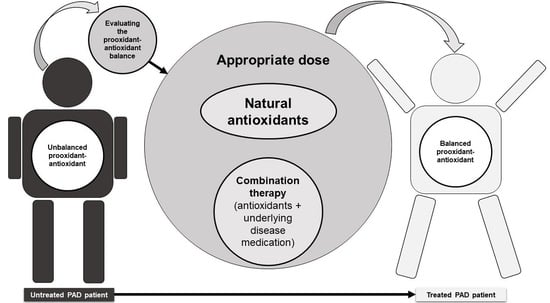
The Potential Role of Antioxidants in the Treatment of Peripheral Arterial Disease: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
3. Data Extraction and Quality Assessment
4. Results
4.1. Search Results
4.2. Study Characteristics
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olinic, D.-M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.-A.; Liew, A.; Schernthaner, G.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334. [Google Scholar] [CrossRef]
- Frank, U.; Nikol, S.; Belch, J.J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on peripheral arterial disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Scully, R.E.; Arnaoutakis, D.J.; Smith, A.D.; Semel, M.; Nguyen, L.L. Estimated annual health care expenditures in individuals with peripheral arterial disease. J. Vasc. Surg. 2018, 67, 558–567. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Ness, J.; Aronow, W.S. Prevalence of Coexistence of Coronary Artery Disease, Ischemic Stroke, and Peripheral Arterial Disease in Older Persons, Mean Age 80 Years, in an Academic Hospital-Based Geriatrics Practice. J. Am. Geriatr. Soc. 1999, 47, 1255–1256. [Google Scholar] [CrossRef]
- Bevan, G.H.; Solaru, K.T.W. Evidence-Based Medical Management of Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Scuto, S.; Marino, E.; Xourafa, A.; Gaudio, A. Oxidative Stress in Peripheral Arterial Disease (PAD) Mechanism and Biomarkers. Antioxidants 2019, 8, 367. [Google Scholar] [CrossRef] [Green Version]
- Stanek, A.; Fazeli, B.; Bartuś, S.; Sutkowska, E. The Role of Endothelium in Physiological and Pathological States: New Data. BioMed Res. Int. 2018, 2018, 1098039. [Google Scholar] [CrossRef] [Green Version]
- Koutakis, P.; Ismaeel, A.; Farmer, P.; Purcell, S.; Smith, R.S.; Eidson, J.L.; Bohannon, W.T. Oxidative stress and antioxidant treatment in patients with peripheral artery disease. Physiol. Rep. 2018, 6, e13650. [Google Scholar] [CrossRef]
- Dalgård, C.; Nielsen, F.; Morrow, J.D.; Enghusen-Poulsen, H.; Jonung, T.; Hørder, M.; De Maat, M.P.M. Supplementation with orange and blackcurrant juice, but not vitamin E, improves inflammatory markers in patients with peripheral arterial disease. Br. J. Nutr. 2008, 101, 263–269. [Google Scholar] [CrossRef]
- Klipstein-Grobusch, K.; Breeijen, J.H.D.; Grobbee, D.E.; Boeing, H.; Hofman, A.; Witteman, J.C.M. Dietary Antioxidants and Peripheral Arterial Disease: The Rotterdam Study. Am. J. Epidemiol. 2001, 154, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woessner, M.; Van Bruggen, M.D.; Pieper, C.F.; Sloane, R.; Kraus, W.E.; Gow, A.J.; Allen, J.D. Beet the Best? Circ. Res. 2018, 123, 654–659. [Google Scholar] [CrossRef]
- Kleijnen, J.; Mackerras, D. Vitamin E for intermittent claudication. Cochrane Database Syst. Rev. 1998, 2000, CD000987. [Google Scholar] [CrossRef] [PubMed]
- Critical Leg Ischaemia Prevention Study (CLIPS) Group; Catalano, M.; Born, G.; Peto, R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: Randomized, double-blind trial. J. Intern. Med. 2007, 261, 276–284. [Google Scholar]
- McDermott, M.M.; Leeuwenburgh, C.; Guralnik, J.M.; Tian, L.; Sufit, R.; Zhao, L.; Criqui, M.H.; Kibbe, M.R.; Stein, J.S.; Lloyd-Jones, D.; et al. Effect of Resveratrol on Walking Performance in Older People with Peripheral Artery Disease: The RESTORE Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 902–907. [Google Scholar] [CrossRef] [Green Version]
- Jepson, R.G.; Kleijnen, J.; Leng, G.C. Garlic for peripheral arterial occlusive disease. Cochrane Database Syst. Rev. 2013, 2013, CD000095. [Google Scholar] [CrossRef] [Green Version]
- Horsch, S.; Walther, C. Ginkgo biloba special extract EGb 761 in the treatment of peripheral arterial occlusive disease (PAOD)—A review based on randomized, controlled studies. Int. J. Clin. Pharmacol. Ther. 2004, 42, 63–72. [Google Scholar] [CrossRef]
- Campbell, A.; Price, J.; Hiatt, W.R. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst. Rev. 2013, 7, CD003833. [Google Scholar] [CrossRef] [Green Version]
