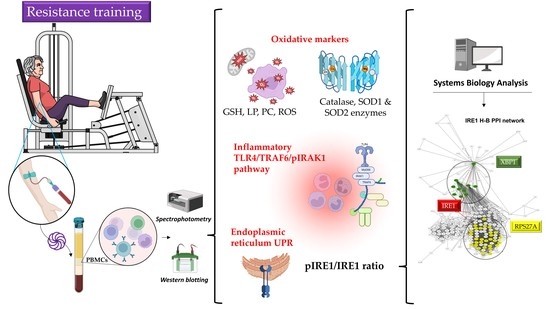
Resistance Training Modulates Reticulum Endoplasmic Stress, Independent of Oxidative and Inflammatory Responses, in Elderly People
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Characteristics
2.2. Maximal Strength Assessment
2.3. Resistance Exercise Training
2.4. Blood Sampling
2.5. Isolation of PBMCs
2.6. Markers of Oxidative Status
2.7. Western Blot Analysis
2.8. Statistical Analysis
2.9. In Silico Analysis
2.9.1. Network Design
2.9.2. Clustering and Gene Ontology Predictions
2.9.3. Centrality Parameter Analysis
3. Results
3.1. Anthropometric and Strength Measurements of the Study Participants
3.2. Oxidative Status, Inflammatory Response, and UPR Pathways at Baseline and Following 8-Week Resistance Training
3.3. Systems Biology Analysis of TLR4, IRE1, and Oxidative Stress Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.; Liu, Y.; Wong, N.K.; Xiao, J.; So, K.F. Oxidative stress in stem cell aging. Cell Transplant. 2017, 26, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Wang, W.; Su, D.M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estébanez, B.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Endoplasmic reticulum unfolded protein response, aging and exercise: An update. Front. Physiol. 2018, 9, 1744. [Google Scholar] [CrossRef] [Green Version]
- Estébanez, B.; Moreira, O.C.; Almar, M.; de Paz, J.A.; Gonzalez-Gallego, J.; Cuevas, M.J. Effects of a resistance-training programme on endoplasmic reticulum unfolded protein response and mitochondrial functions in PBMCs from elderly subjects. Eur. J. Sport Sci. 2019, 19, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An epigenetic role of mitochondria in cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Liu, J.; Reshetov, I.V.; Sukocheva, O.A.; et al. Advances in the prevention and treatment of obesity-driven effects in breast cancers. Front. Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef]
- Victor, P.; Sarada, D.; Ramkumar, K.M. Crosstalk between endoplasmic reticulum stress and oxidative stress: Focus on protein disulfide isomerase and endoplasmic reticulum oxidase 1. Eur. J. Pharmacol. 2021, 892, 173749. [Google Scholar] [CrossRef]
- Van Dam, L.; Dansen, T.B. Cross-talk between redox signalling and protein aggregation. Biochem. Soc. Trans. 2020, 48, 379–397. [Google Scholar] [CrossRef] [Green Version]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Naidoo, N.; Davis, J.G.; Zhu, J.; Yabumoto, M.; Singletary, K.; Brown, M.; Galante, R.; Agarwal, B.; Baur, J.A. Aging and sleep deprivation induce the unfolded protein response in the pancreas: Implications for metabolism. Aging Cell 2014, 13, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Chalil, S.; Jaspers, R.T.; Manders, R.J.; Klein-Nulend, J.; Bakker, A.D.; Deldicque, L. Increased endoplasmic reticulum stress in mouse osteocytes with aging alters Cox-2 response to mechanical stimuli. Calcif. Tissue Int. 2015, 96, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogborn, D.I.; McKay, B.R.; Crane, J.D.; Parise, G.; Tarnopolsky, M.A. The unfolded protein response is triggered following a single, unaccustomed resistance-exercise bout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R664–R669. [Google Scholar] [CrossRef]
- Gavilán, M.P.; Vela, J.; Castaño, A.; Ramos, B.; del Río, J.C.; Vitorica, J.; Ruano, D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol. Aging 2006, 27, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; de Macario, E.C.; Gammazza, A.M.; Bonaventura, G.; Carini, F.; Czarnecka, A.M.; Farina, F.; Zummo, G.; Macario, A.J. Hsp60 and human aging: Les liaisons dangereuses. Front. Biosci. 2013, 18, 626–637. [Google Scholar] [CrossRef] [Green Version]
