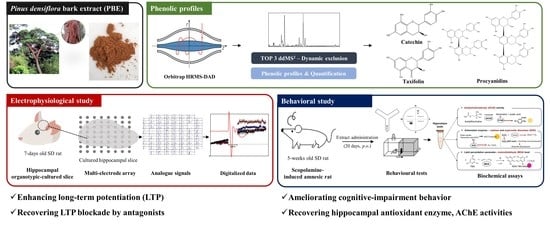
Effects of Phenolic-Rich Pinus densiflora Extract on Learning, Memory, and Hippocampal Long-Term Potentiation in Scopolamine-Induced Amnesic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Identification of Phenolic Compounds in PBE Using UHPLC-Orbitrap MS/MS
2.3. Quantification and the Quality Parameter of Phenolic Compounds in PBE Using UHPLC-DAD
2.4. Animals
2.5. Oral Administration of PBE
2.6. Behavioral Experiments
2.6.1. Y-maze Test
2.6.2. Step-through Passive Avoidance Test
2.6.3. Morris Water Maze Test
2.7. Biochemical Experiments
2.7.1. Collection of Rat Hippocampus
2.7.2. Measurement of SOD and Catalase Activity
2.7.3. Measurement of Total Lipid Oxidation
2.7.4. Measurement of AChE Activity
2.8. Electrophysiological Experiments Using Organotypic Hippocampal Slice Culture
2.8.1. Organotypic Hippocampal Slice Culture
2.8.2. Preparation of Organotypic Hippocampal Slice Tissue on the Microelectrode Array (MEA) Probes
2.8.3. Induction of LTP in Organotypic Hippocampal Slice Tissue
2.8.4. Electrophysiology Data Processing
2.9. Statistical Analysis
3. Results
3.1. Profiling and Determination of Phenolic Compounds Using UHPLC-Orbitrap MS
3.1.1. Identification of Phenolic Compounds Using UHPLC-Orbitrap MS
3.1.2. Quantification of Phenolic Compounds Using UHPLC-Orbitrap MS
3.2. In Vivo Effects of PBE on Memory Deficit in SCOP-Induced SD Rats
3.2.1. PBE Improved SCOP-induced Memory-Cognitive Behavior Disorder
3.2.2. PBE Improved SCOP-induced Enzymatic Antioxidant Defense System Defect and Inhibited AChE Activity
3.3. Effects of PBE on Long-term Synaptic Plasticity in Organotypic Hippocampal Slices
PBE Enhanced LTP and Rescued LTP Induction Failure by Synaptic Channel Antagonists in CA1 Hippocampal Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.W.; Im, S.; Jeong, H.R.; Jung, Y.S.; Lee, I.; Kim, K.J.; Park, S.K.; Kim, D.-O. Neuroprotective effects of Korean red pine (Pinus densiflora) bark extract and its phenolics. J. Microbiol. Biotechnol. 2018, 28, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, D.; Moldes, D.; Fuentealba, C.; Garcia, D.E. Condensed tannins from pine bark: A novel wood surface modifier assisted by laccase. Ind. Crops Prod. 2017, 103, 185–194. [Google Scholar] [CrossRef]
- Janeiro, P.; Brett, A.M.O. Catechin electrochemical oxidation mechanisms. Anal. Chim. Acta 2004, 518, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.; Go, G.W. Pinus densiflora bark extract (PineXol) decreases adiposity in mice by down-regulation of hepatic de novo lipogenesis and adipogenesis in white adipose tissue. J. Microbiol. Biotechnol. 2017, 27, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.J.; Hwang, E.S.; Kim, M.J.; Park, J.H.; Kim, D.-O. Antihypertensive effects of polyphenolic extract from Korean red pine (Pinus densiflora Sieb. et Zucc.) bark in spontaneously hypertensive rats. Antioxidants 2020, 9, 333. [Google Scholar] [CrossRef] [Green Version]
- Štrac, D.Š.; Mück-Šeler, D.; Pivac, N. Neurotransmitter measures in the cerebrospinal fluid of patients with Alzheimer’s disease: A review. Psychiatr. Danub. 2015, 27, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef]
