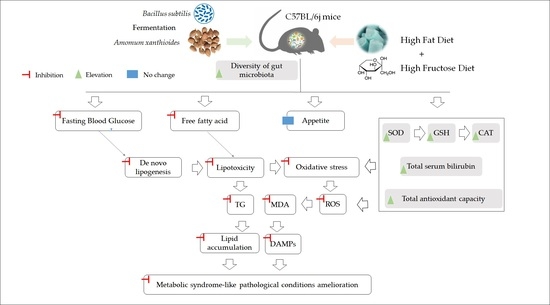
Bacillus subtilis-Fermented Amomum xanthioides Ameliorates Metabolic-Syndrome-Like Pathological Conditions in Long-Term HFHFD-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbal Medicine Preparation
2.2. HPLC Fingerprinting Analysis
2.3. Animal Experimental Design
2.4. Biochemical Analysis in Serum and Hepatic Tissue
2.5. Hepatic Lipid Peroxidation Determination
2.6. Determination of Serum and Hepatic Oxidative Stress and Antioxidative Parameters
2.7. Histopathological Observation and Oil Red O Staining
2.8. Western Blotting
2.9. Fecal Microbiota Analysis by 16s rRNA Gene Amplicon Sequencing
2.10. Statistical Analysis
3. Results
3.1. BSAX Exerted Anti-Obesity and Anti-Hyperlipidemic Effects Superior to Those of EFAX
3.2. BSAX Attenuated Abnormalities in Hepatic Enzymes and Lipid Parameters More Than EFAX
3.3. BSAX Attenuated Fasting Blood Hyperglycemia and Hyperinsulinemia
3.4. BSAX Restored Platelets, Leukocytes, and Lymphocytes
3.5. BSAX Attenuated Hepatic Steatosis More Than EFAX
3.6. BSAX Attenuated Oxidative Stress More Than EFAX
3.7. BSAX Expanded the Diversity of Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, H.J.; Ahn, Y.C.; Wang, J.-H.; Lee, M.M.; Son, C.G. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101526. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes-Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, G.E.; Kim, Y.H.; Han, K.; Jung, J.H.; Rhee, E.J.; Lee, S.S.; Kim, D.J.; Lee, K.W.; Lee, W.Y. Obesity Fact Sheet in Korea, 2019: Prevalence of Obesity and Abdominal Obesity from 2009 to 2018 and Social Factors. J. Obes. Metab. Syndr. 2020, 29, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021; in press. [Google Scholar] [CrossRef]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The Worldwide Obesity Epidemic. Obes. Res. 2001, 9, 228S–233S. [Google Scholar] [CrossRef]
- Lifshitz, F.; Lifshitz, J.Z. Globesity: The root causes of the obesity epidemic in the USA and now worldwide. Pediatr. Endocrinol. Rev. 2014, 12, 17–34. [Google Scholar]
- Suo, S.; Lai, Y.; Li, M.; Song, Q.; Cai, J.; Zhao, J.; Yang, Q.; Ung, C.O.L.; Hu, H. Phytochemicals, pharmacology, clinical application, patents, and products of Amomi fructus. Food Chem. Toxicol. 2018, 119, 31–36. [Google Scholar] [CrossRef]
- Im, H.J.; Hwang, S.J.; Lee, J.S.; Lee, S.B.; Kang, J.Y.; Son, C.G. Ethyl Acetate Fraction of Amomum xanthioides Ameliorates Nonalcoholic Fatty Liver Disease in a High-Fat Diet Mouse Model. Nutrients 2020, 12, 2433. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Wang, J.H.; Bose, S.; Kim, G.C.; Hong, S.U.; Kim, J.H.; Kim, J.E.; Kim, H. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PLoS ONE 2014, 9, e86117. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Bose, S.; Kim, H.G.; Han, K.S.; Kim, H. Fermented Rhizoma Atractylodis Macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci. Rep. 2015, 5, 8391. [Google Scholar] [CrossRef] [Green Version]
- Son, C.G.; Lee, S.K.; Choi, I.K.; Jang, E.S.; Bang, K.J. Herbal Transformation by Fermentation. J. Acupunct. Meridian Stud. 2020, 13, 167–168. [Google Scholar] [CrossRef]