- Chuang, A.W.; Kepp, O.; Kroemer, G.; Bezu, L. Endoplasmic reticulum stress in the cellular release of damage-associated molecular patterns. Int. Rev. Cell Mol. Biol. 2020, 350, 1–28. [Google Scholar] [CrossRef]
- Xiao, T.; Liang, X.; Liu, H.; Zhang, F.; Meng, W.; Hu, F. Mitochondrial stress protein HSP60 regulates ER stress-induced hepatic lipogenesis. J. Mol. Endocrinol. 2020, 64, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Li, X.L.; Wang, Y.L.; Zheng, J.; Zhang, Y.; Zhang, X.F. Inhibiting expression of HSP60 and TLR4 attenuates paraquat-induced microglial inflammation. Chem. Biol. Interact. 2019, 299, 179–185. [Google Scholar] [CrossRef]
- Grootjans, J.; Kaser, A.; Kaufman, R.J.; Blumberg, R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016, 16, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Ye, L.; Ling, M.; Ma, R.; Li, J.; Chen, H.; Pan, L. TLR4/TRAF6/NOX2 signaling pathway is involved in ventilation-induced lung injury via endoplasmic reticulum stress in murine model. Int. Immunopharmacol. 2021, 96, 107774. [Google Scholar] [CrossRef]
- Bi, F.; Liu, W.; Wu, Z.; Ji, C.; Chang, C. Antiaging factor Klotho retards the progress of intervertebral disc degeneration through the Toll-like receptor 4-NF-κB pathway. Int. J. Cell Biol. 2020, 2020, 8319516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Zhao, Y.; Sarkar, P.S.; Rosenblatt, K.P.; Tilton, R.G.; Choudhary, S. Klotho ameliorates chemically induced endoplasmic reticulum (ER) stress signaling. Cell Physiol. Biochem. 2013, 31, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.S.; Muir, K.R.; Doherty, M.; Jones, A.C.; O’Reilly, S.C.; Bassey, E.J. Home based exercise programme for knee pain and knee osteoarthritis: Randomised controlled trial. BMJ 2002, 325, 752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, M.D.; Rhea, M.R.; Sen, A.; Gordon, P.M. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res. Rev. 2010, 9, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Law, T.D.; Clark, L.A.; Clark, B.C. Resistance Exercise to prevent and manage sarcopenia and dynapenia. Annu. Rev. Gerontol. Geriatr. 2016, 36, 205–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beniamini, Y.; Rubenstein, J.J.; Faigenbaum, A.D.; Lichtenstein, A.H.; Crim, M.C. High-intensity strength training of patients enrolled in an outpatient cardiac rehabilitation program. J. Cardiopulm. Rehabil. 1999, 19, 8–17. [Google Scholar] [CrossRef]
- Castaneda, C.; Layne, J.E.; Munoz-Orians, L.; Gordon, P.L.; Walsmith, J.; Foldvari, M.; Roubenoff, R.; Tucker, K.L.; Nelson, M.E. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002, 25, 2335–2341. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, E.; Lee, I.M.; Bennie, J.; Freeston, J.; Hamer, M.; O’Donovan, G.; Ding, D.; Bauman, A.; Mavros, Y. Does strength-promoting exercise confer unique health benefits? A pooled analysis of data on 11 population cohorts with all-cause, cancer, and cardiovascular mortality endpoints. Am. J. Epidemiol. 2018, 187, 1102–1112. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Almar, M.; Mejías, Y.; Rivas, A.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Role of Toll-like receptor 2 and 4 signaling pathways on the inflammatory response to resistance training in elderly subjects. Age 2014, 36, 9734. [Google Scholar] [CrossRef]