- Petry, F.D.; Hoppe, J.B.; Klein, C.P.; dos Santos, B.G.; Hozer, R.M.; Bifi, F.; Matte, C.; Salbego, C.G.; Trindade, V.M.T. Genistein attenuates amyloid-beta-induced cognitive impairment in rats by modulation of hippocampal synaptotoxicity and hyperphosphorylation of Tau. J. Nutr. Biochem. 2021, 87, 108525. [Google Scholar] [CrossRef]
- Evans, P.H. Free radicals in brain metabolism and pathology. Br. Med. Bull. 1993, 49, 577–587. [Google Scholar] [CrossRef]
- El-Sherbiny, D.A.; Khalifa, A.E.; Attia, A.S.; Eldenshary Eel, D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol. Biochem. Behav. 2003, 76, 525–533. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bear, M.F.; Malenka, R.C. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 1994, 4, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Hwang, E.S.; Kim, K.J.; Maeng, S.; Heo, H.J.; Park, J.H.; Kim, D.-O. Anti-amnesic effects of epigallocatechin gallate on scopolamine-induced learning and memory dysfunction in Sprague-Dawley rats. Antioxidants 2021, 11, 1. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Ganzera, M.; Akgun, I.; Sevimli, C.; Korkmaz, K.S.; Bedir, E. Determination of polyphenolic constituents and biological activities of bark extracts from different Pinus species. J. Sci. Food Agric. 2009, 89, 1339–1345. [Google Scholar] [CrossRef]
- De la Luz Cádiz-Gurrea, M.; Fernández-Arroyo, S.; Segura-Carretero, A. Pine bark and green tea concentrated extracts: Antioxidant activity and comprehensive characterization of bioactive compounds by HPLC-ESI-QTOF-MS. Int. J. Mol. Sci. 2014, 15, 20382–20402. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free. Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Ho, I.K.; Ellman, G.L. Triton solubilized acetylcholinesterase of brain. J. Neurochem. 1969, 16, 1505–1513. [Google Scholar] [CrossRef]
- Stoppini, L.; Buchs, P.A.; Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 1991, 37, 173–182. [Google Scholar] [CrossRef]
- Enomoto, H.; Nirasawa, T. Localization of flavan-3-ol species in peanut testa by mass spectrometry imaging. Molecules 2020, 25, 2373. [Google Scholar] [CrossRef]
- Liu, R.X.; Zhao, Z.Y.; Dai, S.J.; Che, X.; Liu, W.H. Identification and quantification of bioactive compounds in Diaphragma juglandis fructus by UHPLC-Q-Orbitrap HRMS and UHPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 3811–3825. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bocalandro, C.; Sanhueza, V.; Gomez-Caravaca, A.M.; Gonzalez-Alvarez, J.; Fernandez, K.; Roeckel, M.; Rodriguez-Estrada, M.T. Comparison of the composition of Pinus radiata bark extracts obtained at bench- and pilot-scales. Ind. Crops Prod. 2012, 38, 21–26. [Google Scholar] [CrossRef]
- Sarria-Villa, R.A.; Gallo-Corredor, J.A.; Páse, M.I. Isolation of catechin and gallic acid from Colombian bark of Pinus patula. Chem. Sci. J. 2017, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Nisca, A.; Stefănescu, R.; Stegărus, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative study regarding the chemical composition and biological activity of pine (Pinus nigra and P. sylvestris) bark extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Rong, S.; Xie, B.; Sun, Z.; Zhang, L.; Wu, H.; Yao, P.; Zhang, Y.; Liu, L. Procyanidins extracted from the lotus seedpod ameliorate scopolamine-induced memory impairment in mice. Phytother. Res. 2009, 23, 1742–1747. [Google Scholar] [CrossRef]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Small, D.H.; Avila, J.; Sáez-Válero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with P-tau and β-amyloid. Front. Mol. Neurosci. 