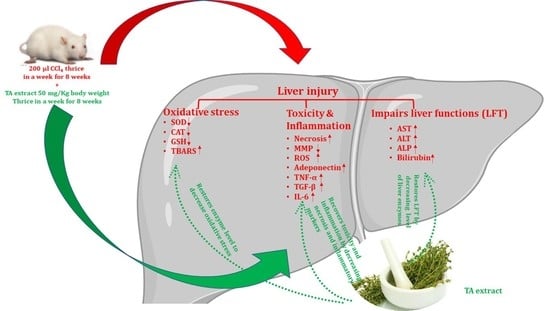
1. Introduction
The liver performs a key role in the regulation of numerous biochemical functions associated with metabolism [
1]. Liver injury is a multi-factorial disease caused by environmental and chemical toxins, drugs, and alcohol intake, which induce oxidative stress, leading to complicated pathophysiology. Typically, the end result of this is cirrhosis and hepatocellular carcinoma (HCC) [
2,
3]. The liver is rich in mitochondria that aid in aerobic metabolism through the electron transport chain (ETC). During the process of oxidative phosphorylation, reactive oxygen species (ROS) are produced, and this means that hepatocytes are highly susceptible to oxidative stress, which ultimately leads to hepatocellular damage. Owing to a number of side effects of the existing therapeutic modalities used for liver diseases, the management of hepatocellular diseases is often poor. Thus, the need to develop efficient, effective, and reliable hepatoprotective drugs from plant sources with fewer adverse effects is seemingly important [
4] This notion is supported by various reports that suggest that vegetarian diets and fruits from plant sources are rich in antioxidants. The consumption of such a diet drastically curtails the risk of developing chronic hepatocellular disorders [
5].
Environmental toxicants have been documented as inducing oxidative stress by promoting ROS production, which leads to liver injury, necrosis, and tissue damage. [
6]. One of the potent environmental toxicants is carbon tetrachloride (CCl
4). Upon the administration of CCl
4into the body orally or through systemic circulation, the detoxifying enzyme cytochrome P-450 present in the liver converts CCl
4into a more toxic form called the trichloromethyl radical (CCl
3+) through a process called biotransformation [
7]. In the presence of oxygen, CCl
3+ is converted into a highly reactive radical form called trichloromethylperoxy (CCl
3OO
+) [
8]. These highly reactive free radical species covalently bind with phospholipid membranes, which in turn induce lipid peroxidation that firstly impairs cell membrane integrity and secondly enhances the permeability of cells, thus causing severe damage to them [
9]. Owing to its toxic effects, the use of CCl
4is restricted, yet it is still one of the most widely used chemicals for the induction of hepatotoxicity in animal studies. It serves to evaluate the modulation of the pro-inflammatory and anti-scavenging effects of plant products abundant in antioxidants that could act as promising hepatoprotective agents [
10].
Recently, antioxidants obtained from various plant remedies have been introduced in therapeutics for liver fibrosis [
11]. Such anti-fibrotic therapies are used to modulate oxidative stress and have been entered into clinical trials [
12]. Morin, a flavonoid compound with antioxidant activity, primarily isolated from
Maclura pomifera, prevents CCl
4-mediated liver fibrosis by attenuating the levels of nitric oxide (NO) and MDA to restore the GSR of hepatocytes to its normal levels [
13]. Silymarin—a mixture of flavonolignan compounds—exhibits promising antioxidant activity. When administered orally, a 100 mg/kg silymarin dose prevents CCl
4-mediated liver fibrosis by reducing the MDA levels, restoring the GSR activity of hepatocytes, and preventing the depolarization of MMP to counter oxidative stress [
14]. Epigallocatechin (EGCG) is another polyphenolic compound with antioxidant activity that prevents liver fibrosis in a plethora of CCl
4-mediated animal models [
15]. Together, these studies suggest that natural antioxidant compounds show much potential to reverse fibrosis by attenuating oxidative stress, and preclinical investigations as part of clinical trials are required for future therapeutics.
