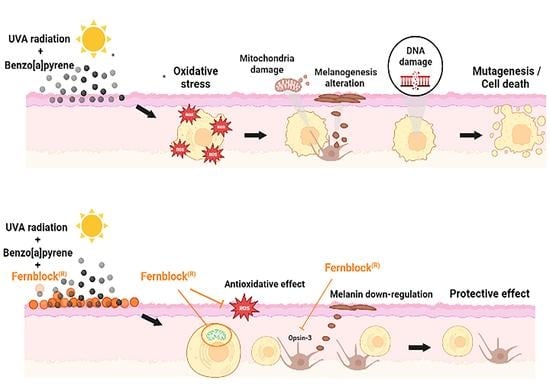
Protective Effect of the Hydrophilic Extract of Polypodium leucotomos, Fernblock®, against the Synergistic Action of UVA Radiation and Benzo[a]pyrene Pollutant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. UVA Light Irradiation
2.3. Combined Treatment with BaP (+/−FB) and UVA Light Exposure
2.4. Cell Viability Assessment
2.5. Reactive Oxygen Species Determination
2.6. Indirect Immunofluorescence
2.7. Mitochondrial Morphology
2.8. Quantification of the Expression of Opsin-3 by Real Time Polymerase Chain Reaction
2.9. Statistical Analyses
3. Results
3.1. Characterization of the Photoprotective Effects of FB on Cell Viability and Morphology
3.2. Evaluation of Oxidative Stress Levels
3.3. Mitochondrial Morphology and Localization of Cytochrome C
3.4. Evaluation of DNA Damage
3.5. Effect on the Expression of Opsin-3 in B16-F10 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 12, 1816–1841. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silveira, J.E.; Myaki-Pedroso, D.M. UV light and skin aging. Rev. Environ. Health 2014, 3, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 2, 248–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Infamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.A.; Cassidy, P. Dark CPDs and photocarcinogenesis: The party continues after the lights go out. Pigment Cell Melanoma Res. 2015, 4, 373–374. [Google Scholar] [CrossRef]
- Ikehata, H.; Ono, T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 3, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UVR exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Global Ambient Air Quality Database; World Health Organization: Geneva, Switzerland, 2018. Available online: http://www.who.int/airpollution/data/cities/en/ (accessed on 17 August 2022).
- World Health Organization. Burden of Disease from the Joint Effects of Household and Ambient Air Pollution for 2012; World Health Organization: Geneva, Switzerland, 2012. Available online: http://www.who.int/phe/health_topics/outdoorair/databases/AP_jointeffect_BoD_results_Nov2016.pf?ua=1 (accessed on 17 August 2022).
- Working Group on Polycyclic Aromatic Hydrocarbons. Ambient Air Pollution by Polycyclic Aromatic Hydrocarbons (PAH); Position Paper; Office for Official Publications of the European Communities: Luxembourg, 2001; Available online: http://eceuropa.eu/environment/air/pdf/pp_pah.pdf (accessed on 17 August 2022).
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J. Dermatol. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wang, S.Q. Recognizing the impact of ambient air pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrot, L. Pollution and sun exposure: A deleterious synergy. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef] [PubMed]
- Saladi, R.; Austin, L.; Gao, D.; Lu, Y.; Phelps, R.; Lebwohl, M.; Wei, H. The combination of benzo[a]pyrene and ultraviolet a causes an in vivo time-related accumulation of DNA damage in mouse skin. Photochem. Photobiol. 2003, 77, 413–419. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, D.; Atencio, D.P.; Perez, E.; Saladi, R.; Moore, J.; Guevara, D.; Rosenstein, B.S.; Lebwohl, M.; Wei, H. Combined subcarcinogenic benzo[a]pyrene and UVA synergistically caused high tumor incidence and mutations in H-ras gene, but not p53, in SKH-1 hairless mouse skin. Int. J. Cancer 2005, 116, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E.; Wei, H. Synergistic damage by UVA radiation and pollutants. Toxicol. Ind. Health 2009, 25, 219–224. [Google Scholar] [CrossRef]
- Soeur, J.; Belaïdi, J.-P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Toyooka, T.; Ibuki, Y. New method for testing phototoxicity of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2006, 40, 3603–3608. [Google Scholar] [CrossRef]
- Xia, Q.; Chiang, H.-M.; Yin, J.-J.; Chen, S.; Cai, L.; Yu, H.; Fu, P.P. UVA photoirradiation of benzo[a]pyrene metabolites: Induction of cytotoxicity, reactive oxygen species, and lipid peroxidation. Toxicol. Ind. Health 2015, 31, 898–910. [Google Scholar] [CrossRef]
- Alotaibi, A.G.; Li, J.V.; Gooderham, N.J. Tumour necrosis factor-α (TNF-α) enhances dietary carcinogen-induced DNA damage in colorectal cancer epithelial cells through the activation of the JNK signaling pathway. Toxicology 2021, 457, 152806. [Google Scholar] [CrossRef]
