Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals
Abstract
:1. Introduction
2. Oxidation Process and Radicals
3. Classification of Natural Antioxidants
4. Natural Antioxidant Mechanism in Radical Scavenging
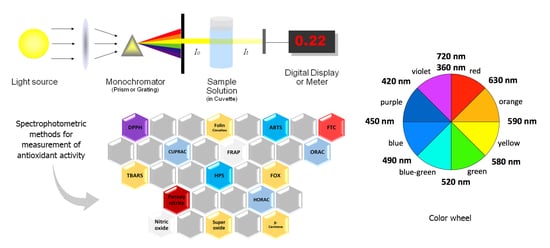
5. Spectrophotometric Methods for Measuring Antioxidant Activity
5.1. HAT and ET methods
5.1.1. DPPH
5.1.2. Folin–Ciocalteu (FC)
5.1.3. CUPRAC
5.1.4. FRAP
5.1.5. FOX
5.1.6. FTC
5.1.7. β-Carotene Bleaching Assay
5.1.8. ABTS
5.1.9. ORAC
5.1.10. TBA-TBARS
5.2. Targeted Scavenging Activities
5.2.1. Hydrogen Peroxide Scavenging Assay Activity
5.2.2. Superoxide Radical Scavenging Activity
5.2.3. Nitric Oxide Radical Scavenging
5.2.4. Peroxynitrite Scavenging Capacity
6. Advantages and Limitations of Spectrophotometric Assays
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Effect of Different Cooking Methods on Lipid Oxidation and Formation of Volatile Compounds in Foal Meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ali Pambuk, C.I. Free Radicals: The Types Generated in Biological System. MOJ Cell Sci. Rep. 2018, 5, 72–73. [Google Scholar] [CrossRef] [Green Version]
- Dreher, D.; Junod, A.F. Role of Oxygen Free Radicals in Cancer Development. Eur. J. Cancer 1996, 32, 30–38. [Google Scholar] [CrossRef]
- Maddu, N. Diseases Related to Types of Free Radicals. In Antioxidants; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C.F.R. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant By-Product Antioxidants: Control of Protein-Lipid Oxidation in Meat and Meat Products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Wiseman, A. Dietary Alkyl Thiol Free Radicals (RSS) Can Be as Toxic as Reactive Oxygen Species (ROS). Med. Hypotheses 2004, 63, 667–670. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Jia, C.; Zhang, X.; Xia, W. Optimized Microwave-Assisted Extraction of Phenolic Acids from Citrus Mandarin Peels and Evaluation of Antioxidant Activity in Vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants: The Basics—What They Are and How To. In Antioxidants in Disease Mechanisms and Therapy: Antioxidants in Disease Mechanisms and Therapeutic Strategies; Academic Press: Cambridge, MA, USA, 1996; Volume 38, p. 3. [Google Scholar]
- Halliwell, B. How to Characterize a Biological Antioxidant. Free Radic. Res. 1990, 9, 1–32. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the Potential of Antioxidants from Fruits and Vegetables and Strategies for Their Recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Gueffai, A.; Gonzalez-serrano, D.J.; Christodoulou, M.C.; Orellana-palacios, J.C.; Ortega, M.L.S.; Ouldmoumna, A.; Kiari, F.Z.; Ioannou, G.D.; Kapnissi-christodoulou, C.P.; Moreno, A.; et al. Phenolics from Defatted Black Cumin Seeds (Nigella Sativa L.): Ultrasound-Assisted Extraction Optimization, Comparison, and Antioxidant Activity. Biomolecules 2022, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Haghani, S.; Hadidi, M.; Pouramin, S.; Adinepour, F.; Hasiri, Z.; Moreno, A.; Munekata, P.E.S.; Lorenzo, J.M. Application of Cornelian Cherry (Cornus Mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage. Antioxidants 2021, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of Plant Extracts to Meat and Meat Products to Extend Shelf-Life and Health-Promoting Attributes: An Overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Falco, B.; Grauso, L.; Fiore, A.; Bonanomi, G.; Lanzotti, V. Metabolomics and Chemometrics of Seven Aromatic Plants: Carob, Eucalyptus, Laurel, Mint, Myrtle, Rosemary and Strawberry Tree. Phytochem. Anal. 2022, 33, 696–709. [Google Scholar] [CrossRef]
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A.; et al. Anti-Cancer Activity and Phenolic Content of Extracts Derived from Cypriot Carob (Ceratonia siliqua L.) Pods Using Different Solvents. Molecules 2021, 26, 5017. [Google Scholar] [CrossRef]