- Curtis, P.; Potter, J.; Kroon, P.; Wilson, P.; Dhatariya, K.; Sampson, M.; Cassidy, A. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 936–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loffredo, L.; Perri, L.; Catasca, E.; Pignatelli, P.; Brancorsini, M.; Nocella, C.; De Falco, E.; Bartimoccia, S.; Frati, G.; Carnevale, R.; et al. Dark Chocolate Acutely Improves Walking Autonomy in Patients with Peripheral Artery Disease. J. Am. Heart Assoc. 2014, 3, e001072. [Google Scholar] [CrossRef] [Green Version]
- Hammer, A.; Koppensteiner, R.; Steiner, S.; Niessner, A.; Goliasch, G.; Gschwandtner, M.; Hoke, M. Dark chocolate and vascular function in patients with peripheral artery disease: A randomized, controlled cross-over trial. Clin. Hemorheol. Microcirc. 2015, 59, 145–153. [Google Scholar] [CrossRef]
- McDermott, M.M.; Criqui, M.H.; Domanchuk, K.; Ferrucci, L.; Guralnik, J.M.; Kibbe, M.R.; Kosmac, K.; Kramer, C.M.; Leeuwenburgh, C.; Li, L.; et al. Cocoa to Improve Walking Performance in Older People with Peripheral Artery Disease: The COCOA-PAD Pilot Randomized Clinical Trial. Circ. Res. 2020, 126, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Belch, J.; MacCuish, A.; Campbell, I.; Cobbe, S.; Taylor, R.; Prescott, R.; Lee, R.; Bancroft, J.; MacEwan, S.; Shepherd, J.; et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: Factorial randomized placebo-controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008, 337, a1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loffredo, L.; Pignatelli, P.; Cangemi, R.; Andreozzi, P.; Panico, M.A.; Meloni, V.; Violi, F. Imbalance between nitric oxide generation and oxidative stress in patients with peripheral arterial disease: Effect of an antioxidant treatment. J. Vasc. Surg. 2006, 44, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Loffredo, L.; Marcoccia, A.; Pignatelli, P.; Andreozzi, P.; Borgia, M.C.; Cangemi, R.; Chiarotti, F.; Violi, F. Oxidative-stress-mediated arterial dysfunction in patients with peripheral arterial disease. Eur. Heart J. 2007, 5, 608–612. [Google Scholar] [CrossRef]
- Singh, J.A.; Cleveland, J. Allopurinol and the risk of incident peripheral arterial disease in the elderly: A US Medicare claims data study. Rheumatology 2018, 57, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Poggesi, L.; Scarti, L.; Boddi, M.; Masotti, G.; Serneri, G.G.N. Pentoxifylline Treatment in Patients with Occlusive Peripheral Arterial Disease. Circulatory Changes and Effects on Prostaglandin Synthesis. Angiology 1985, 36, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Majid, K.A.; Lohana, P.; Anvekar, P.; Mustafa, S.H.; Kumar, R.; Adnan, L.N.U.; Bhimani, P.; Ali, S.R.; Lnu, A.; Shah, S.H.A. Risk Factors of Peripheral Vascular Disease in Diabetes Mellitus in Abbottabad, Pakistan: A Cross-Sectional Study. Cureus 2021, 13, e17556. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 2248. [Google Scholar] [CrossRef]
- Wen, C.; Ying, Y.; Yu, F.; Zhou, J. Research Progress of Oxidative Stress and MicroRNAs in the Prevention of Catheter-Related Thrombus Under Resistance Exercise. Clin. Appl. Thromb. 2020, 26, 1076029620931931. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Impact of Micronutrients on Hypertension: Evidence from Clinical Trials with a Special Focus on Meta-Analysis. Nutrients 2021, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, D.H.; Paletas, K.; Pegiou, T.; Sarigianni, M.; Befani, C.; Koliakos, G. A novel assay for the evaluation of the prooxidant–antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin. Biochem. 2007, 40, 248–254. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Ghayour-Mobarhan, M.; Tavallaie, S.; Parizadeh, M.R.; Moohebati, M.; Ghafoori, F.; Kazemi-Bajestani, S.M.R.; Paletas, K.; Pegiou, T.; Koliakos, G. Prooxidant–antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin. Biochem. 2008, 41, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Chaparro, S.; López-Carmona, M.D.; Cobos-Palacios, L.; Sanz-Cánovas, J.; Bernal-López, M.R.; Gómez-Huelgas, R. Statins and Peripheral Arterial Disease: A Narrative Review. Front. Cardiovasc. Med. 2021, 8, 777016. [Google Scholar] [CrossRef]