- Ibuki, Y.; Goto, R. Phototoxicity of benzo[a]pyrene by ultraviolet A irradiation: Induction of apoptosis in Jurkat cells. Environ. Toxicol. Pharmacol. 2002, 11, 101–109. [Google Scholar] [CrossRef]
- Block, F.J.; Tait, S.W.G. Mitochondria as multifaced regulators of cell death. Nat. Rev. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Ozdeslik, R.N.; Olinski, L.E.; Trieu, M.M.; Oprian, D.D.; Oancea, E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc. Natl. Acad. Sci. USA 2019, 4, 11508–11517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olinski, L.E.; Lin, E.M.; Oancea, E. Illuminating insights into opsin 3 function in the skin. Adv. Biol. Regul. 2020, 75, 100668. [Google Scholar] [CrossRef] [PubMed]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tyminska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Y.; Lu, H. Opsin 3 is a key regulator of ultraviolet A-induced photoageing in human dermal fibroblast cells. Br. J. Dermatol. 2020, 182, 1228–1244. [Google Scholar] [CrossRef] [Green Version]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Li, H.; Dang, F.; Chen, L.; Liu, X.; Gao, J. Induction of proliferative and mutagenic activity by benzo(a)pyrene in PC-3 cells via JAK2/STAT3 pathway. Mutat. Res. 2020, 821, 111720. [Google Scholar] [CrossRef]
- King, G.N.; Healy, C.M.; Glover, M.T.; Kwan, J.T.; Williams, D.M.; Leigh, I.M.; Worthington, H.V.; Thornhill, M.H. Increased prevalence of dysplastic and malignant lip lesions in renal-transplant recipients. N. Engl. J. Med. 1995, 332, 1052–1057. [Google Scholar] [CrossRef]
- Kaur, C.D.; Saraf, S. Topical vesicular formulations of Curcuma longa extract on recuperating the ultraviolet radiation-damaged skin. J. Cosmet. Dermatol. 2011, 10, 260–265. [Google Scholar] [CrossRef]
- Sotler, R.; Adamic, M.; Jarni, K.; Dahmane, R.; Trebse, P.; Kralj, M.B. Analyzing the Photoprotection Efficiency of Sunscreens Containing Antioxidants under Disinfection Conditions. Antioxidants 2021, 10, 1720. [Google Scholar] [CrossRef]
- Gonzalez, S.; Gilaberte, Y.; Philips, N.; Juarranz, A. Fernblock, a Nutriceutical with Photoprotective Properties and Potential Preventive Agent for Skin Photoaging and Photoinduced Skin Cancers. Int. J. Mol. Sci. 2011, 12, 8466–8475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schalka, S.; Coelho Donato, L. Evaluation of effectiveness of a sunscreen containing Polypodium leucatomos extract in reducing the sun damage to the skin. Surg. Cosmet. Dermatol. 2019, 11, 310–318. [Google Scholar] [CrossRef]
- Parrado, C.; Nicolas, J.; Juarranz, A.; Gonzalez, S. The role of the aqueous extract Polypodium leucotomos in photoprotection. Photochem. Photobiol. Sci. 2020, 19, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Jańczyk, A.; Garcia-Lopez, M.A.; Fernandez-Peñas, P.; Alonso-Lebrero, J.L.; Benedicto, I.; López-Cabrera, M.; Gonzalez, S. A Polypodium leucotomos extract inhibits solar-simulated radiation-induced TNF-alpha and iNOS expression, tran-scriptional activation and apoptosis. Exp. Dermatol. 2007, 16, 823–829. [Google Scholar] [CrossRef]
- Delgado-Wicke, P.; Rodríguez-Luna, A.; Ikeyama, Y.; Honma, Y.; Kume, T.; Gutiérrez, M.; Lorrio, S.; Juarranz, A.; González, S. Fernblock® Upregulates NRF2 Antioxidant Pathway and Protects Keratinocytes from PM2.5-Induced Xenotoxic Stress. Oxid. Med. Cell. Longev. 2020, 14, 2908108. [Google Scholar] [CrossRef] [Green Version]
- Portillo-Esnaola, M.; Rodríguez-Luna, A.; Nicolás-Morala, J.; Gallego-Rentero, M.; Villalba, M.; Juarranz, A.; González, S. Formation of Cyclobutane Pyrimidine Dimers after UVA Exposure (Dark-CPDs) Is Inhibited by a Hydrophilic Extract of Polypodium leucotomos. Antioxidants 2021, 10, 1961. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Philips, N.; Conte, J.; Chen, Y.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial regulation of matrix metalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-beta by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487–495. [Google Scholar] [CrossRef]