Tamarix articulata (TA) is a halophytic plant (belongingto the family
Tamaricaceae) commonly found in the deserts of Saudi Arabia. Traditionally called “Athal” in the Arabic language, TA grows extremely well in drought, harsh, and arid conditions. The plant grows to a height of 15 m and a girth of 2 m, as described in our previously published study [
16]. Ethnobotanical studies have revealed that TA has been extensively used as folk medicine by the Tafilalet, a tribal population in the South-Eastern area of Morocco. Traditionally, the plant is used to treat various ailments, such as gastrointestinal, skin, heart diseases, and other ailments [
17]. Phytochemical analysis revealed the major constituents of TA extract thatdisplay pharmacological activities, as mentioned in our previous study [
18]. Crude extracts of the plant have been reported to exhibit anticancer activities by inhibiting cell viability in various types of cancer cells [
19]. Owing to the large amount of polyphenolic and flavonoid compounds, the methanolic extract of TA is apromising antioxidant with antiproliferative activities, as stated in our previous research [
16,
20]. Although TA exhibits some promising pharmacological activities, including antioxidant, antiproliferative, and antilipidemic activities [
16,
18,
19,
20,
21], there is not a single report suggesting that TA extract exhibits hepatoprotective activity. Therefore, the current study evaluates its hepatoprotective activity against the well-established carbontetrachloride-induced hepatotoxicity in Wistar rats. We found that TA extract reverses liver fibrosis induced by CCl
4in animals and restores pro-inflammatory as well as serum enzymes and antioxidant enzymes. Together, these results suggest that TA has a promising hepatoprotective effect and has much potential as a remedy against fibrosis and liver-associated diseases.
2. Materials and Methods
2.1. Chemicals, Reagents, and Kits
Carbon tetrachloride (CCl4) (#PHR1063), silymarin (#S0292), and other chemicals and reagents were purchased from Sigma Aldrich Chemicals Co., St. Louis, Missouri, United States. Kits for the pro-inflammatory (TGF-β #ab119558, TNF-α #ab236712, IL-6 #ab234570, and adiponectin #ab239421) and anti-inflammatory cytokine markers (SOD #ab65354, CAT #ab118184, GSR #ab65322, and TBARS/MDA #ab238537), liver function test (LFT) (AST #ab263882, ALT #234579, ALP #ab83369, and Bil #ab235627) enzymes to detect free radical scavenging activity, mitochondrial membrane potential (MMP) (MMP #ab113852), and ROS (ab #ab186027) detection were purchased from Abcam, Cambridge, United Kingdom.
2.2. Plant Material
TA plant was collected in August 2019 from Qassim Province in Saudi Arabia. The leaves of the TA plant were air-dried in the shade to remove all of the moisture [
20]. The data from our previous work identified the plant extracts, while the phytochemical analysis of major constituents of TA extracts displayed various pharmacological activities. A comparative assessment of different parts of the plant was conducted as described in our recently published studies [
18,
20,
21].
2.3. Preparation of Extract
Methanolic extract of TA was prepared as per the standard protocol [
21]. Dry TA leaves were collected from the floor and washed with distilled water. After cleaning, the TA leaves were shade-dried for 10 days to ensure complete drying followed by grinding in a kitchen blender to produce a fine powder. The dried TA leaf (100 g) powder was soaked in methanol (300 mL). Using the manual method of extraction, the mixture of dry leafpowder in methanol was constantly stirred on amagnetic stirrer at room temperature for 5 days. The obtained extract was filtered through Whatman filter paper and was concentrated by evaporating the solvent to attain a fine residue powder. The yield of the methanolic extract of the dry leaves of TA amounted to 8.33% and the dry powder of the extract was stored at 4 °C for future use.