- Wang, Q.; Zennadi, R. Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. Int. J. Mol. Sci. 2020, 21, 4259. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef] [PubMed]

| Terms 1 | Search Strategy Terms |
|---|---|
| Term 1 | “Antioxidants” OR “Antioxidant treatments” “Antioxidant therapy” |
| Term 2 | “Antioxidants drugs” OR “vitamin c” OR “Ascorbic acid” OR “Vitamin A” OR “Vitamin E” OR “Lipoic acid” OR “Masoprocol” OR “Pramipexole” OR “Nitric Oxide” OR “Allopurinol” OR “Pentoxifylline” OR “Melatonin” OR “Dimethyl sulfoxide” OR “Probucol” OR “Resveratrol” OR “3-hydroxyanthranilic acid” OR Acetylcysteine OR Nicaraven OR Lodoxamide OR Mequinol OR “Hydroquinone” OR “Selenic acid” OR “Selenium” OR “Lycopene” OR “Tocopherol” OR “Rebamipide” OR “Allicin” OR “Anisodamine” OR “Bucillamine” OR “Carvedilol” OR “Pentoxifylline” |
| Term 3 | Consumption OR “Dietary intake” OR Supplement OR Supplementation OR “Nutritional supplement” |
| Term 4 | “Peripheral arterial disease” OR “Peripheral artery disease” OR “PAD” OR “Peripheral vascular disease” OR “PVD”, “Atherosclerosis” OR “Coronary artery disease” OR “CAD” |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies that are randomized controlled trials | Studies including participants at risk of PAD, including otherwise healthy smokers and hypertensive or diabetic patients |
| Studies with a sample size ≤ 10 participants | Studies that administer mixed nutrient supplementation where no group receives any specific antioxidant supplement alone |
| Studies including participants who either are healthy or have established PAD | Studies incorporating dual treatments such as exercise and supplementation |
| Studies that orally administer a single antioxidant intervention through supplementation or dietary or drug interventions | studies on animal models |
| Study | Year | Sample Size (N) | Age (y) | Gender | Intervention | Form of Intervention (Natural or Synthetic) | Dose/Day | Duration | Control | Status after Intervention | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dalgad et al. [10] | 2009 | 48 | Not mentioned | Not mentioned | Orange and blackcurrant juice (vitamin C); orange and blackcurrant juice + vitamin E | Natural: orange and blackcurrant juice | 210 mg | 4 weeks | Sugar-containing reference beverage (0 mg vit c) | There were significant effects in orange and blackcurrant juice-treated patients but not in combination with vitamin E |
| 2 | Klipstein-Grobusch et al. [11] | 2001 | 574 | 55–94 | 204 men and 370 women | Dietary β-carotene, vitamin C, and vitamin E | Natural: 70 food items in 13 food groups | Was not considered due to insufficient accuracy in the recording of the dose and duration | 1 year | There were no controls in this study | In women, vitamin C intake was significantly inversely associated with the risk of PAD and lower ankle–arm systolic blood pressure Index (AAI); in men, inverse associations of PAD and AAI with vitamin E were observed; no association of αtocopherol and β-carotene with intermittent claudication |
| 3 | Woessner et al. [12] | 2018 | 24 | 40–80 | Not mentioned | Beetroot juice | Natural | 70 mL | 12 weeks | Placebo beverage | There was a significant improvement in walking distance |
| 4 | Kleijnen et al. [13] | 1998 | 154 | 50–60 | Men | Vitamin E | Synthetic: dietary supplement | 300–900 mg | 8–10 months | Placebo | There were no significant effects |
| 5 | Catalano et al. [14] | 2007 | 210 | 60–70 | Men | Oral antioxidant vitamins (vitamin E, vitamin C, and beta-carotene) | Synthetic: dietary supplement | 600 mg vitamin E, 250 mg vitamin C, and 20 mg beta-carotene | >20 months | Placebo | There were no significant effects |
| 6 | McDermott [15] | 2017 | 66 | 65 | 45 men and 65 women | Resveratrol | Synthetic medicinal supplement | Group 1: 500 mg (N = 22) Group 2: 125 mg (N = 22) | 6 months | Placebo | There were no significant effects |
