Untargeted Metabolomics and Antioxidant Capacities of Muscadine Grape Genotypes during Berry Development
Abstract
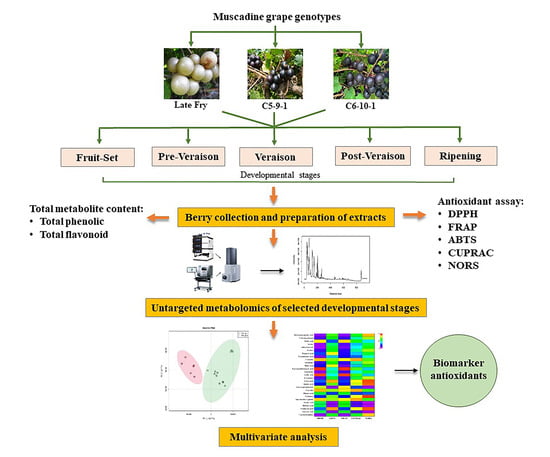
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Muscadine Grape Materials
2.3. Preparation of Muscadine Extracts
2.4. Analysis of Total Phenolic and Flavonoid Contents
2.5. Analysis of Antioxidant Activities
2.5.1. DPPH Radical-Scavenging Activity
2.5.2. Ferric Reducing Antioxidant Potential (FRAP) Assay
2.5.3. ABTS Assay
2.5.4. Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Assay
2.5.5. Nitric Oxide Radical-Scavenging (NORS) Assay
2.6. Untargeted Metabolomics Using UPLC-TOF-MS Analysis
2.7. Data Processing and Statistical Analysis
2.8. Metabolite Identification
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Content
3.2. Total Antioxidant Activities
3.3. The Metabolite Profiling of Muscadine Grape Genotypes at Selected Developmental Stages
3.4. Multivariate Analysis of Candidate Metabolites and Antioxidant Activities of Muscadine Grapes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olien, W.C. The muscadine grape: Botany, viticulture, history, and current industry. Hort. Sci. 1990, 25, 732–739. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit quality, nutraceutical and antimicrobial properties of 58 muscadine grape varieties (Vitis rotundifolia Michx.) grown in United States. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- Bralley, E.E.; Hargrove, J.L.; Greenspan, P.; Hartle, D.K. Topical anti-inflammatory activities of Vitis rotundifolia (muscadine grape) extracts in the tetradecanoylphorbol acetate model of ear inflammation. J. Med. Food 2007, 10, 636–642. [Google Scholar] [CrossRef] [PubMed]
- God, J.M.; Tate, P.; Larcom, L.L. Anticancer effects of four varieties of muscadine grape. J. Med. Food 2007, 10, 54–59. [Google Scholar] [CrossRef]
- Hudson, T.S.; Hartle, D.K.; Hursting, S.D.; Nunez, N.P.; Wang, T.T.; Young, H.A.; Arany, P.; Green, J.E. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007, 67, 8396–8405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonca, P.; Darwish, A.G.; Tsolova, V.; El-Sharkawy, I.; Soliman, K.F.A. The anticancer and antioxidant effects of muscadine grape extracts on racially different triple-negative breast cancer cells. Anticancer Res. 2019, 39, 4043–4053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellen, P.B.; Daniel, K.R.; Brosnihan, K.B.; Hansen, K.J.; Herrington, D.M. Effect of muscadine grape seed supplementation on vascular function in subjects with or at risk for cardiovascular disease: A randomized crossover trial. J. Am. Coll. Nutr. 2010, 29, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wang, G.P.; Whitlock, J.A.; Zhao, S.; Yagiz, Y.; Gu, L. Muscadine grapes (Vitis rotundifolia) and dealcoholized muscadine wine alleviated symptoms of colitis and protected against dysbiosis in mice exposed to dextran sulfate sodium. J. Funct. Foods 2020, 65, 103746. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds—Biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Sharp, J.L.; Wang, X.; You, Y.; Zhang, C. High-performance liquid chromatography–mass spectrometry and evaporative light-scattering detector to compare phenolic profiles of muscadine grapes. J. Chromatogr. A 2012, 1240, 96–103. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gu, L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (Muscadine Grapes) as determined by HPLC-DAD-ESI-MS(n). J. Agric. Food. Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef]