- Hesami, S.; Safi, S.; Larijani, K.; Badi, H.N.; Abdossi, V.; Hadidi, M. Synthesis and Characterization of Chitosan Nanoparticles Loaded with Greater Celandine (Chelidonium Majus L.) Essential Oil as an Anticancer Agent on MCF-7 Cell Line. Int. J. Biol. Macromol. 2022, 194, 974–981. [Google Scholar] [CrossRef]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O.I. The Characterization of Antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative Stress Induced-Neurodegenerative Diseases: The Need for Antioxidants That Penetrate the Blood Brain Barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, R.; Zhang, R.; Zhang, N. Simple Spectrophotometric Determination of Sulfate Free Radicals. Anal. Methods 2018, 10, 3470–3474. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Riley, P.A. Free Radicals in Biology: Oxidative Stress and the Effects of Ionizing Radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Giles, G.I.; Jacob, C. Reactive Sulfur Species: An Emerging Concept in Oxidative Stress. Biol. Chem. 2002, 383, 375–388. [Google Scholar] [CrossRef]
- Rahman, K. Studies on Free Radicals, Antioxidants, and Co-Factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar]
- Inoue, M.; Sato, E.F.; Nishikawa, M.; Park, A.-M.; Kira, Y.; Imada, I.; Utsumi, K. Mitochondrial Generation of Reactive Oxygen Species and Its Role in Aerobic Life. Curr. Med. Chem. 2005, 10, 2495–2505. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Akaike, T.; Sawa, T.; Miyamoto, Y.; van der Vliet, A.; Maeda, H. Activation of Matrix Metalloproteinases by Peroxynitrite-Induced Protein S-Glutathiolation via Disulfide S-Oxide Formation. J. Biol. Chem. 2001, 276, 29596–29602. [Google Scholar] [CrossRef]
- Abedinzadeh, Z. Sulfur-Centered Reactive Intermediates Derived from the Oxidation of Sulfur Compounds of Biological Interest. Can. J. Physiol. Pharmacol. 2001, 79, 166–170. [Google Scholar] [CrossRef]
- Møller, P.; Loft, S. The Role of Antioxidants in the Prevention of Oxidative Damage to Nucleic Acids. In Oxidative Damage to Nucleic Acids; Springer: New York, NY, USA, 2007; pp. 207–223. [Google Scholar] [CrossRef]
- Nguyen, T.; Brunson, D.; Crespi, C.L.; Penman, B.W.; Wishnok, J.S.; Tannenbaum, S.R. DNA Damage and Mutation in Human Cells Exposed to Nitric Oxide in Vitro. Proc. Natl. Acad. Sci. USA 1992, 89, 3030–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Schuman, E.M.; Madison, D. V Nitric Oxide And. Am. J. Physiol. 1994, 272, 31–35. [Google Scholar]
- Nordberg, J.; Arnér, E.S.J. Reactive Oxygen Species, Antioxidants, and the Mammalian Thioredoxin System. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, Y. Lipid Metabolism in Alzheimer’s and Parkinson’s Disease. Future Lipidol. 2006, 1, 441–453. [Google Scholar] [CrossRef]
- Porter, N.A.; Caldwell, S.E.; Mills, K.A. Mechanisms of Free Radical Oxidation of Unsaturated Lipids. Lipids 1995, 30, 277–290. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [Green Version]
- Flieger, J.; Flieger, W.; Baj, J. Antioxidants: Classification, Natural Sources, Activity/Capacity. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Zhang, W.; Dominguez, R.; Xing, L.; Fierro, E.M.; Lorenzo, J.M. Health Benefits, Extraction and Development of Functional Foods with Curcuminoids. J. Funct. Foods 2021, 79, 104392. [Google Scholar] [CrossRef]
- López-Fernández, O.; Bohrer, B.M.; Munekata, P.E.S.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Improving Oxidative Stability of Foods with Apple-Derived Polyphenols. Compr. Rev. Food Sci. Food Saf. 2022, 21, 296–320. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Yilmaz, B.; Pateiro, M.; Kumar, M.; Domínguez, R.; Shariati, M.A.; Hano, C.; Lorenzo, J.M. Valorization of By-Products from Prunus Genus Fruit Processing: Opportunities and Applications. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant Activities of Chitosans and Its Derivatives in In Vitro and In Vivo Studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus Nigra L.) as Potential Source of Antioxidants. Characterization, Optimization of Extraction Parameters and Bioactive Properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, Characterization and Study of Antioxidant Activity of Quercetin-Magnesium Complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Hadidi, M.; Rostamabadi, H.; Moreno, A.; Jafari, S.M. Nanoencapsulation of Essential Oils from Industrial Hemp (Cannabis Sativa L.) by-Products into Alfalfa Protein Nanoparticles. Food Chem. 2022, 386, 132765. [Google Scholar] [CrossRef]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Majidiyan, N.; Hadidi, M.; Azadikhah, D.; Moreno, A. Protein Complex Nanoparticles Reinforced with Industrial Hemp Essential Oil: Characterization and Application for Shelf-Life Extension of Rainbow Trout Fillets. Food Chem. X 2022, 13, 100202. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.M.; Basak, A. Human Catalase: Looking for Complete Identity. Protein Cell 2010, 1, 888–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Shahidi, F. Methods for the Assessment of Antioxidant Activity in Foods; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782420972. [Google Scholar]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Nerdy, N.; Manurung, K. Spectrophotometric Method for Antioxidant Activity Test and Total Phenolic Determination of Red Dragon Fruit Leaves and White Dragon Fruit Leaves. Rasayan J. Chem. 2018, 11, 1183–1192. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric Determination of Antioxidant Activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A Critical Review of Analytical Methods Used for the Chemical Characterisation and Quantification of Phlorotannin Compounds in Brown Seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric Assays for Total Antioxidant Capacity (TAC) in Dog Serum: An Update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Evaluation of the Copper(II) Reduction Assay Using Bathocuproinedisulfonic Acid Disodium Salt for the Total Antioxidant Capacity Assessment: The CUPRAC-BCS Assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Tanner, C.; Mechoulam, R.; Pertwee, R.G.; Ross, R.A. Inhibition of Human Neutrophil Chemotaxis by Endogenous Cannabinoids and Phytocannabinoids: Evidence for a Site Distinct from CB1 and CB 2. Mol. Pharmacol. 2008, 73, 441–450. [Google Scholar] [CrossRef]
- Opitz, S.E.W.; Smrke, S.; Goodman, B.A.; Yeretzian, C. Methodology for the Measurement of Antioxidant Capacity of Coffee: A Validated Platform Composed of Three Complementary Antioxidant Assays. In Processing and Impact on Antioxidants in Beverages; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780124047389. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Dox, A.W.; Plaisance, G.P. Condensation of Thiobarbituric Acid with Aromatic Aldehydes. J. Am. Chem. Soc. 1916, 38, 2164–2166. [Google Scholar] [CrossRef] [Green Version]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 2020, 61122. [Google Scholar] [CrossRef]
- Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Inflammatory and Oxidative Stress Markers in Skeletal Muscle of Obese Subjects. In Obesity: Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–189. [Google Scholar] [CrossRef]
- DeLong, J.M.; Prange, R.K.; Hodges, D.M.; Forney, C.F.; Bishop, M.C.; Quilliam, M. Using a Modified Ferrous Oxidation-Xylenol Orange (FOX) Assay for Detection of Lipid Hydroperoxides in Plant Tissue. J. Agric. Food Chem. 2002, 50, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.D.C.; Tejeda, A.; Duque, A.L.; Macías, P. Determination of Lipoxygenase Activity in Plant Extracts Using a Modified Ferrous Oxidation-Xylenol Orange Assay. J. Agric. Food Chem. 2007, 55, 5956–5959. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, T.A.; Liebler, D.C. Peroxyl Radical Oxidation of β-Carotene: Formation of β-Carotene Epoxides. Chem. Res. Toxicol. 1991, 4, 290–295. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and Free Radical Scavenging Activity of Spondias Pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.N.A.; Kunchandy, E. Oxygen Radical Scavenging Activity of Curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar]
