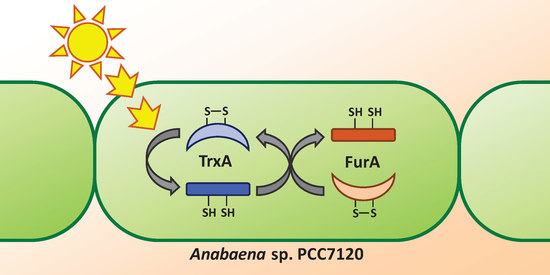
Thioredoxin Dependent Changes in the Redox States of FurA from Anabaena sp. PCC 7120
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cloning, Expression and Protein Purification
2.2. FurA Cross-Linking Assays
2.3. Reconstitution of the NTR/TrxA/FurA Redox Cascade and Determination of Protein Redox States In Vitro
2.4. Determination of FurA Redox States In Vivo
2.5. Analysis of Protein–Protein Interaction Using the Bacterial Two-Hybrid System
3. Results
3.1. FurA Interacts with TrxA
3.1.1. Cross-Linking of FurA with TrxA
3.1.2. In Vivo Analysis of FurA–TrxA Interaction
3.2. Thioredoxin A Is Able to Reduce FurA in Anabaena sp. PCC7120
3.3. Thioredoxin A Preferentially Reduces the Disulfide Bridge between C141 and C144 in Recombinant Wild-Type FurA
3.4. Light-Dark Modulation of FurA Thiol Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fillat, M.F. The FUR (ferric uptake regulator) superfamily: Diversity and versatility of key transcriptional regulators. Arch Biochem. Biophys. 2014, 546, 41–52. [Google Scholar] [CrossRef]
- Bagg, A.; Neilands, J.B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 1987, 26, 5471–5477. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; López-Gomollón, S.; Muro-Pastor, A.; Valladares, A.; Bes, M.T.; Peleato, M.L.; Fillat, M.F. Interaction of FurA from Anabaena sp. PCC 7120 with DNA: A Reducing Environment and the Presence of Mn2+ are Positive Effectors in the Binding to isiB and furA Promoters. Biometals 2006, 19, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Pich, O.Q.; Carpenter, B.M.; Gilbreath, J.J.; Merrell, D.S. Detailed analysis of Helicobacter pylori Fur-regulated promoters reveals a Fur box core sequence and novel Fur-regulated genes. Mol. Microbiol. 2012, 84, 921–941. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Bes, M.T.; Valladares, A.; Peleato, M.L.; Fillat, M.F. FurA is the master regulator of iron homeostasis and modulates the expression of tetrapyrrole biosynthesis genes in Anabaena sp. PCC 7120. Environ. Microbiol. 2012, 14, 3175–3187. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.; Carpenter, B.M.; Gancz, H.; Merrell, D.S. Helicobacter pylori apo-Fur regulation appears unconserved across species. J. Microbiol. 2010, 48, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontenot, C.R.; Tasnim, H.; Valdes, K.A.; Popescu, C.V.; Ding, H. Ferric uptake regulator (Fur) reversibly binds a [2Fe-2S] cluster to sense intracellular iron homeostasis in Escherichia coli. J. Biol. Chem. 2020, 295, 15454–15463. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.K.; Tabor, S.; Richardson, C.C. Proteomic analysis of thioredoxin-targeted proteins in Escherichia coli. Proc. Natl. Acad. Sci. USA 2004, 101, 3759–3764. [Google Scholar] [CrossRef] [Green Version]
- Arts, I.S.; Vertommen, D.; Baldin, F.; Laloux, G.; Collet, J.F. Comprehensively Characterizing the Thioredoxin Interactome In Vivo Highlights the Central Role Played by This Ubiquitous Oxidoreductase in Redox Control. Mol. Cell. Proteom. 2016, 15, 2125–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsburgh, M.J.; Ingham, E.; Foster, S.J. In Staphylococcus aureus, fur is an interactive regulator with PerR, contributes to virulence, and Is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 2001, 183, 468–475. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Bes, M.T.; Peleato, M.L.; Fillat, M.F. Unravelling the regulatory function of FurA in Anabaena sp. PCC 7120 through 2-D DIGE proteomic analysis. J. Proteom. 2011, 74, 660–671. [Google Scholar] [CrossRef]
- González, A.; Bes, M.T.; Peleato, M.L.; Fillat, M.F. Expanding the Role of FurA as Essential Global Regulator in Cyanobacteria. PLoS ONE 2016, 11, e0151384. [Google Scholar] [CrossRef] [Green Version]
- Imlay, J.A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006, 59, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Bes, M.T.; Barja, F.; Peleato, M.L.; Fillat, M.F. Overexpression of FurA in Anabaena sp. PCC 7120 reveals new targets for this regulator involved in photosynthesis, iron uptake and cellular morphology. Plant Cell Physiol. 2010, 51, 1900–1914. [Google Scholar] [CrossRef] [Green Version]