- Kovács, Á.T. Bacillus subtilis. Trends Microbiol. 2019, 27, 724–725. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef]
- Moon, K.; Lee, S.; Cha, J. Bacillus subtilis Fermentation of Malva verticillata Leaves Enhances Antioxidant Activity and Osteoblast Differentiation. Foods 2020, 9, 671. [Google Scholar] [CrossRef]
- Kim, H.-K.; Choe, Y.-H.; Kim, G.-S.; Kim, H.-Y.; Kim, B.-S. Effect of Korean red ginseng marc fermented by Bacillus subtilis on swine immunity. Korean J. Vet. Serv. 2018, 41, 141–147. [Google Scholar] [CrossRef]
- Hsu, M.F.; Chiang, B.H. Stimulating effects of Bacillus subtilis natto-fermented Radix astragali on hyaluronic acid production in human skin cells. J. Ethnopharmacol. 2009, 125, 474–481. [Google Scholar] [CrossRef]
- Choi, Y.A.; Choi, J.K.; Jang, Y.H.; Lee, S.; Lee, S.R.; Choi, J.H.; Park, J.H.; Shin, T.Y.; Kim, S.H. Antiinflammatory effect of Amomum xanthioides in a mouse atopic dermatitis model. Mol. Med. Rep. 2017, 16, 8964–8972. [Google Scholar] [CrossRef] [Green Version]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin. Med. 2011, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Hwang, S.J.; Son, C.G. Comparative Analysis of the Antioxidative and Hepatoprotective Activities of Dimethyl Diphenyl Bicarboxylate in Four Animal Models of Hepatic Injury. Antioxidants 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Lee, S.-B.; Lee, D.-S.; Son, C.-G. Total Antioxidant Capacity in HBV Carriers, a Promising Biomarker for Evaluating Hepatic Fibrosis: A Pilot Study. Antioxidants 2021, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaki-Jouybari, F.; Shanaki, M.; Delfan, M.; Gorgani-Firouzjae, S.; Khakdan, S. High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Arch. Physiol. Biochem. 2020, 126, 242–249. [Google Scholar] [CrossRef]
- Wang, J.H.; Hwang, S.J.; Lim, D.W.; Son, C.G. Cynanchum atratum Alleviates Non-Alcoholic Fatty Liver by Balancing Lipogenesis and Fatty Acid Oxidation in a High-Fat, High-Fructose Diet Mice Model. Cells 2021, 11, 23. [Google Scholar] [CrossRef]
- Im, Y.R.; Hunter, H.; de Gracia Hahn, D.; Duret, A.; Cheah, Q.; Dong, J.; Fairey, M.; Hjalmarsson, C.; Li, A.; Lim, H.K.; et al. A Systematic Review of Animal Models of NAFLD Finds High-Fat, High-Fructose Diets Most Closely Resemble Human NAFLD. Hepatology 2021, 74, 1884–1901. [Google Scholar] [CrossRef]
- Jones, J.G. Hepatic glucose and lipid metabolism. Diabetologia 2016, 59, 1098–1103. [Google Scholar] [CrossRef] [Green Version]
- Muriel, P.; López-Sánchez, P.; Ramos-Tovar, E. Fructose and the Liver. Int. J. Mol. Sci. 2021, 22, 6969. [Google Scholar] [CrossRef]
- Pirimoğlu, B.; Sade, R.; Polat, G.; İşlek, A.; Kantarcı, M. Analysis of correlation between liver fat fraction and AST and ALT levels in overweight and obese children by using new magnetic resonance imaging technique. Turk. J. Gastroenterol. 2020, 31, 156–162. [Google Scholar] [CrossRef]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef]