2.4. Liquid Chromatography–Mass Spectrometry (LC–MS) Metabolomic Analysis and Data Processing
The analysis was carried out as described in our recently published studies [
18,
20,
21]. LC–MS metabolomic analysis was undertaken consisting of an ACQUITY UPLC I-Class System (Waters Technologies, Milford, MA, USA) coupled with a 6500Qtrap(AB Sciex, Concord, ON, Canada). Chromatographic separation was completed on a Zorbax XDB C18 column (2.1 × 150 mm. 3.5 µm) (Agilent, Santa Clara, CA, USA) maintained at 40 °C with a flow rate of 300 µL/min. The mobile phase consisted of A (0.1% formic acid in HPLC grade water) and B (0.1% formic acid in HPLC grade acetonitrile). The linear gradient elution was as follows: 2% B (from 0 to 2), 95% B (from 2 to 24), 95% B (held for 2 min), and then 4 min equilibration time. Electrospray ionization mass spectra (ESI-MS) were acquired in the positive mode (ES+), with an electrode voltage of 5500 V. The declustering potential was set to 90 V and the entrance potential was 10 V. Nitrogen was used as the curtain gas (30 psi) and nebulizer gas on the MS. Spectra were collected with a mass range of 100–900 m/z. Data files from the LC were converted to MZxml format using MS Convert (ProteoWizard 3.0.20270). Analysis of the data was conducted using MZ mine software (version 2.53). After importing the data into the MZ mine, a minimum intensity cutoff of 1000 was applied and the retention time was adjusted with a tolerance of 0.2 min. Adjusted peaks were then aligned to one mass list to facilitate identification and comparison. The KEGG Database was used to identify compounds of interest in the finalized list based on m/z with a tolerance of 30 ppm.
2.5. Experimental Animals
Male Wistar rats weighing 100–110 g were procured from KAUST, Saudi Arabia. All animals (Wistar rats) were acclimatized in the institute animal house for at least one week prior to experimentation with a 12 h dark and light cycle at room temperature. Animals were fed with a standard pellet diet and water ad libitum. This research was endorsed by the Ethics Committee of the College of Applied Medical Sciences, Qassim University (cams1-2019-1-14-s-3360).
2.6. CCl4-Induced Hepatotoxicity
The Wistar male rats acclimatized in the animal house were randomly grouped into 7 groups, with each group having 6 rats (42 Wistar rats) per experiment (n = 3, meaning 126 animals for the whole study). Animals were orally dosed with CCl
4as mentioned previously [
22,
23], and other respective concentrations of TA extracts (30, 40, and 50 mg/kg b.w.) using gastric gavage without the administration of any anesthesia agent. Group A was designated as normal, without any chemical or extract being administered. Group B was designated as the CCl
4-treated group and the animals were administered 40% CCl
4mixed in olive oil orally for 3 alternate days a week for 8 weeks. Group C was designated as 40% CCl
4mixed in olive oil and TA extract 30 mg/kg administered orally for 3 alternate days a week for 8 weeks. Group D was designated as 40% CCl
4mixed in olive oil and TA extract 40 mg/kg administered orally for 3 alternate days a week for 8 weeks. Group E was designed as 40% CCl
4mixed in olive oil and TA extract 50 mg/kg administered orally for 3 alternate days a week for 8 weeks. Group F was designated as 40% CCl
4mixed in olive oil and TA extract 60 mg/kg administered orally for 3 alternate days a week for 8 weeks. Group G was designed as 40% CCl
4mixed in olive oil and 100 mg/kg b.w. silymarin [
24] administered orally for 3 alternate days a week for 8 weeks (Table 3).