2011, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Bartus, R.T.; Dean, R.L., III; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Su, T.; Paradiso, B.; Long, Y.S.; Liao, W.P.; Simonato, M. Evaluation of cell damage in organotypic hippocampal slice culture from adult mouse: A potential model system to study neuroprotection. Brain Res. 2011, 1385, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, E.; Park, M.; Lee, E.; Namkoong, K. Organotypic hippocampal slice culture from the adult mouse brain: A versatile tool for translational neuropsychopharmacology. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 41, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Legradi, A.; Varszegi, S.; Szigeti, C.; Gulya, K. Adult rat hippocampal slices as in vitro models for neurodegeneration: Studies on cell viability and apoptotic processes. Brain Res. Bull. 2011, 84, 39–44. [Google Scholar] [CrossRef]
- Turovskaya, M.V.; Gaidin, S.G.; Mal’tseva, V.N.; Zinchenko, V.P.; Turovsky, E.A. Taxifolin protects neurons against ischemic injury in vitro via the activation of antioxidant systems and signal transduction pathways of GABAergic neurons. Mol. Cell. Neurosci. 2019, 96, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Olverman, H.J.; Jones, A.W.; Watkins, J.C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding-sites in rat-brain membranes. Nature 1984, 307, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.A.; Niu, Y.; Liu, Y.; Lee, N.H.; Hara, Y.; Thames, G.D.; Minutti, R.R.; Carpenter, C.L.; Wang, H.; Heber, D. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J. Nutr. Biochem. 2005, 16, 610–616. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Liu, C.S.; Nam, T.G.; Han, M.W.; Ahn, S.M.; Choi, H.S.; Kim, T.Y.; Chun, O.K.; Koo, S.I.; Kim, D.O. Protective effect of detoxified Rhus verniciflua stokes on human keratinocytes and dermal fibroblasts against oxidative stress and identification of the bioactive phenolics. Biosci. Biotechnol. Biochem. 2013, 77, 1682–1688. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agr. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agr. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.L.; Tuchilus, C.; Aprotosoaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Garcia, M.; Rosales-Castro, M. Effect of solvent and solvent-to-solid ratio on the phenolic extraction and the antioxidant capacity of extracts from Pinus durangensis and Quercus sideroxyla bark. Maderas-Cienc. Tecnol. 2016, 18, 701–714. [Google Scholar] [CrossRef] [Green Version]
- Chupin, L.; Maunu, S.L.; Reynaud, S.; Pizzi, A.; Charrier, B.; Charrier-ELBouhtoury, F. Microwave assisted extraction of maritime pine (Pinus pinaster) bark: Impact of particle size and characterization. Ind. Crops Prod. 2015, 65, 142–149. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Jeon, J.-S.; Um, B.-H. A comparison of pycnogenol and bark extracts from Pinus thunbergii and Pinus densiflora: Extractability, antioxidant activity and proanthocyanidin composition. J. Med. Plant Res. 2012, 6, 2839–2849. [Google Scholar] [CrossRef]