- Martín-Luna, B.; Sevilla, E.; González, A.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Expression of fur and its antisense α-fur from Microcystis aeruginosa PCC7806 as response to light and oxidative stress. J. Plant Physiol. 2011, 168, 2244–2250. [Google Scholar] [CrossRef]
- Hernández, J.A.; Bes, M.T.; Fillat, M.F.; Neira, J.L.; Peleato, M.L. Biochemical analysis of the recombinant Fur (ferric uptake regulator) protein from Anabaena PCC 7119: Factors affecting its oligomerization state. Biochem. J. 2002, 366, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botello-Morte, L.; Pellicer, S.; Sein-Echaluce, V.C.; Contreras, L.M.; Neira, J.L.; Abian, O.; Velázquez-Campoy, A.; Peleato, M.L.; Fillat, M.F.; Bes, M.T. Cysteine Mutational Studies Provide Insight into a Thiol-Based Redox Switch Mechanism of Metal and DNA Binding in FurA from Anabaena sp. PCC 7120. Antioxid Redox Signal 2016, 24, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Pellicer, S.; González, A.; Peleato, M.L.; Martinez, J.I.; Fillat, M.F.; Bes, M.T. Site-directed mutagenesis and spectral studies suggest a putative role of FurA from Anabaena sp. PCC 7120 as a heme sensor protein. FEBS J. 2012, 279, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Guío, J.; Sarasa-Buisán, C.; Velázquez-Campoy, A.; Bes, M.T.; Fillat, M.F.; Peleato, M.L.; Sevilla, E. 2-oxoglutarate modulates the affinity of FurA for the ntcA promoter in Anabaena sp. PCC 7120. FEBS Lett. 2020, 594, 278–289. [Google Scholar] [CrossRef]
- Botello-Morte, L.; Bes, M.T.; Heras, B.; Fernández-Otal, A.; Peleato, M.L.; Fillat, M.F. Unraveling the redox properties of the global regulator FurA from Anabaena sp. PCC 7120: Disulfide reductase activity based on its CXXC motifs. Antioxid. Redox Signal. 2014, 20, 1396–1406. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Nguyen, A.Y.; Dai, Z.; Su, D.; Gaffrey, M.J.; Moore, R.J.; Jacobs, J.M.; Monroe, M.E.; Smith, R.D.; Koppenaal, D.W.; et al. Proteome-wide Light/Dark Modulation of Thiol Oxidation in Cyanobacteria Revealed by Quantitative Site-specific Redox Proteomics. Mol. Cell Proteom. 2014, 13, 3270–3285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadler, N.C.; Melnicki, M.R.; Serres, M.H.; Merkley, E.D.; Chrisler, W.B.; Hill, E.A.; Romine, M.F.; Kim, S.; Zink, E.M.; Datta, S.; et al. Live Cell Chemical Profiling of Temporal Redox Dynamics in a Photoautotrophic Cyanobacterium. ACS Chem. Biol. 2014, 9, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Ansong, C.; Sadler, N.C.; Hill, E.A.; Lewis, M.P.; Zink, E.M.; Smith, R.D.; Beliaev, A.S.; Konopka, A.E.; Wright, A.T. Characterization of protein redox dynamics induced during light-to-dark transitions and nutrient limitation in cyanobacteria. Front. Microbiol. 2014, 5, 325. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Florencio, F.J.; Pérez-Pérez, M.E.; López-Maury, L.; Mata-Cabana, A.; Lindahl, M. The diversity and complexity of the cyanobacterial thioredoxin systems. Photosynth. Res. 2006, 89, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Gleason, F.K.; Holmgren, A. Thioredoxin and related proteins in procaryotes. FEMS Microbiol. Rev. 1988, 4, 271–297. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Schurmann, P.; Wolosiuk, R.A.; Jacquot, J.P. The ferredoxin/thioredoxin system: From discovery to molecular structures and beyond. Photosynth. Res. 2002, 73, 215–222. [Google Scholar] [CrossRef]
- Buey, R.M.; Fernández-Justel, D.; González-Holgado, G.; Martínez-Júlvez, M.; González-López, A.; Velázquez-Campoy, A.; Medina, M.; Buchanan, B.B.; Balsera, M. Unexpected diversity of ferredoxin-dependent thioredoxin reductases in cyanobacteria. Plant. Physiol. 2021, 186, 285–296. [Google Scholar] [CrossRef]
- Gleason, F.K.; Holmgren, A. Isolation and characterization of thioredoxin from the cyanobacterium, Anabaena sp. J. Biol. Chem. 1981, 256, 8306–8309. [Google Scholar] [CrossRef]
- Buey, R.M.; Galindo-Trigo, S.; López-Maury, L.; Velázquez-Campoy, A.; Revuelta, J.L.; Florencio, F.J.; De Pereda, J.M.; Schürmann, P.; Buchanan, B.B.; Balsera, M. A New Member of the Thioredoxin Reductase Family from Early Oxygenic Photosynthetic Organisms. Mol. Plant 2017, 10, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Pellicer, S.; Bes, M.T.; González, A.; Neira, J.L.; Peleato, M.L.; Fillat, M.F. High-recovery one-step purification of the DNA-binding protein Fur by mildguanidinium chloride treatment. Process. Biochem. 2010, 45, 292–296. [Google Scholar] [CrossRef]