- Bailly, F.; Zoete, V.; Vamecq, J.; Catteau, J.P.; Bernier, J.L. Antioxidant Actions of Ovothiol-Derived 4-Mercaptoimidazoles: Glutathione Peroxidase Activity and Protection against Peroxynitrite-Induced Damage. FEBS Lett. 2000, 486, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-Mediated Oxidation of Dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Riley, R.; Chapman, V. Genetic Control of the Cytologically Diploid Behaviour of Hexaploid Wheat. Nature 1958, 182, 713–715. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staško, A.; Brezová, V.; Biskupič, S.; Mišík, V. The Potential Pitfalls of Using 1,1-Diphenyl-2-Picrylhydrazyl to Characterize Antioxidants in Mixed Water Solvents. Free Radic. Res. 2007, 41, 379–390. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical Scavenging Ability of Polyphenolic Compounds towards DPPH Free Radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Molyneux, P. Molineux 07-DPPH. Songklanakarin J. Sci. Technol 2004, 26, 211–219. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- González-Serrano, D.J.; Hadidi, M.; Varcheh, M.; Zarei Jelyani, A.; Moreno, A.; Lorenzo, J.M. Bioactive Peptide Fractions from Collagen Hydrolysate of Common Carp Fish Byproduct: Antioxidant and Functional Properties. Antioxidants 2022, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Zhang, H.Y. A Theoretical Investigation on DPPH Radical-Scavenging Mechanism of Edaravone. Bioorg. Med. Chem. Lett. 2003, 13, 3789–3792. [Google Scholar] [CrossRef] [PubMed]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martínez, G.A. Use of LED Light for Brussels Sprouts Postharvest Conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteau, V. Tyrosine and Tryptophane in Proteins. J. Biol. Chem. 1927, 73, 627–648. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; de Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [Green Version]
- Ikawa, M.; Schaper, T.D.; Dollard, C.A.; Sasner, J.J. Utilization of Folin-Ciocalteu Phenol Reagent for the Detection of Certain Nitrogen Compounds. J. Agric. Food. Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in Carobs: A Review on Their Composition, Antioxidant Capacity and Cytotoxic Effects, and Health Impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Barrows, J.N.; Jameson, G.B.; Pope, M.T. Structure of a Heteropoly Blue, The Four-Electron Reduced β-12-Molybdophosphate Anion. J. Am. Chem. Soc. 1985, 107, 1771–1773. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Torres-Aguirre, G.A.; Núñez-Gastélum, J.A.; de la Rosa, L.A.; Rodrigo-García, J.; Ayala-Zavala, J.F.; Álvarez-Parrilla, E. Nuevo Acercamiento a La Interacción Del Reactivo De Folin-Ciocalteu Con Azúcares Durante La Cuantificación De Polifenoles Totales. Tip 2017, 20, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E.; Altun, M. Total Antioxidant Capacity Assay of Human Serum Using Copper(II)-Neocuproine as Chromogenic Oxidant: The CUPRAC Method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Bener, M.; Özyürek, M.; Güçlü, K.; Apak, R. Development of a Low-Cost Optical Sensor for Cupric Reducing Antioxidant Capacity Measurement of Food Extracts. Anal. Chem. 2010, 82, 4252–4258. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Romero, M.P.R.; Brito, R.E.; Palma, A.; Montoya, M.R.; Mellado, J.M.R.; Rodríguez-Amaro, R. An Electrochemical Method for the Determination of Antioxidant Capacities Applied to Components of Spices and Condiments. J. Electrochem. Soc. 2017, 164, B97–B102. [Google Scholar] [CrossRef]
- Amoli, P.I.; Hadidi, M.; Hasiri, Z.; Rouhafza, A.; Jelyani, A.Z.; Hadian, Z.; Khaneghah, A.M.; Lorenzo, J.M. Incorporation of Low Molecular Weight Chitosan in a Low-Fat Beef Burger: Assessment of Technological Quality and Oxidative Stability. Foods 2021, 10, 1959. [Google Scholar] [CrossRef]
- Ebner, H.; Dienstbach, F.; Sandritter, W. Hormonelle Beeinflussung Des Experimentellen Portiocarcinoms. Verh. Dtsch. Ges. Inn. Med. 1967, 73, 366–369. [Google Scholar]
- Sharma, S.; Vig, A.P. Evaluation of In Vitro Antioxidant Properties of Methanol and Aqueous Extracts of Parkinsonia Aculeata L. Leaves. Sci. World J. 2013, 2013, 604865. [Google Scholar] [CrossRef] [Green Version]