- Mejías-Peña, Y.; Estébanez, B.; Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Almar, M.; de Paz, J.A.; González-Gallego, J.; Cuevas, M.J. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging 2017, 9, 408–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimauro, I.; Scalabrin, M.; Fantini, C.; Grazioli, E.; Beltran Valls, M.R.; Mercatelli, N.; Parisi, A.; Sabatini, S.; Di Luigi, L.; Caporossi, D. Resistance training and redox homeostasis: Correlation with age-associated genomic changes. Redox Biol. 2016, 10, 34–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Portilla-Cueto, K.; Medina-Pérez, C.; Romero-Pérez, E.M.; Hernández-Murúa, J.A.; Oliveira, C.E.P.; de Souza-Teixeira, F.; González-Bernal, J.J.; Vila-Chã, C.; de Paz, J.A. Reference values for isometric, dynamic, and asymmetry leg extension strength in patients with multiple sclerosis. Int. J. Environ. Res. Public Health 2020, 17, 8083. [Google Scholar] [CrossRef]
- Gearhart, R.F.; Lagally, K.M.; Riechman, S.E.; Andrews, R.D.; Robertson, R.J. Strength tracking using the OMNI resistance exercise scale in older men and women. J. Strength Cond. Res. 2009, 23, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, B.; Rodriguez, A.L.; Visavadiya, N.P.; Whitehurst, M.; Cuevas, M.J.; González-Gallego, J.; Huang, C.J. Aerobic training down-regulates Pentraxin 3 and Pentraxin 3/Toll-like receptor 4 ratio, irrespective of oxidative stress response, in elderly subjects. Antioxidants 2020, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Laliena, A.; San Miguel, B.; Crespo, I.; Alvarez, M.; González-Gallego, J.; Tuñón, M.J. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J. Pineal Res. 2012, 53, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scardoni, G.; Tosadori, G.; Faizan, M.; Spoto, F.; Fabbri, F.; Laudanna, C. Biological network analysis with CentiScaPe: Centralities and experimental dataset integration. F1000Research 2014, 3, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scardoni, G.; Laudanna, C. Centralities based analysis of complex networks. In New Frontiers in Graph Theory; Zhang, Y., Ed.; IntechOpen: London, UK, 2012; pp. 323–348. [Google Scholar]
- Boltz, T.A.; Devkota, P.; Wuchty, S. Collective influencers in protein interaction networks. Sci. Rep. 2019, 9, 3948. [Google Scholar] [CrossRef] [Green Version]
- Pawluk, H.; Pawluk, R.; Robaczewska, J.; Kędziora-Kornatowska, K.; Kędziora, J. Biomarkers of antioxidant status and lipid peroxidation in elderly patients with hypertension. Redox Rep. 2017, 22, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Yavuzer, H.; Yavuzer, S.; Cengiz, M.; Erman, H.; Doventas, A.; Balci, H.; Erdincler, D.S.; Uzun, H. Biomarkers of lipid peroxidation related to hypertension in aging. Hypertens. Res. 2016, 39, 342–348. [Google Scholar] [CrossRef]
- Maher, P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005, 4, 288–314. [Google Scholar] [CrossRef]
- Loguercio, C.; Taranto, D.; Vitale, L.M.; Beneduce, F.; Del Vecchio Blanco, C. Effect of liver cirrhosis and age on the glutathione concentration in the plasma, erythrocytes, and gastric mucosa of man. Free Radic. Biol. Med. 1996, 20, 483–488. [Google Scholar] [CrossRef]
- Lang, C.A.; Naryshkin, S.; Schneider, D.L.; Mills, B.J.; Lindeman, R.D. Low blood glutathione levels in healthy aging adults. J. Lab. Clin. Med. 1992, 120, 720–725. [Google Scholar]
- Toroser, D.; Sohal, R.S. Age-associated perturbations in glutathione synthesis in mouse liver. Biochem. J. 2007, 405, 583–589. [Google Scholar] [CrossRef]
- Chen, C.N.; Brown-Borg, H.M.; Rakoczy, S.G.; Ferrington, D.A.; Thompson, L.V. Aging impairs the expression of the catalytic subunit of glutamate cysteine ligase in soleus muscle under stress. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, N.; Das, S.; Mahapatra, S.K.; Chakraborty, S.P.; Kundu, P.K.; Roy, S. Age associated oxidative damage in lymphocytes. Oxidative Med. Cell. Longev. 2010, 3, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Pansarasa, O.; Bertorelli, L.; Vecchiet, J.; Felzani, G.; Marzatico, F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic. Biol. Med. 1999, 27, 617–622. [Google Scholar] [CrossRef]
