; Lavalette, S.; Roubeix, C.; Montassar, F.; Housset, M.; Levy, O.; Delarasse, C.; Paques, M.; Sahel, J.A.; et al. Upregulation of P2RX7 in Cx3cr1-deficient mononuclear phagocytes leads to increased interleukin-1beta secretion and photoreceptor neurodegeneration. J. Neurosci. 2015, 35, 6987–6996. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, P.; Hsiao, G.; Chen, Y.-S.; Lin, W.-W.; Chan, C.-M. P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types. Antioxidants 2023, 12, 141. https://doi.org/10.3390/antiox12010141
Sekar P, Hsiao G, Chen Y-S, Lin W-W, Chan C-M. P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types. Antioxidants. 2023; 12(1):141. https://doi.org/10.3390/antiox12010141
Chicago/Turabian StyleSekar, Ponarulselvam, George Hsiao, Yuan-Shen Chen, Wan-Wan Lin, and Chi-Ming Chan. 2023. "P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types" Antioxidants 12, no. 1: 141. https://doi.org/10.3390/antiox12010141