- Fillat, M.F.; Borrias, W.E.; Weisbeek, P.J. Isolation and overexpression in Escherichia coli of the flavodoxin gene from Anabaena PCC 7119. Biochem. J. 1991, 280, 187–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pueyo, J.J.; Gomez-Moreno, C.; Mayhew, S.G. Oxidation-reduction potentials of ferredoxin-NADP+ reductase and flavodoxin from Anabaena PCC 7119 and their electrostatic and covalent complexes. Eur. J. Biochem. 1991, 202, 1065–1071. [Google Scholar] [CrossRef]
- Leichert, L.I.; Jakob, U. Global Methods to Monitor the Thiol–Disulfide State of Proteins In Vivo. Antioxid. Redox Signal. 2006, 8, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Karimova, G.; Pidoux, J.; Ullmann, A.; Ladant, D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 5752–5756. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Lindahl, M.; Florencio, F.J. Thioredoxin-linked processes in cyanobacteria are as numerous as in chloroplasts, but targets are different. Proc. Natl. Acad. Sci. USA 2003, 100, 16107–16112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Pérez, M.E.; Florencio, F.J.; Lindahl, M. Selecting thioredoxins for disulphide proteomics: Target proteomes of three thioredoxins from the cyanobacterium Synechocystis sp. PCC 6803. Proteomics 2006, 6 (Suppl. S1), S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Mata-Cabana, A.; Florencio, F.J.; Lindahl, M. Membrane proteins from the cyanobacterium Synechocystis sp. PCC 6803 interacting with thioredoxin. Proteomics 2007, 7, 3953–3963. [Google Scholar] [CrossRef]
- Kadowaki, T.; Nishiyama, Y.; Hisabori, T.; Hihara, Y. Identification of OmpR-family response regulators interacting with thioredoxin in the Cyanobacterium Synechocystis sp. PCC 6803. PLoS ONE 2015, 10, e0119107. [Google Scholar]
- Kujirai, J.; Nanba, S.; Kadowaki, T.; Oka, Y.; Nishiyama, Y.; Hayashi, Y.; Arai, M.; Hihara, Y. Interaction of the GntR-family transcription factor Sll1961 with thioredoxin in the cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2018, 8, 6666. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Nakamura, K.; Kojima, K.; Nishiyama, Y.; Hatakeyama, W.; Hisabori, T.; Hihara, Y. The PedR transcriptional regulator interacts with thioredoxin to connect photosynthesis with gene expression in cyanobacteria. Biochem. J. 2010, 431, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Ehira, S.; Ohmori, M. The Redox-sensing Transcriptional Regulator RexT Controls Expression of Thioredoxin A2 in the Cyanobacterium Anabaena sp. Strain PCC 7120. J. Biol. Chem. 2012, 287, 40433–40440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolšek, K.; Aponte-Santamaria, C.; Gräter, F. Accessibility explains preferred thiol-disulfide isomerization in a protein domain. Sci. Rep. 2017, 7, 9858. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, J.; Ballou, D.P.; Williams, C.H. Reactivity of Thioredoxin as a Protein Thiol-Disulfide Oxidoreductase. Chem. Rev. 2011, 111, 5768–5783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihara, S.; Sugiura, K.; Yoshida, K.; Hisabori, T. Thioredoxin targets are regulated in heterocysts of cyanobacterium Anabaena sp. PCC 7120 in a light-independent manner. J. Exp. Bot. 2020, 71, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hara, S.; Hisabori, T. Thioredoxin Selectivity for Thiol-based Redox Regulation of Target Proteins in Chloroplasts. J. Biol. Chem. 2015, 290, 14278–14288. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guío, J.; Bes, M.T.; Balsera, M.; Calvo-Begueria, L.; Sevilla, E.; Peleato, M.L.; Fillat, M.F. Thioredoxin Dependent Changes in the Redox States of FurA from Anabaena sp. PCC 7120. Antioxidants 2021, 10, 913. https://doi.org/10.3390/antiox10060913
Guío J, Bes MT, Balsera M, Calvo-Begueria L, Sevilla E, Peleato ML, Fillat MF. Thioredoxin Dependent Changes in the Redox States of FurA from Anabaena sp. PCC 7120. Antioxidants. 2021; 10(6):913. https://doi.org/10.3390/antiox10060913
Chicago/Turabian StyleGuío, Jorge, María Teresa Bes, Mónica Balsera, Laura Calvo-Begueria, Emma Sevilla, María Luisa Peleato, and María F. Fillat. 2021. "Thioredoxin Dependent Changes in the Redox States of FurA from Anabaena sp. PCC 7120" Antioxidants 10, no. 6: 913. https://doi.org/10.3390/antiox10060913