- Lu, C.Y.; Lee, H.C.; Fahn, H.J.; Wei, Y.H. Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat. Res. 1999, 423, 11–21. [Google Scholar] [CrossRef]
- Allen, R.G.; Keogh, B.P.; Gerhard, G.S.; Pignolo, R.; Horton, J.; Cristofalo, V.J. Expression and regulation of superoxide dismutase activity in human skin fibroblasts from donors of different ages. J. Cell Physiol. 1995, 165, 576–587. [Google Scholar] [CrossRef]
- Guemouri, L.; Artur, Y.; Herbeth, B.; Jeandel, C.; Cuny, G.; Siest, G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin. Chem. 1991, 37, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Núñez, V.M.; Ruiz-Ramos, M.; Sánchez-Rodríguez, M.A.; Retana-Ugalde, R.; Muñoz-Sánchez, J.L. Aging-related oxidative stress in healthy humans. Tohoku J. Exp. Med. 2007, 213, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Junqueira, V.B.; Barros, S.B.; Chan, S.S.; Rodrigues, L.; Giavarotti, L.; Abud, R.L.; Deucher, G.P. Aging and oxidative stress. Mol. Asp. Med. 2004, 25, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Kasapoglu, M.; Ozben, T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp. Gerontol. 2001, 36, 209–220. [Google Scholar] [CrossRef]
- Niwa, Y.; Iizawa, O.; Ishimoto, K.; Akamatsu, H.; Kanoh, T. Age-dependent basal level and induction capacity of copper-zinc and manganese superoxide dismutase and other scavenging enzyme activities in leukocytes from young and elderly adults. Am. J. Pathol. 1993, 143, 312–320. [Google Scholar] [PubMed]
- Marzani, B.; Felzani, G.; Bellomo, R.G.; Vecchiet, J.; Marzatico, F. Human muscle aging: ROS-mediated alterations in rectus abdominis and vastus lateralis muscles. Exp. Gerontol. 2005, 40, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Medinas, D.B.; Rozas, P.; Traub, F.M.; Woehlbier, U.; Brown, R.H.; Bosco, D.A.; Hetz, C. Endoplasmic reticulum stress leads to accumulation of wild-type SOD1 aggregates associated with sporadic amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, 8209–8214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecocci, P.; Polidori, M.C.; Troiano, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Straatman, M.; Monti, D.; Stahl, W.; Sies, H.; et al. Plasma antioxidants and longevity: A study on healthy centenarians. Free Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef]
- Chandwaney, R.; Leichtweis, S.; Leeuwenburgh, C.; Ji, L.L. Oxidative stress and mitochondrial function in skeletal muscle: Effects of aging and exercise training. Age 1998, 21, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.L.; Dillon, D.; Wu, E. Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am. J. Physiol. 1990, 258, R918–R923. [Google Scholar] [CrossRef]
- Zuo, Z.; Lei, H.; Wang, X.; Wang, Y.; Sonntag, W.; Sun, Z. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Age 2011, 33, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Sullivan-Gunn, M.J.; Lewandowski, P.A. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. 2013, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Sudheesh, N.P.; Ajith, T.A.; Ramnath, V.; Janardhanan, K.K. Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin. Nutr. 2010, 29, 406–412. [Google Scholar] [CrossRef]