- Wood, J.E.; Senthilmohana, S.T.; Peskinb, A.V. Antioxidant activity of procyanidin-containing plant extracts at different pHs. Food Chem. 2002, 77, 155–161. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Procyanidins as antioxidants and tumor cell growth modulators. J. Agr. Food Chem. 2006, 54, 2392–2397. [Google Scholar] [CrossRef]
- Grzesik, M.; Namiesnik, J.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of ferrous catechin complexes. Free Radical Bio. Med. 2018, 120, S111. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]




| Peak | Retention Time (min) | Proposed Compound | Molecular Formula [M − H]− | Calculated Mass [M − H]− | Measured Mass [M − H]− | Δ ppm | MS2 Fragments (Relative Abundance, %) |
|---|---|---|---|---|---|---|---|
| 1 | 2.22 | Hydroxymandelic acid | C8H7O4− | 167.0345 | 167.0344 | −0.60 | 149.0235 (11), 139.0393 (26), 123.0444 (100) |
| 2 | 2.39 | Syringaldehyde | C9H9O4− | 181.0501 | 181.0500 | −0.55 | 151.0392 (19), 133.0287 (53), 123.0444 (100) |
| 3 | 2.61 | Protocatechuic acid a | C7H5O4− | 153.0188 | 153.0189 | 0.65 | 109.0288 (100) |
| 4 | 4.85 | Procyanidin B1 a | C30H25O12− | 577.1347 | 577.1337 | −1.73 | 451.1010 (8), 425.0853 (10), 407.0753 (80), 289.0702 (100), 245.0806 (16), 161.0235 (15), 125.0236 (38) |
| 5 | 5.13 | 3-p-Coumaroylquinic acid | C16H17O8− | 337.0924 | 337.0915 | −2.67 | 191.0556 (11), 173.0449 (5), 163.0395 (100), 155.0345 (3), 119.0497 (20) |
| 6 | 5.45 | Procyanidin B3 a | C30H25O12− | 577.1347 | 577.1337 | −1.73 | 451.1009 (7), 425.0853 (12), 407.0753 (90), 289.0702 (100), 245.0806 (12), 161.0235 (15), 125.0236 (42) |
| 7 | 5.66 | (+)-Catechin a | C15H13O6− | 289.0712 | 289.0706 | −2.08 | 245.0808 (100), 203.0704 (86), 125.0237 (60), 109.0289 (55) |
| 8 | 6.03 | Procyanidin trimer | C45H37O18− | 865.1980 | 865.1957 | −2.66 | 695.1343 (7), 577.1334 (9), 451.0993 (20), 425.0847 (22), 407.0753 (89), 289.0703 (100), 243.0287 (39), 125.0238 (62) |
| 9 | 6.47 | Caffeic acid a | C9H7O4− | 179.0345 | 179.0344 | −0.56 | 135.0443 (100) |
| 10 | 6.67 | Procyanidin trimer | C45H37O18− | 865.1980 | 865.1957 | −2.66 | 577.1334 (17), 451.1007 (20), 425.0864 (22), 407.0753 (85), 289.0703 (100), 243.0287 (38), 125.0238 (72) |
| 11 | 8.18 | (−)-Epicatechin b | C15H13O6− | 289.0712 | 289.0706 | −2.08 | 245.0808 (100), 203.0704 (86), 125.0237 (70), 109.0289 (62) |
| 12 | 8.46 | 4-p-Coumaroylquinic acid | C16H17O8− | 337.0924 | 337.0915 | −2.67 | 191.0553 (1), 173.0449 (100), 163.0394 (19), 155.0344 (5), 119.0497 (5) |
| 13 | 8.80 | Procyanidin trimer | C45H37O18− | 865.1980 | 865.1957 | −2.66 | 695.1345 (4), 577.1334 (17), 451.1021 (17), 425.0860 (18), 407.0753 (85), 289.0703 (100), 125.0238 (81) |
| 14 | 9.29 | Unknown | C19H29O11 | 433.1704 | 433.1694 | −1.33 | 181.0857 (100), 166.0623 (95) |
| 15 | 9.73 | Procyanidin B2 b | C30H25O12− | 577.1347 | 577.1337 | −1.73 | 451.1010 (7), 425.0853 (9), 407.0753 (68), 289.0702 (100), 245.0806 (16), 161.0235 (15), 125.0236 (38) |