To evaluate the effective dose of TA extract with the least toxicity to the animals, we intended to perform a preliminary experiment on animals. This was performed to optimize the dose of TA extract that could exhibit effective hepatoprotective activity with less deleterious effects on animals. After properly acclimatizing the animals, we categorized them randomly into seven groups, as shown in Table 3. The disease control (Group B) animals were orally administered 200 µL 40% CCl4dissolved in olive oil for three alternate days a week for 8 weeks, whereas the control group (Group A) animals were orally administered 200 µL of vehicle. The other groups (Group C, D, E, and F) were orally administered 200 µL of 40% CCl4dissolved in olive oil and 200 µL of TA extract with varying doses (30, 40, 50, and 60 mg/kg b.w., respectively) for three alternate days a week for 8 weeks. However, Group G animals were orally administered 200 µL of standard silymarin compound for three alternate days a week for 8 weeks; this was in addition to 200 µL of40% CCl4dissolved in olive oil. To evaluate the effective dose in terms of the hepatoprotective effect and safe toxicity in animals, we observed the hepatoprotective activity of TA extract against CCl4-mediated liver toxicity in a dose-dependent manner (Tables 4 and 5). Intriguingly, our results suggest that the ideal activity of TA extract was determined at 50 mg/kg b.w. (Group E), and the results were significantly compared with those of standard silymarin (Group G). When tested at 60 mg/kg b.w. (Group F), TA extract displayed toxicity-associated symptoms in animals, such as lethargy, diarrhea, muscle tremors, loss of appetite, etc., and they died before the completion of the study (Table 4 and Table 5). Collectively, the preliminary optimization results suggest that a 50 mg/kg b.w. oral dose of TA extract has the maximum hepatoprotective effect against CCl4-mediated liver toxicity.
2.7. Evaluation of Liver Function Test
Following the completion of the treatment schedules, the animals in each group were sacrificed following methods approved by the Ethics Committee of the College of Applied Medical Sciences, Qassim University (cams1-2019-1-14-s-3360). Once the study was completed, diethyl ether was used to anesthetize the animals, followed by cervical dislocation and operating procedures to extract internal organs for the current study. The blood (1.0 mL) was collected from the tail vein before sacrificing the animals. The collected blood was allowed to clot and was centrifuged at 3000 rpm and 4 °C for 10 min [
25]. The supernatant (serum) was collected in sterile microfuge tubes and stored at −80 °C for the analysis of various biochemical parameters. The liver function enzyme levels of alkaline phosphatase (ALP), alanine transaminase (ALT), and aspartate aminotransferase (AST), and the bilirubin levels in serum were estimated as previously described [
26]. However, the quantification of pro-inflammatory cytokine markers (TNF-α, IL-6, and TGF-β) and the anti-inflammatory cytokine marker adiponectin levels were investigated with the use of an enzyme-linked immunosorbent assay (ELISA) kit.
2.8. Histopathological Analysis
The liver tissue was extracted from the animals of each group (n = 6) and thoroughly cleaned in PBS at pH 7.4. A small chunk of liver tissue was excised and fixed in 10% formalin to preserve the morphology of the cells from putrification. After processing the samples for paraffin blocks, the thin paraffin sections were cut with ultra-microtome. The sections were subjected to hematoxylin–eosin for inflammation and Masson’s trichome staining to analyze the histopathological changes in the collagen fibers of the hepatic tissue using a microscope [
27].
2.9. Detection of In Vivo Antioxidant Enzymes
Briefly, a small chunk of liver tissue (approximately 10%) was chopped into smaller pieces, followed by homogenization (Fisherband 150 efficient homogenizer, #15-340-167, Waltham, MA, USA) in a 2 mL sterile tube by using 1 mL of PBS (pH 7.4). The mixture obtained was subjected to centrifugation at 10,000 rpm for 10 min at 4 °C. The supernatant obtained was used to evaluate the antioxidant enzymes and pro-inflammatory markers. The enzymatic activity of catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GSR), and malondialdehyde (MDA) per milligram of the protein concentration of hepatic tissue homogenate was estimated utilizing a spectrophotometric method (Bradford method) [
28].
2.10. Determination of Reactive Oxygen Species (ROS)
After extracting the livers from the animals in each group (n = 6), part of the liver was excised, washed thoroughly with PBS followed by resuspension in culture media, and subjected to tissue dissociation with a tissue dissociator. After counting the hepatocytes using a hemocytometer (Neubauer chamber; #02-671-6, Waltham, MA, USA), 1 × 10
6 hepatocytes obtained were exposed to DCFH-DA for 15 min at 37 °C in a 5% CO
2incubator in the dark. The presence of free radicals or ROS converting DCFH-DA into dichlorofluorescein emits a green color; here, it was recorded with a fluorescence microplate reader at an excitation wavelength of 488 nm and emission wavelength of 525 nm [
29].