- Estébanez, B.; Visavadiya, N.P.; de Paz, J.A.; Whitehurst, M.; Cuevas, M.J.; González-Gallego, J.; Huang, C.J. Resistance training diminishes the expression of exosome CD63 protein without modification of plasma miR-146a-5p and cfDNA in the elderly. Nutrients 2021, 13, 665. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirupathi, A.; De Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ma, J.; Li, J.; Wang, D.; Wang, Z.; Wang, S. Mitochondrial phosphatase PGAM5 modulates cellular senescence by regulating mitochondrial dynamics. Nat. Commun. 2020, 11, 2549. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-stimulated ROS sensitive signaling pathways in skeletal muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Bouzid, M.A.; Filaire, E.; McCall, A.; Fabre, C. Radical oxygen species, exercise and aging: An update. Sports Med. 2015, 45, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate intensity resistive training reduces oxidative stress and improves muscle mass and function in older individuals. Antioxidants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, B.; Sblendorio, V. Oxidative stress tests: Overview on reliability and use. Part I Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 309–342. [Google Scholar]
- Pesce, M.; Tatangelo, R.; La Fratta, I.; Rizzuto, A.; Campagna, G.; Turli, C.; Ferrone, A.; Franceschelli, S.; Speranza, L.; Patruno, A.; et al. Aging-related oxidative stress: Positive effect of memory training. Neuroscience 2018, 370, 246–255. [Google Scholar] [CrossRef]
- Morucci, G.; Ryskalin, L.; Pratesi, S.; Branca, J.J.V.; Modesti, A.; Modesti, P.A.; Gulisano, M.; Gesi, M. Effects of a 24-week exercise program on functional fitness, oxidative stress, and salivary cortisol levels in elderly subjects. Medicina 2022, 58, 1341. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Wang, S.; Sun, Q.W.; Zhang, B.; Ullah, M.; Sun, Z. Klotho deficiency causes heart aging via impairing the Nrf2-GR pathway. Circ. Res. 2021, 128, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Guo, H.; Meng, S.; Zhu, B.; Fang, J.; Huang, J.; Chen, J.; Wang, Y.; Wang, L.; Yao, X.; et al. Klotho ameliorates diabetic nephropathy by activating Nrf2 signaling pathway in podocytes. Biochem. Biophys. Res. Commun. 2021, 534, 450–456. [Google Scholar] [CrossRef]
- Guo, Y.; Zhuang, X.; Huang, Z.; Zou, J.; Yang, D.; Hu, X.; Du, Z.; Wang, L.; Liao, X. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Zhai, Y.; Ao, L.; Cleveland, J.C.; Liu, H.; Fullerton, D.A.; Meng, X. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget 2017, 8, 15663–15676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Espuch-Oliver, A.; de Haro, T.; Gutierrez, A.; Ruiz, J.R.; Castillo, M.J. Exercise training increases the S-Klotho plasma levels in sedentary middle-aged adults: A randomised controlled trial. The FIT-AGEING study. J. Sports Sci. 2019, 37, 2175–2183. [Google Scholar] [CrossRef]
- Matsubara, T.; Miyaki, A.; Akazawa, N.; Choi, Y.; Ra, S.G.; Tanahashi, K.; Kumagai, H.; Oikawa, S.; Maeda, S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H348–H355. [Google Scholar] [CrossRef] [Green Version]
- Ji, N.; Luan, J.; Hu, F.; Zhao, Y.; Lv, B.; Wang, W.; Xia, M.; Zhao, X.; Lao, K. Aerobic exercise-stimulated Klotho upregulation extends life span by attenuating the excess production of reactive oxygen species in the brain and kidney. Exp. Ther. Med. 2018, 16, 3511–3517. [Google Scholar] [CrossRef] [Green Version]
- Mytych, J.; Sołek, P.; Będzińska, A.; Rusinek, K.; Warzybok, A.; Tabęcka-Łonczyńska, A.; Koziorowski, M. Towards age-related anti-inflammatory therapy: Klotho suppresses activation of er and golgi stress response in senescent monocytes. Cells 2020, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Morton, J.P.; Maclaren, D.P.; Cable, N.T.; Campbell, I.T.; Evans, L.; Kayani, A.C.; McArdle, A.; Drust, B. Trained men display increased basal heat shock protein content of skeletal muscle. Med. Sci. Sports Exerc. 2008, 40, 1255–1262. [Google Scholar] [CrossRef]