| 7 | Jepson et al. [16] | 2013 | 78 | 40–70 | Men and women | Allium sativum (garlic) | Synthetic: dietary supplement | 800 mg | 12 weeks | Placebo | There were no significant effects |
| 8 | Horsch et al. [17] | 2004 | 619 | Not mentioned | Not mentioned | Ginkgo biloba (ginkgo) | Synthetic: dietary supplement | 120–160 mg | 6–24 weeks | Placebo | There was a significant improvement in pain-free walking |
| 9 | Sommerfield et al. [18] | 2007 | 425 | Not mentioned | Not mentioned | Omega-3 fatty acids | Synthetic: dietary supplement | 45 mg to 3 g | 4 months to 2 years | Placebo | There were no significant effects |
| 10 | Curtis et al. [19] | 2013 | 93 | Not mentioned | Not mentioned | Combined isoflavone and flavan-3-ols | Synthetic combination of isoflavone and flavan-3-ols | 100 mg isoflavone; 850 mg flavan-3-ols | 1 year | Placebo | There were no significant effects |
| 11 | Loffredo et al. [20] | 2014 | 20 | 60–70 | 14 men and 6 women | Cocoa | Synthetic dark chocolate (>85% cocoa) | 40 g | 2 h after chocolate ingestion | Milk chocolate (≤35% cocoa) | There was a significant improvement in maximal walking distance serum NOx and decreased serum isoprostanes |
| 12 | Hammer et al. [21] | 2015 | 21 | 60–70 | 17 men and 4 women | Cocoa | Synthetic dark chocolate | 50 g | 2 h after chocolate ingestion | Placebo | There was no significant effect |
| 13 | McDermott et al. [22] | 2020 | 44 | 70–80 | Men | Cocoa | Synthetic cocoa beverage | 15 g | 6 months | Placebo beverage | There was a significant improvement in walking distance |
| 14 | Belch et al. [23] | 2008 | 320 | 40 or more | Men and women | Antioxidant capsule contained α-tocopherol, ascorbic acid, pyridoxine hydrochloride, zinc sulfate, nicotinamide, lecithin, and sodium selenite | Synthetic antioxidant capsule combination | α-tocopherol 200 mg, ascorbic acid 100 mg, pyridoxine hydrochloride 25 mg, zinc sulfate 10 mg, nicotinamide 10 mg, lecithin 9.4 mg, and sodium selenite 0.8 m | 1–5 years | Placebo | There was a significant decrease in deaths from coronary heart disease |
| 15 | Loffredo et al. [24] | 2006 | 40 | 40–80 | Not mentioned | Propionyl -L-carnitine | Synthetic intravenous propionyl-L-carnitine | 6 mg | 7 days | Placebo | There was a significant improvement in maximum walking distance |
| 16 | Loffredo et al. [25] | 2007 | 25 | 40–80 | Not mentioned | Propionyl-L-carnitine | Synthetic intravenous propionyl-L-carnitine | 6 mg | 7 days | Placebo | There was a significant improvement in the oxidative stress marker and flow-mediated dilation |
| 17 | Singh JA et al. [26] | 2018 | 3167 | 75–85 | Men and women | Allopurinol | Synthetic | Not enough evidence to determine | 5 years | There were no controls in this study | There was a significantly lower risk of PAD in the longer allopurinol users. |
| 18 | Poggesi et al. [27] | 1985 | 10 | 32–50 | 8 men and 2 women | Pentoxifylline | Synthetic pentoxifylline ampules; pentoxifylline tablets | 100 mg pentoxifylline ampules; 400 mg pentoxifylline tablets | 1–20 days | Placebo | There was a significant improvement in arterial blood flow and antithrombotic effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keramat, S.; Sharebiani, H.; Patel, M.; Fazeli, B.; Stanek, A. The Potential Role of Antioxidants in the Treatment of Peripheral Arterial Disease: A Systematic Review. Antioxidants 2022, 11, 2126. https://doi.org/10.3390/antiox11112126
Keramat S, Sharebiani H, Patel M, Fazeli B, Stanek A. The Potential Role of Antioxidants in the Treatment of Peripheral Arterial Disease: A Systematic Review. Antioxidants. 2022; 11(11):2126. https://doi.org/10.3390/antiox11112126
Chicago/Turabian StyleKeramat, Shayan, Hiva Sharebiani, Malay Patel, Bahare Fazeli, and Agata Stanek. 2022. "The Potential Role of Antioxidants in the Treatment of Peripheral Arterial Disease: A Systematic Review" Antioxidants 11, no. 11: 2126. https://doi.org/10.3390/antiox11112126