- Marco, G.J. A Rapid Method for Evaluation of Antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Revisiting the Polar Paradox Theory: A Critical Overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene Assay Revisited. Application to Characterize and Quantify Antioxidant and Prooxidant Activities in a Microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [Green Version]
- Mikami, I.; Yamaguchi, M.; Shinmoto, H.; Tsushida, T. Development and Validation of a Microplate-Based ß-Carotene Bleaching Assay and Comparison of Antioxidant Activity (Aoa) in Several Crops Measured by ß-Carotene Bleaching, Dpph and Orac Assays. Food Sci. Technol. Res. 2009, 15, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Litescu, S.C.; Eremia, S.A.V.; Tache, A.; Vasilescu, I.; Radu, G.L. The Use of Oxygen Radical Absorbance Capacity (ORAC) and Trolox Equivalent Antioxidant Capacity (TEAC) Assays in the Assessment of Beverages’ Antioxidant Properties. In Processing and Impact on Antioxidants in Beverages; Elsevier: New York City, NY, USA, 2014; ISBN 9780124047389. [Google Scholar]
- Cao, G.; Prior, R.L. Measurement of Oxygen Radical Absorbance Capacity in Biological Samples. Methods Enzymol. 1999, 299, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant Evaluation Protocols: Food Quality or Health Effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; Intechopen: London, UK, 2019; pp. 1–28. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel Fluorometric Assay for Hydroxyl Radical Prevention. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Číž, M.; Čížová, H.; Denev, P.; Kratchanova, M.; Slavov, A.; Lojek, A. Different Methods for Control and Comparison of the Antioxidant Properties of Vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Office, T. Commentary: Comments on the Mechanism of the “Fenton-like” Reaction. Acc. Chem. Res. 1999, 32, 547–550. [Google Scholar] [CrossRef]
- Boveris, A.; Martino, E.; Stoppani, A.O.M. Evaluation of the Horseradish Peroxidase-Scopoletin Method for the Measurement of Hydrogen Peroxide Formation in Biological Systems. Anal. Biochem. 1977, 80, 145–158. [Google Scholar] [CrossRef]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review Scopoletin—A Coumarin Phytoalexin with Medicinal Properties. CRC Crit. Rev. Plant. Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Koppenol, W.H. The Basic Chemistry of Nitrogen Monoxide and Peroxynitrite. Free Radic. Biol. Med. 1998, 25, 385–391. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids Are Scavengers of Superoxide Anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Zachariah, S.M.; Viswanad, V. Evaluation of Antioxidante and Total Flavanoid Content of Mirabilis Jalpa Linn Using In Vitro Models. Int. Res. J. Pharm. 2012, 3, 187–192. [Google Scholar]
- Pannala, A.; Razaq, R.; Halliwell, B.; Singh, S.; Rice-Evans, C.A. Inhibition of Peroxynitrite Dependent Tyrosine Nitration by Hydroxycinnamates: Nitration or Electron Donation? Free Radic. Biol. Med. 1998, 24, 594–606. [Google Scholar] [CrossRef]
- Beckman, J.S.; Chen, J.; Ischiropoulos, H.; Crow, J.P. Oxidative Chemistry of Peroxynitrite. Methods Enzymol. 1994, 233, 229–240. [Google Scholar] [CrossRef]
- Fernández-Rubio, J.; Rodríguez-Gil, J.L.; Postigo, C.; Mastroianni, N.; López de Alda, M.; Barceló, D.; Valcárcel, Y. Psychoactive Pharmaceuticals and Illicit Drugs in Coastal Waters of North-Western Spain: Environmental Exposure and Risk Assessment. Chemosphere 2019, 224, 379–389. [Google Scholar] [CrossRef]
- Erel, O. A Novel Automated Direct Measurement Method for Total Antioxidant Capacity Using a New Generation, More Stable ABTS Radical Cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Happyana, N.; Agnolet, S.; Muntendam, R.; van Dam, A.; Schneider, B.; Kayser, O. Analysis of Cannabinoids in Laser-Microdissected Trichomes of Medicinal Cannabis Sativa Using LCMS and Cryogenic NMR. Phytochemistry 2013, 87, 51–59. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Sripriya, N.; Udaya Prakash, N.K.; Deepa, S. Studies on Antioxidant Activities of Six Cultivars of Piper Betle Linn. Int. J. Pharm. Pharm. Sci. 2014, 6, 270–273. [Google Scholar]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]