- Morton, J.P.; MacLaren, D.P.; Cable, N.T.; Bongers, T.; Griffiths, R.D.; Campbell, I.T.; Evans, L.; Kayani, A.; McArdle, A.; Drust, B. Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. J. Appl. Physiol. 2006, 101, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Khassaf, M.; Child, R.B.; McArdle, A.; Brodie, D.A.; Esanu, C.; Jackson, M.J. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J. Appl. Physiol. 2001, 90, 1031–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakic, B.; Carlsson, M.; Buszko, M.; Cappellano, G.; Ploner, C.; Onestingel, E.; Foti, M.; Hackl, H.; Demetz, E.; Dietrich, H.; et al. The Effects of endurance exercise and diet on atherosclerosis in young and aged ApoE-/- and wild-type mice. Gerontology 2019, 65, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative stress in exercise training: The involvement of inflammation and peripheral signals. Free Radic. Res. 2019, 53, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, R.P.; Royes, L.F.F.; Gonzalez-Gallego, J.; Bresciani, G. Oxidative stress and inflammation: Liver responses and adaptations to acute and regular exercise. Free Radic. Res. 2017, 51, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Banday, M.; Qadri, O.; Bashir, A.; Hilal, N.; Nida-I-Fatima; Rader, S.; Fazili, K.M. The molecular mechanism and functional diversity of UPR signaling sensor IRE1. Life Sci. 2021, 265, 118740. [Google Scholar] [CrossRef]
- Maurel, M.; Chevet, E.; Tavernier, J.; Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014, 39, 245–254. [Google Scholar] [CrossRef]
- Tokutake, Y.; Yamada, K.; Hayashi, S.; Arai, W.; Watanabe, T.; Yonekura, S. IRE1-XBP1 pathway of the unfolded protein response is required during early differentiation of C2C12 myoblasts. Int. J. Mol. Sci. 2019, 21, 182. [Google Scholar] [CrossRef] [Green Version]
- Sha, H.; Yang, L.; Liu, M.; Xia, S.; Liu, Y.; Liu, F.; Kersten, S.; Qi, L. Adipocyte spliced form of X-box-binding protein 1 promotes adiponectin multimerization and systemic glucose homeostasis. Diabetes 2014, 63, 867–879. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xie, Y.; Zhao, K.; Chen, K.; Cao, Y.; Zhang, J.; Han, M.; Hu, L.; He, R.; Wang, D.; et al. Endoplasmic reticulum stress exacerbates inflammation in chronic rhinosinusitis with nasal polyps via the transcription factor XBP1. Clin. Immunol. 2021, 223, 108659. [Google Scholar] [CrossRef]
- Park, S.M.; Kang, T.I.; So, J.S. Roles of XBP1s in transcriptional regulation of target genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Cai, M.Y.; Ma, K.; Yang, J.; Zhou, J.; Fu, W.; Wei, F.Z.; Wang, L.; Xie, D.; et al. XBP-1u suppresses autophagy by promoting the degradation of FoxO1 in cancer cells. Cell Res. 2013, 23, 491–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.; Li, Y.; Yang, J.; Wang, G.; Margariti, A.; Jiang, Z.; Yu, H.; Zampetaki, A.; Hu, Y.; Xu, Q.; et al. Unspliced X-box-binding protein 1 (XBP1) protects endothelial cells from oxidative stress through interaction with histone deacetylase 3. J. Biol. Chem. 2014, 289, 30625–30634. [Google Scholar] [CrossRef]