2.11. Determination of Depolarization Mitochondrial Membrane Potential (MMP)
Briefly, 1 × 10
6 hepatocytes obtained from the tissue dissociation process were exposed to JC-1 dye for 15 min at 37 °C in a 5% CO
2incubator in the dark. Immediately, suspended stained cell mixtures were analyzed by flow cytometry to evaluate the depolarization of MMP with anexcitation wavelength of 488 nm and emission wavelength of 590 nm [
29].
2.12. Statistical Analysis
All the results obtained represent the mean ± standard error of the mean (SEM), calculated and processed by one-way ANOVA. A p-value equal to or less than 0.05 was designated as significant.
4. Discussion
In the previous study, we reported that TA extract has promising antiproliferative and antioxidant activities. Owing to the presence of high contents of flavonoid and polyphenolic compounds in TA extract [
20], the present hypothesis supports the notion that TA extract might have hepatoprotective activity and anti-scavenging effect to restore antioxidant enzymes and neutralize ROS production induced by CCl
4. Therefore, the current study aimed to evaluate the hepatoprotective activity of TA extract against CCl
4-mediated hepatotoxicity in Wistar rats. Our results demonstrate that TA extract protects against CCl
4-mediated hepatotoxicity by restoring serum liver biochemistry, thereby regulating the level of the pro-inflammatory cytokines TNF-α, IL-6, and TGF-β. This was checked by elevating the level of the anti-inflammatory cytokine adiponectin. It attenuates ROS production and maintains the integrity of mitochondrial membrane potential, which ultimately aids in reducing the necrotic population and fibrosis of hepatocytes.
Among the environmental toxicants, CCl
4is considered one of the prominent toxicants used to study the hepatoprotective effect of various plant-based products for in vivo animal models [
39]. CCl
4, upon administration to the animal body, is converted into the more potent free radical CCl
3OO
+ with the help of a group of liver enzyme systems called cytochrome P-450 [
40]. This conversion of a toxicant substance into a more toxicant substance by liver enzymes is known as biotransformation [
41]. The free radical CCl
3OO
+ generated after the biotransformation process in the liver induces lipid peroxidation by interacting covalently with cellular macromolecules (membrane phospholipids) [
42]. This interaction causes the disruption of membrane integrity and results in pore formation in the cell membrane, thereby releasing the hepatic enzymes AST, ALP, ALT, and bilirubin from hepatocytes [
43]. Thus, CCl
4administration causes injury to hepatocytes and thereby elevates the levels of liver enzymes in serum [
29]. In the current study, we conducted preliminary experiments to evaluate the ideal dose of TA extract and the route of administration in terms of effective hepatoprotective activity with a safe toxicity profile in animals. Our preliminary results suggest that TA extract showed hepatoprotective activity against CCl
4-mediated liver toxicity in a dose-dependent manner. The optimum activity of TA extract was observed at 50 mg/kg b.w. orally (Group E); the results were significant compared with those obtained with 100 mg/kg b.w. standard silymarin. Additionally, we observed that a dose higher than 60 mg/kg b.w.c aused the animals to show toxicity symptoms, such as lethargy, diarrhea, muscle tremors, loss of appetite, etc., and they died before the completion of the study. The administration of 50 mg/kg b.w. of TA extract (Group E) restored the levels of serum enzymes of the liver to normal by inhibiting the potency of the CCl
3OO
+ free radical, which in turn reduced lipid peroxidation, as evidenced by the reduced level of MDA upon the treatment of animals with TA extract compared with those administered CCl
4 (Group B). The hepatoprotective activity of TA extract was further confirmed by the histopathology results of hepatic tissues. Remarkable hepatocellular damage was observed in animals administered CCl
4 (Group B) when compared with untreated animals (Group A). Microscopic analysis demonstrated that a significant number of necrotic populations of hepatocytes with large vacuolization, infiltration of inflammatory cells, portal biliary damage, and hepatocyte blooming were observed in animals administeredCCl
4orally (Group B). However, animals treated with an oral TA extract dose of 50 mg/kg b.w. (Group E) showed significant protection against CCl
4-mediated hepatotoxicity, which was accompanied by a reduction in the necrosis of hepatocytes, infiltrated cells, less vacuolization, and hepatocyte blooming.