| 16 | 10.01 | Taxifolin 3-O-glucoside | C21H21O12− | 465.1033 | 465.1020 | −2.80 | 447.0911 (20), 437.1066 (83), 304.0529 (2), 303.0494 (27), 285.0389 (70), 275.0547 (32), 259.0598 (42) |
| 17 | 11.71 | Taxifolin a | C15H11O7− | 303.0505 | 303.0502 | −0.99 | 285.0392 (48), 241.0493 (17), 217.0496 (19), 199.0392 (12), 175.0392 (27), 125.0237 (100) |
| 18 | 12.36 | Unknown | C26H33O12− | 537.1967 | 537.1958 | −1.59 | 524.0999 (1), 327.1220 (24), 315.1220 (100) |
| 19 | 13.12 | Taxifolin isomer | C15H11O7− | 303.0505 | 303.0502 | −0.99 | 285.0393 (47), 241.0493 (16), 217.0497(23), 199.0392 (15), 175.0393 (26), 125.0237 (100) |
| 20 | 16.45 | Dehydroxyltaxifolin | C15H11O6− | 287.0556 | 287.0549 | −2.44 | 259.0599 (87), 243.0651 (17), 125.0236 (100) |
| 21 | 18.71 | Quercetin 3-O-rhamnoside | C21H19O11− | 447.0928 | 447.0915 | −2.91 | 302.0374 (10), 301.0336 (85), 300.0261 (100), 174.9551 (30) |
| 22 | 22.21 | Quercetin 7-O-glucoside | C21H19O12− | 463.0877 | 463.0864 | −2.81 | 301.0338 (100), 174.9551 (9) |
| 23 | 25.08 | Quercetin b | C15H9O7− | 301.0349 | 301.0340 | −2.99 | 273.0389 (10), 178.9976 (50), 151.0028 (100) |
| Retention Time (min) | Phenolic Compound | Concentration (mg/g) a | Calibration Curve | Correlation Coefficient (R) | LOD b (μg/mL) | LOQ c (μg/mL) |
|---|---|---|---|---|---|---|
| 2.61 | Protocatechuic acid | 5.51 ± 0.00 | y = 10448.33x + 1472.27 | 0.9997 | 0.03 | 0.08 |
| 4.85 | Procyanidin B1 | 13.40 ± 0.18 | y = 1893.37x − 61.05 | 0.9993 | 0.25 | 0.77 |
| 5.45 | Procyanidin B3 | 3.75 ± 0.14 | y = 1863.07x − 24.93 | 0.9998 | 0.12 | 0.36 |
| 5.66 | (+)-Catechin | 10.08 ± 0.68 | y = 1995.57x + 129.22 | 0.9998 | 0.01 | 0.03 |
| 6.47 | Caffeic acid | 0.63 ± 0.01 | y = 14706.33x + 205.35 | 0.9996 | 0.05 | 0.15 |
| 8.18 | (−)-Epicatechin | 0.62 ± 0.01 | y = 1865.40x + 135.90 | 0.9997 | 0.09 | 0.27 |
| 9.73 | Procyanidin B2 | 0.80 ± 0.00 | y = 2351.27x − 89.67 | 0.9998 | 0.04 | 0.13 |
| 11.71 | Taxifolin | 12.91 ± 0.06 | y = 8428.27x + 85.21 | 0.9999 | 0.04 | 0.13 |
| 13.12 | Taxifolin isomer | 2.24 ± 0.01 | y = 8428.27x + 85.21 | 0.9999 | 0.04 | 0.13 |
| 25.08 | Quercetin | 0.26 ± 0.02 | y = 5315.23x − 58.53 | 0.9998 | 0.09 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.J.; Hwang, E.-S.; Kim, M.-J.; Rha, C.-S.; Song, M.C.; Maeng, S.; Park, J.-H.; Kim, D.-O. Effects of Phenolic-Rich Pinus densiflora Extract on Learning, Memory, and Hippocampal Long-Term Potentiation in Scopolamine-Induced Amnesic Rats. Antioxidants 2022, 11, 2497. https://doi.org/10.3390/antiox11122497
Kim KJ, Hwang E-S, Kim M-J, Rha C-S, Song MC, Maeng S, Park J-H, Kim D-O. Effects of Phenolic-Rich Pinus densiflora Extract on Learning, Memory, and Hippocampal Long-Term Potentiation in Scopolamine-Induced Amnesic Rats. Antioxidants. 2022; 11(12):2497. https://doi.org/10.3390/antiox11122497
Chicago/Turabian StyleKim, Kwan Joong, Eun-Sang Hwang, Min-Jeong Kim, Chan-Su Rha, Myoung Chong Song, Sungho Maeng, Ji-Ho Park, and Dae-Ok Kim. 2022. "Effects of Phenolic-Rich Pinus densiflora Extract on Learning, Memory, and Hippocampal Long-Term Potentiation in Scopolamine-Induced Amnesic Rats" Antioxidants 11, no. 12: 2497. https://doi.org/10.3390/antiox11122497