- Huang, C.; Wu, S.; Ji, H.; Yan, X.; Xie, Y.; Murai, S.; Zhao, H.; Miyagishi, M.; Kasim, V. Identification of XBP1-u as a novel regulator of the MDM2/p53 axis using an shRNA library. Sci. Adv. 2017, 3, e1701383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Fu, Y.; Cai, Z.; Yu, F.; Gong, Z.; Dai, R.; Hu, Y.; Zeng, L.; Xu, Q.; Kong, W. Unspliced XBP1 confers VSMC homeostasis and prevents aortic aneurysm formation via FoxO4 interaction. Circ. Res. 2017, 121, 1331–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Dai, R.; Wu, H.; Cai, Z.; Xie, N.; Zhang, X.; Shen, Y.; Gong, Z.; Jia, Y.; Yu, F.; et al. Unspliced XBP1 counteracts β-catenin to inhibit vascular calcification. Circ. Res. 2022, 130, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Riepe, C.; Zelin, E.; Frankino, P.A.; Meacham, Z.A.; Fernandez, S.G.; Ingolia, N.T.; Corn, J.E. Double stranded DNA breaks and genome editing trigger loss of ribosomal protein RPS27A. FEBS J. 2022, 289, 3101–3114. [Google Scholar] [CrossRef]
- Redman, K.L.; Rechsteiner, M. Identification of the long ubiquitin extension as ribosomal protein S27a. Nature 1989, 338, 438–440. [Google Scholar] [CrossRef]
- Zheng, N.; Shabek, N. Ubiquitin ligases: Structure, function, and regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Kopito, R.R.; Christianson, J.C. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb. Perspect. Biol. 2013, 5, a013185. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Chen, X.; Lee, A.H.; Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010, 11, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Savic, S.; Ouboussad, L.; Dickie, L.J.; Geiler, J.; Wong, C.; Doody, G.M.; Churchman, S.M.; Ponchel, F.; Emery, P.; Cook, G.P.; et al. TLR dependent XBP-1 activation induces an autocrine loop in rheumatoid arthritis synoviocytes. J. Autoimmun. 2014, 50, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Joe, Y.; Kim, H.J.; Kim, Y.S.; Jeong, S.O.; Pae, H.O.; Ryter, S.W.; Surh, Y.J.; Chung, H.T. Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J. Immunol. 2015, 194, 4498–4506. [Google Scholar] [CrossRef] [PubMed]
- Gendrisch, F.; Völkel, L.; Fluck, M.; Apostolova, P.; Zeiser, R.; Jakob, T.; Martin, S.F.; Esser, P.R. IRE1 and PERK signaling regulates inflammatory responses in a murine model of contact hypersensitivity. Allergy 2022, 77, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Mao, L.; Yang, L.; Zou, J.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget 2017, 8, 36449–36461. [Google Scholar] [CrossRef] [Green Version]
- Foley, K.P.; Chen, Y.; Barra, N.G.; Heal, M.; Kwok, K.; Tamrakar, A.K.; Chi, W.; Duggan, B.M.; Henriksbo, B.D.; Liu, Y.; et al. Inflammation promotes adipocyte lipolysis via IRE1 kinase. J. Biol. Chem. 2021, 296, 100440. [Google Scholar] [CrossRef]
- Roy, A.; Tomaz da Silva, M.; Bhat, R.; Bohnert, K.R.; Iwawaki, T.; Kumar, A. The IRE1/XBP1 signaling axis promotes skeletal muscle regeneration through a cell non-autonomous mechanism. eLife 2021, 10, e73215. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Zhu, M.; Gu, J.; Song, J.; Cui, L.; Liu, D.; Ning, Q.; Jia, X.; Feng, L. Paeoniflorin prevents endoplasmic reticulum stress-associated inflammation in lipopolysaccharide-stimulated human umbilical vein endothelial cells via the IRE1α/NF-κB signaling pathway. Food Funct. 2018, 9, 2386–2397. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Zhu, Y.; Qiao, S.; Cai, J.; Zhang, Z. Melatonin relieves liver fibrosis induced by Txnrd3 knockdown and nickel exposure via IRE1/NF-kB/NLRP3 and PERK/TGF-β1 axis activation. Life Sci. 2022, 301, 120622. [Google Scholar] [CrossRef]


| Young (N = 11) | CG (N = 8) | TG (N = 22) | ||
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | p-Value | |
| Age (years) | 22.4 ± 2.2 | 74.13 ± 0.9 | 72.7 ± 0.4 | 0.082 |
| Height (cm) | 176.3 ± 1.5 | 153.4 ± 2.8 | 164.9 ± 2.4 | 0.018 * |
| Weight (kg) | 77.6 ± 1.8 | 69.6 ± 5.4 | 74.1 ± 3.6 | 0.540 |
| BMI (kg/m2) | 25.1 ± 1.4 | 29.6 ± 2.2 | 26.9 ± 0.9 | 0.192 |