Another striking feature of hepatic tissue damage caused by CCl
4is the induction of oxidative stress that affects the levels of antioxidant enzymes, such as SOD, CAT, and GSR [
44]. These enzymes together create an anti-scavenging system by catalyzing biochemical reactions to neutralize any free radicals generated under normal physiological conditions into nontoxic compounds to nullify any detrimental effects of toxic compounds, thus helping in the regulation of cell homeostasis [
45]. Upon the administration of CCl
4to animals, the generation of free radicals increased many-fold [
46]. This over-production of free radicals caused oxidative stress, which in turn affected the levels of antioxidant enzymes (SOD, CAT, and GSR). However, the animals treated with 50 mg/kg b.w. of TA extract (Group E) exhibited decreased CCl
4-mediated oxidative stress, specifically by elevating the levels of antioxidant enzymes significantly when compared with the CCl
4-treated group (Group B). This induction of antioxidant enzymes prevented oxidative stress by neutralizing the CCl
4-mediated generation of free radicals.
The oxidative stress is induced by CCl
4-stimulated liver fibrosis in animals dosed with CCl
4 [
47]. As evidenced by the high level of hydroxyproline, a major amino acid was found in collagen tissue by Masson’s trichome staining [
48]. Another striking feature of fibrotic tissue was the elevated expression of pro-inflammatory cytokines, which play a crucial role in the stimulation of portal fibroblasts, thereby aiding in the synthesis of more fibrous tissue and associated extracellular matrix substances [
49]. Herein, we observed, upon the administration of 50 mg/kg b.w. of TA extract (Group E), significantly decreased fibrosis markers, such as TNF-α, IL-6, and TGF-β, in serum samples when compared with CCL
4-treated animals (Group B). However, after treating the animals with 50 mg/kg b.w. of TA extract (Group E), we observed a significant increase in the serum level of the anti-inflammatory cytokine adiponectin. Collectively, these results suggest that TA extract has two effects: firstly, it restores the level of anti-inflammatory adiponectin, and secondly, it decreases the level of the pro-inflammatory cytokine proteins TNF-α, IL-6, and TGF-β to control CCl
4-mediated hepatocellular fibrosis and inflammation.
Besides other factors that cause the necrosis of hepatocytes, CCl
4administration induces ROS production and mitochondrial membrane potential (MMP) depolarization that lead to hepatic tissue damage and induce the necrosis of hepatocytes [
50]. In the current study, our results highlight that hepatocytes of rat livers initially dosed with CCl
4had significantly elevated levels of ROS and mitochondrial membrane depolarization when compared with hepatocytes obtained from livers from rats in the control group. However, the hepatocytes collected from the animal group treated with TA extract showed a remarkable reduction in ROS generation and inhibited depolarization of mitochondrial membrane potential when compared with the CCl
4-treated group and control group. Conversely, a significant reduction in ROS generation and the depolarization of mitochondrial membrane potential was observed for the groups treated with lower doses of TA extract (30 and 40 mg/kg b.w.; Groups C and D) when compared with the CCl
4-treated group (Group B) and control group (Group A). Therefore, in effect, our results demonstrate that TA extract effectively normalizes ROS generation and helps to protect hepatocytes by reducing the depolarization of the mitochondrial membrane potential. Together, these hepatoprotective outcomes may be due to the presence of bioactive compounds in the TA extract. Although the TA extracts exhibited a promising hepatoprotective effect against CCl
4-induced hepatotoxicity, the limitation of the study was the inability to evaluate the mechanism of action, pharmacokinetics, and bioavailability of its bioactive constituents.