- Qiu, H.; Schlegel, V. Impact of nutrient overload on metabolic homeostasis. Nutr. Rev. 2018, 76, 693–707. [Google Scholar] [CrossRef] [Green Version]
- James, W.P.T.; Johnson, R.J.; Speakman, J.R.; Wallace, D.C.; Frühbeck, G.; Iversen, P.O.; Stover, P.J. Nutrition and its role in human evolution. J. Intern. Med. 2019, 285, 533–549. [Google Scholar] [CrossRef] [Green Version]
- Mastorci, F.; Vassalle, C.; Chatzianagnostou, K.; Marabotti, C.; Siddiqui, K.; Eba, A.O.; Mhamed, S.A.S.; Bandopadhyay, A.; Nazzaro, M.S.; Passera, M.; et al. Undernutrition and Overnutrition Burden for Diseases in Developing Countries: The Role of Oxidative Stress Biomarkers to Assess Disease Risk and Interventional Strategies. Antioxidants 2017, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, B.; Lukic, S.; Mijac, D.; Marjanovic-Haljilji, M.; Vojnovic, M.; Bogdanovic, J.; Glisic, T.; Filipovic, N.; Al Kiswani, J.; Djokovic, A.; et al. The New Therapeutic Approaches in the Treatment of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 13219. [Google Scholar] [CrossRef]
- Han, J.; Li, E.; Chen, L.; Zhang, Y.; Wei, F.; Liu, J.; Deng, H.; Wang, Y. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature 2015, 524, 243–246. [Google Scholar] [CrossRef]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK Activation Reduces Hepatic Lipid Content by Increasing Fat Oxidation In Vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar] [CrossRef] [Green Version]
- Gillani, S.W.; Ghayedi, N.; Roosta, P.; Seddigh, P.; Nasiri, O. Effect of Metformin on Lipid Profiles of Type 2 Diabetes Mellitus: A Meta-analysis of Randomized Controlled Trials. J. Pharm. Bioallied Sci. 2021, 13, 76–82. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H.; Giles, W.H.; Brown, D.W. The Metabolic Syndrome and Antioxidant Concentrations: Findings From the Third National Health and Nutrition Examination Survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The Role of Nrf2 in Liver Disease: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2018, 9, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxidative Med. Cell. Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef] [Green Version]
- Samocha-Bonet, D.; Justo, D.; Rogowski, O.; Saar, N.; Abu-Abeid, S.; Shenkerman, G.; Shapira, I.; Berliner, S.; Tomer, A. Platelet counts and platelet activation markers in obese subjects. Mediat. Inflamm. 2008, 2008, 834153. [Google Scholar] [CrossRef] [Green Version]
- Fang, K.C.; Cheng, Y.L.; Su, C.W.; Wang, Y.J.; Lan, K.H.; Huo, T.I.; Huang, Y.H.; Chu, C.J.; Lin, C.C.; Hou, M.C.; et al. Higher platelet counts are associated with metabolic syndrome independent of fatty liver diagnosis. J. Chin. Med. Assoc. 2017, 80, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Mertoglu, C.; Gunay, M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S127–S131. [Google Scholar] [CrossRef]