| CG (Pre) | CG (Post) | TG (Pre) | TG (Post) | ||
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | p-Value | |
| 1RM bench-press seated (kg) | 55.1 ± 5.2 | 44.8 ± 3.1 | 54.5 ± 4.6 | 72.2 ± 5.0 | <0.001 * |
| 1RM leg extension (kg) | 55.9 ± 6. 7 | 57.7 ± 4.6 | 72.4 ± 5.4 | 88.0 ± 5.6 | 0.042 * |
| Young (N = 11) | Elderly (N = 30) | ||
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | p-Value | |
| GSH (nM/mg protein) | 1.25 ± 0.02 | 1.33 ± 0.03 | 0.057 |
| LP (nM/mg protein) | 0.52 ± 0.02 | 0.66 ± 0.03 | 0.001 * |
| PC (nM/mg protein) | 4.00 ± 0.37 | 5.05 ± 0.47 | 0.210 |
| ROS (DCF RFUX103/mg protein) | 712.1 ± 53.7 | 716.1 ± 55.0 | 0.968 |
| Catalase (O.D.) | 0.90 ± 0.06 | 0.84 ± 0.04 | 0.476 |
| NRF2 (O.D.) | 0.79 ± 0.14 | 0.64 ± 0.05 | 0.311 |
| SOD1 (O.D.) | 1.17 ± 0.08 | 1.20 ± 0.10 | 0.871 |
| SOD2 (O.D.) | 0.93 ± 0.14 | 0.63 ± 0.07 | 0.037 * |
| HSP60 (O.D.) | 0.82 ± 0.04 | 0.86 ± 0.03 | 0.511 |
| Klotho (O.D.) | 0.61 ± 0.11 | 0.86 ± 0.12 | 0.222 |
| pIRE1/IRE1 ratio (O.D.) | 1.45 ± 0.21 | 1.67 ± 0.12 | 0.347 |
| pIRAK1 (O.D.) | 0.46 ± 0.09 | 0.64 ± 0.06 | 0.115 |
| TLR4 (O.D.) | 0.58 ± 0.11 | 0.69 ± 0.69 | 0.361 |
| TRAF6 (O.D.) | 0.58 ± 0.09 | 0.43 ± 0.06 | 0.218 |
| CG (Pre) | CG (Post) | TG (Pre) | TG (Post) | p-Value | ||
|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Baseline | Group × Time | |
| GSH (nM/mg protein) | 1.25 ± 0.06 | 1.33 ± 0.04 | 1.36 ± 0.03 | 1.40 ± 0.03 | 0.050 | 0.532 |
| LP (nM/mg protein) | 0.56 ± 0.06 | 0.65 ± 0.06 | 0.69 ± 0.04 | 0.65 ± 0.03 | 0.083 | 0.174 |
| PC (nM/mg protein) | 3.45 ± 0.67 | 4.00 ± 0.73 | 5.63 ± 0.56 | 5.05 ± 0.54 | 0.039 * | 0.401 |
| ROS (DCF RFUX103/mg protein) | 812.4 ± 148.2 | 1028.2 ± 231.1 | 681.1 ± 53.0 | 726.4 ± 47.8 | 0.299 | 0.419 |
| Catalase (O.D.) | 0.92 ± 0.09 | 1.08 ± 0.11 | 0.81 ± 0.05 | 0.81 ± 0.05 | 0.288 | 0.074 |
| NRF2 (O.D.) | 0.80 ± 0.10 | 0.82 ± 0.12 | 0.58 ± 0.05 | 0.49 ± 0.06 | 0.034 * | 0.279 |
| SOD1 (O.D.) | 1.43 ± 0.24 | 1.19 ± 0.18 | 1.12 ± 0.10 | 1.24 ± 0.23 | 0.186 | 0.295 |
| SOD2 (O.D.) | 0.73 ± 0.17 | 0.79 ± 0.18 | 0.60 ± 0.07 | 0.61 ± 0.06 | 0.382 | 0.496 |
| HSP60 (O.D.) | 0.80 ± 0.06 | 0.88 ± 0.06 | 0.87 ± 0.04 | 0.93 ± 0.05 | 0.325 | 0.816 |
| Klotho (O.D.) | 1.02 ± 0.22 | 1.01 ± 0.16 | 0.81 ± 0.14 | 0.78 ± 0.16 | 0.437 | 0.930 |
| pIRE1/IRE1 ratio (O.D.) | 1.88 ± 0.25 | 1.53 ± 0.26 | 1.60 ± 0.13 | 1.69 ± 0.14 | 0.308 | 0.040 * |
| pIRAK1 (O.D.) | 0.68 ± 0.14 | 0.59 ± 0.11 | 0.63 ± 0.07 | 0.56 ± 0.07 | 0.718 | 0.836 |
| TLR4 (O.D.) | 0.74 ± 0.12 | 0.78 ± 0.13 | 0.68 ± 0.06 | 0.68 ± 0.05 | 0.591 | 0.744 |
| TRAF6 (O.D.) | 0.64 ± 0.12 | 0.46 ± 0.09 | 0.36 ± 0.06 | 0.34 ± 0.06 | 0.037 * | 0.167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estébanez, B.; Visavadiya, N.P.; Vargas, J.E.; Rivera-Viloria, M.; Khamoui, A.V.; de Paz, J.A.; Huang, C.-J. Resistance Training Modulates Reticulum Endoplasmic Stress, Independent of Oxidative and Inflammatory Responses, in Elderly People. Antioxidants 2022, 11, 2242. https://doi.org/10.3390/antiox11112242
Estébanez B, Visavadiya NP, Vargas JE, Rivera-Viloria M, Khamoui AV, de Paz JA, Huang C-J. Resistance Training Modulates Reticulum Endoplasmic Stress, Independent of Oxidative and Inflammatory Responses, in Elderly People. Antioxidants. 2022; 11(11):2242. https://doi.org/10.3390/antiox11112242
Chicago/Turabian StyleEstébanez, Brisamar, Nishant P. Visavadiya, José E. Vargas, Marta Rivera-Viloria, Andy V. Khamoui, José A. de Paz, and Chun-Jung Huang. 2022. "Resistance Training Modulates Reticulum Endoplasmic Stress, Independent of Oxidative and Inflammatory Responses, in Elderly People" Antioxidants 11, no. 11: 2242. https://doi.org/10.3390/antiox11112242