- Philips, N.; Smith, J.; Keller, T.; Gonzalez, S. Predominant effects of Polypodium leucotomos on membrane integrity, lipid peroxidation, and expression of elastin and matrix metalloproteinase-1 in ultraviolet radiation exposed fibroblasts, and keratinocytes. J. Dermatol. Sci. 2003, 32, 1–9. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Boiteux, S.; Coste, F.; Castaing, B. Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic. Biol. Med. 2017, 107, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Chen, F.; Chen, H.; Peng, H.; Wang, K. Benzo[a]pyrene (BaP) exposure generates persistent reactive oxygen species (ROS) to inhibit the NF-κB pathway in medaka (Oryzias melastigma). Environ. Pollut. 2019, 251, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Esnaola, M.; Mataix, M.; Alonso-Juarranz, M.; Lorrio, S.; Villalba, M.; Rodríguez-Luna, A.; González, S. The Aqueous Extract of Polypodium leucotomos (Fernblock®) Regulates Opsin 3 and Prevents Photooxidation of Melanin Precursors on Skin Cells Exposed to Blue Light Emitted from Digital Devices. Antioxidants 2021, 10, 400. [Google Scholar] [CrossRef]
- Winkelmann, R.R.; Rosso, J.; Rigel, D.S. Polypodium leucotomos extract: A status report on clinical efficacy and safety. J. Drugs Dermatol. 2015, 14, 254–261. Available online: https://jddonline.com/articles/polypodium-leucotomos-extract-a-status-report-on-clinical-efficacy-and-safety-S1545961615P0254X/ (accessed on 17 August 2022). [PubMed]
- Palomino, O.M. Current knowledge in Polypodium leucotomos effect on skin protection. Arch. Dermatol. Res. 2015, 307, 199–209. [Google Scholar] [CrossRef]
- Ryšavá, A.; Čížková, K.; Franková, J.; Roubalová, L.; Ulrichová, J.; Vostálová, J.; Vrba, J.; Zálešák, B.; Svobodová, A.R. Effect of UVA radiation on the Nrf2 signaling pathway in human skin cells. J. Photochem. Photobiol. B 2020, 209, 111948. [Google Scholar] [CrossRef]
- Reed, L.; Jarvis, I.W.H.; Phillips, D.H.; Arlt, V.M. Enhanced DNA adduct formation by benzo[a]pyrene in human liver cells lacking cytochrome P450 oxidoreductase. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 852, 503162. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, X.; Chen, X.; Yang, G.; Wang, Q.; Rao, K.; Xiong, W.; Yuan, J. Benzo(a)pyrene-induced mitochondrial dysfunction and cell death in p53-null Hep3B cells. Mutat. Res. 2011, 726, 75–83. [Google Scholar] [CrossRef]
- Gao, D.; Luo, Y.; Guevara, D.; Wang, Y.; Rui, M.; Goldwyn, B.; Lu, Y.; Smith, E.C.A.; Lebwohl, M.; Wei, H. Benzo[a]pyrene and its metabolites combined with ultraviolet A synergistically induce 8-hydroxy-2’-deoxyguanosine via reactive oxygen species. Free Radic. Biol. Med. 2005, 39, 1177–1183. [Google Scholar] [CrossRef]
- Shyong, E.Q.; Lu, Y.; Goldstein, A.; Lebwohl, M.; Wei, H. Synergistic enhancement of H2O2 production in human epidermoid carcinoma cells by Benzo[a]pyrene and ultraviolet A radiation. Toxicol. Appl. Pharmacol. 2003, 188, 104–109. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Rosenstein, B.; Lebwohl, M.; Wei, H. Benzo[a]pyrene enhances the formation of 8-hydroxy-2′-deoxyguanosine by ultraviolet A radiation in calf thymus DNA and human epidermoid carcinoma cells. Biochemistry 1998, 37, 10307–10312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, M.; Choi, Y.H.; Kim, B.K.; Kim, K.S.; Park, K.J.; Lee, N.H.; Hyun, C. Down-regulation of tyrosinase, TRP-1, TRP-2 and MITF expressions by citrus press-cakes in murine B16 F10 melanoma. Asian Pac. J. Trop. Biomed. 2013, 3, 617–622. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego-Rentero, M.; Nicolás-Morala, J.; Alonso-Juarranz, M.; Carrasco, E.; Portillo-Esnaola, M.; Rodríguez-Luna, A.; González, S. Protective Effect of the Hydrophilic Extract of Polypodium leucotomos, Fernblock®, against the Synergistic Action of UVA Radiation and Benzo[a]pyrene Pollutant. Antioxidants 2022, 11, 2185. https://doi.org/10.3390/antiox11112185
Gallego-Rentero M, Nicolás-Morala J, Alonso-Juarranz M, Carrasco E, Portillo-Esnaola M, Rodríguez-Luna A, González S. Protective Effect of the Hydrophilic Extract of Polypodium leucotomos, Fernblock®, against the Synergistic Action of UVA Radiation and Benzo[a]pyrene Pollutant. Antioxidants. 2022; 11(11):2185. https://doi.org/10.3390/antiox11112185
Chicago/Turabian StyleGallego-Rentero, María, Jimena Nicolás-Morala, Miguel Alonso-Juarranz, Elisa Carrasco, Mikel Portillo-Esnaola, Azahara Rodríguez-Luna, and Salvador González. 2022. "Protective Effect of the Hydrophilic Extract of Polypodium leucotomos, Fernblock®, against the Synergistic Action of UVA Radiation and Benzo[a]pyrene Pollutant" Antioxidants 11, no. 11: 2185. https://doi.org/10.3390/antiox11112185