| Reactive Species | |||
|---|---|---|---|
| ROS | RNS | RSS | |
| Radical | Peroxyl ROO• | Nitric oxide NO• | Alkoxyl-thiyl RS• |
| Alkoxyl RO• | Nitrogen dioxide NO2• | Sulfide cation (RSR)•+ | |
| Hydroxyl •OH | Disulfide anion (RSSR)•− | ||
| Superoxide O2•− | Disulfide cation (R2S∴SR2)•+ | ||
| Bicarbonate HCO3• | Perthiyl RSS• | ||
| Sulfinyl RSO• | |||
| Sulfonyl RS(O)2• | |||
| Sulfur trioxide anion SO3•− | |||
| Sulfate anion SO4•− | |||
| Non-Radical | Hydrogen peroxide | Peroxynitrite ONOO− | Sulfate SO42− |
| H2O2 | Nitrosyl cation NO+ | Dithionate S2O62− | |
| Singlet oxygen 1O2 | Nitrous acid HNO2 | Sulfite SO32− | |
| Ozone O3 | Nitryl chloride NO2CL | Disulfide R2S | |
| Peroxide R2O2 | Nitroxyl anion NO− | Hydrogen sulphide H2S | |
| Alcohol ROH | Dinitrogen trioxide N2O3 | Disulfide-S-dioxide RS(O2) SR | |
| Organic peroxide ROOH | Dinitrogen tetraoxide N2O4 | Disulfide-S-monoxide RS(O)SR | |
| Peroxynitrous acid ONOOH | Sulfenic acid RSOH | ||
| Thiol RSR | |||
| Tetrathionate S4O62− | |||
| Peroxodisulfate S2O82− | |||
| Assay | nm | Principle of Method | Determination | Color Shifting | Reference | |
|---|---|---|---|---|---|---|
| From | To | |||||
| DPP | 515–520 | Antioxidant reaction with free organic radicals | Colorimetry |  | [10,59,61] | |
| Folin–Ciocalteu | 760–765 | The reductive capacity of antioxidants to determine the total phenolic content | Colorimetry |  | [59,62,63] | |
| CUPRAC | 450–490 | Measures TAC of the reduction of Cu (II) to Cu (I) by antioxidants | Colorimetry |  | [26,64,65] | |
| FRAP | 593 | Measures the antioxidant potential through the reduction of Fe (III) to Fe (II) by antioxidants | Colorimetry |  | [23,26,46,65] | |
| ABTS | 414, 645–650, 734, 815–820 | Measures the relative ability of antioxidants to scavenge the ABTS generated in the aqueous phase | Colorimetry |  | [6,23,64,65,66,67] | |
| ORAC and HORAC | 485–525 and 485–535 | Antioxidant reaction with peroxyl radicals and quench OH radicals generated by a Co(II)-based Fenton-like system | Loss of fluorescence of fluorescein |  | [5,24,26,66,68] | |
| TBA-TBARS | 532–535 | Based on the reactivity of malondialdehyde (MDA) with TBA to produce a red color | Colorimetry |  | [23,69,70,71] | |
| FOX | 550–560 | Measure the levels of hydrogen peroxide in biological systems by the oxidation of Fe(II) to Fe(III) | Colorimetry |  | [23,72,73] | |
| FTC | 500 | Measure the levels of hydrogen peroxide as the ferric ion is converted by an oxidant from a ferrous ion | Colorimetry |  | [23,71,74] | |
| β-Carotene Bleaching Assay | 440 | Measure the levels of peroxyl radicals as β-carotene blenched | Colorimetry |  | [23,75] | |
| Hydrogen peroxide scavenging | 460 | Total oxidant scavenging capacity of antioxidants | Fluorescence |  | [10,76] | |
| Superoxide radical scavenging | 560–562 | Total oxidant scavenging capacity of antioxidants | Colorimetry |  | [76,77] | |
| Nitric oxide radical scavenging | 540 | Total oxidant scavenging capacity of antioxidants | Colorimetry |  | [76] | |
| Peroxynitrite Scavenging | 485, 505, 529–530, 611 | Total oxidant scavenging capacity of antioxidants | Fluorescence |  | [78,79] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. https://doi.org/10.3390/antiox11112213
Christodoulou MC, Orellana Palacios JC, Hesami G, Jafarzadeh S, Lorenzo JM, Domínguez R, Moreno A, Hadidi M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants. 2022; 11(11):2213. https://doi.org/10.3390/antiox11112213
Chicago/Turabian StyleChristodoulou, Marios C., Jose C. Orellana Palacios, Golnaz Hesami, Shima Jafarzadeh, José M. Lorenzo, Rubén Domínguez, Andres Moreno, and Milad Hadidi. 2022. "Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals" Antioxidants 11, no. 11: 2213. https://doi.org/10.3390/antiox11112213