- Hur, K.Y.; Lee, M.S. Gut Microbiota and Metabolic Disorders. Diabetes Metab. J. 2015, 39, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Zouiouich, S.; Loftfield, E.; Huybrechts, I.; Viallon, V.; Louca, P.; Vogtmann, E.; Wells, P.M.; Steves, C.J.; Herzig, K.-H.; Menni, C.; et al. Markers of metabolic health and gut microbiome diversity: Findings from two population-based cohort studies. Diabetologia 2021, 64, 1749–1759. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut microbiota phenotypes of obesity. npj Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Kim, H.G.; Kim, H.S.; Lee, J.S.; Im, H.J.; Kim, W.Y.; Son, C.G. Ethyl Acetate Fraction of Amomum xanthioides Exerts Antihepatofibrotic Actions via the Regulation of Fibrogenic Cytokines in a Dimethylnitrosamine-Induced Rat Model. Evid. Based Complement. Altern. Med. 2016, 2016, 6014380. [Google Scholar] [CrossRef]





| Contents | Normal | HFHFD | EFAX | BSAX | MET |
|---|---|---|---|---|---|
| Food intake (g/day/mouse) | 3.21 ± 0.07 | 1.99 ± 0.11 ## | 1.94 ± 0.08 | 1.90 ± 0.06 | 1.88 ± 0.10 |
| Fructose intake (mL/week/mouse) | 12.8 ± 1.0 | 11.3 ± 1.2 # | 11.0 ± 1.0 | 11.0 ± 0.3 | 10.5 ± 0.8 |
| FER (%) | 1.15 ± 0.20 | 6.33 ± 0.82 ## | 5.10 ± 1.43 | 4.12 ± 0.70 ** | 4.56 ± 1.84 |
| AST (IU/L) | 50.8 ± 5.5 | 83.3 ± 13.7 ## | 71.0 ± 13.8 | 61.4 ± 6.8 * | 66.6 ± 9.5 * |
| ALT (IU/L) | 16.4 ± 2.0 | 48.2 ± 17.2 ## | 31.3 ± 14.7 | 29.9 ± 5.3 * | 31.5 ± 13.8 |
| FFA (mEq/L) | 0.88 ± 0.11 | 1.02 ± 0.01 # | 0.89 ± 0.09 * | 0.91 ± 0.04 ** | 1.01 ± 0.22 |
| HOMA-IR | 0.54 ± 0.19 | 3.44 ± 1.11 ## | 1.78 ± 0.88 * | 0.85 ± 0.83 ** | 1.52 ± 1.24 * |
| Hematological Indices | Normal | HFHFD | EFAX | BSAX | MET |
|---|---|---|---|---|---|
| Erythrocytes (109/mL) | 8.0 ± 0.7 | 8.2 ± 0.4 | 8.4 ± 0.6 | 8.7 ± 0.8 | 8.8 ± 0.7 |
| Hemoglobin (g/dL) | 12.1 ± 1.1 | 12.5 ± 0.5 | 13.0 ± 1.1 | 13.3 ± 1.1 | 13.7 ± 1.2 |
| Platelets (106/mL) | 264 ± 147 | 630 ± 227 ## | 409 ± 314 | 377 ± 104 * | 300 ± 146 * |
| Leukocytes (106/mL) | 13.4 ± 3.5 | 6.1 ± 1.2 ## | 9.1 ± 2.8 * | 14.0 ± 2.6 ** | 12.3 ± 3.7 ** |
| Lymphocytes (106/mL) | 9.2 ± 2.3 | 4.3 ± 1.8 ## | 6.9 ± 2.0 * | 10.9 ± 1.9 ** | 9.4 ± 3.1 * |
| Platelet–lymphocyte ratio | 31.0 ± 17.7 | 163.2 ± 68.2 ## | 60.3 ± 36.4 * | 34.8 ± 9.8 ** | 34.6 ± 21.4 ** |
| Monocytes (106/mL) | 0.9 ± 0.4 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.9 ± 0.2 ** | 0.8 ± 0.2 ** |
| Granulocytes (106/mL) | 3.4 ± 1.0 | 1.8 ± 0.2 # | 1.7 ± 0.7 | 2.3 ± 0.5 * | 2.1 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-H.; Hwang, S.-J.; Shin, K.-S.; Lim, D.-W.; Son, C.-G. Bacillus subtilis-Fermented Amomum xanthioides Ameliorates Metabolic-Syndrome-Like Pathological Conditions in Long-Term HFHFD-Fed Mice. Antioxidants 2022, 11, 2254. https://doi.org/10.3390/antiox11112254
Wang J-H, Hwang S-J, Shin K-S, Lim D-W, Son C-G. Bacillus subtilis-Fermented Amomum xanthioides Ameliorates Metabolic-Syndrome-Like Pathological Conditions in Long-Term HFHFD-Fed Mice. Antioxidants. 2022; 11(11):2254. https://doi.org/10.3390/antiox11112254
Chicago/Turabian StyleWang, Jing-Hua, Seung-Ju Hwang, Kwang-Soo Shin, Dong-Woo Lim, and Chang-Gue Son. 2022. "Bacillus subtilis-Fermented Amomum xanthioides Ameliorates Metabolic-Syndrome-Like Pathological Conditions in Long-Term HFHFD-Fed Mice" Antioxidants 11, no. 11: 2254. https://doi.org/10.3390/antiox11112254