- Anesi, A.; Stocchero, M.; Dal Santo, S.; Commisso, M.; Zenoni, S.; Ceoldo, S.; Tornielli, G.B.; Siebert, T.E.; Herderich, M.; Pezzotti, M.; et al. Towards a scientific interpretation of the terroir concept: Plasticity of the grape berry metabolome. BMC Plant. Biol. 2015, 15, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshef, N.; Walbaum, N.; Agam, N.; Fait, A. Sunlight modulates fruit metabolic profile and shapes the spatial pattern of compound accumulation within the grape cluster. Front. Plant. Sci. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, S.; Wu, Y.; Fu, R.; Wang, L.; Chen, Y.; Xu, W.; Zhang, C.; Ma, C.; Shi, J.; Wang, S. Comparative metabolic profiling of grape skin tissue along grapevine berry developmental stages reveals systematic influences of root restriction on skin metabolome. Int. J. Mol. Sci. 2019, 20, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Coombe, B.G. Research on development and ripening of the grape berry. Am. J. Enol. Vitic. 1992, 43, 101–110. [Google Scholar]
- Wang, L.; Sun, X.; Weiszmann, J.; Weckwerth, W. System-level and granger network analysis of integrated proteomic and metabolomic dynamics identifies key points of grape berry development at the interface of primary and secondary metabolism. Front. Plant. Sci. 2017, 8, 1066. [Google Scholar] [CrossRef] [Green Version]
- Marshall, D.A.; Stringer, S.J.; Spiers, J.D. Stilbene, ellagic acid, flavonol, and phenolic content of muscadine grape (Vitis rotundifolia Michx.) cultivars. Pharm. Crop. 2012, 3, 69–77. [Google Scholar] [CrossRef]
- Pinu, F.R. Grape and wine metabolomics to develop new insights using untargeted and targeted approaches. Fermentation 2018, 4, 92. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Cao, X.; Yuan, Z.; Guo, G. Untargeted metabolomics coupled with chemometrics approach for Xinyang Maojian green tea with cultivar, elevation and processing variations. Food Chem. 2021, 352, 129359. [Google Scholar] [CrossRef]
- Das, P.R.; Kim, Y.; Hong, S.-J.; Eun, J.-B. Profiling of volatile and non-phenolic metabolites—Amino acids, organic acids, and sugars of green tea extracts obtained by different extraction techniques. Food Chem. 2019, 296, 69–77. [Google Scholar] [CrossRef]
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004, 52, 8021–8030. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Darwish, A.G.G.; Samy, M.N.; Sugimoto, S.; Otsuka, H.; Abdel- Salam, H.; Matsunami, K. Effects of hepatoprotective compounds from the leaves of Lumnitzera racemosa on acetaminophen-induced liver damage in vitro. Chem. Pharm. Bull. 2016, 64, 360–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.-S.; Kim, S.-H.; Kim, Y.-B.; Kim, Y.-C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [Green Version]
- Zheleva-Dimitrova, D.Z. Antioxidant and acetylcholinesterase inhibition properties of Amorpha fruticosa L. and Phytolacca americana L. Pharmacogn. Mag. 2013, 9, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Güçlü, G.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.; Hazra, B.; Sarkar, R.; Biswas, S.; Mandal, D. Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista leaf. Evid. Based Compl. Alt. Med. 2011, 173768, 11. [Google Scholar]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. Metlin: A metabolite mass spectral database. Ther. Drug Monit. 2005, 6, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human metabolome database. Nucleic Acids Res. 2007, 35, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Chem. 2016, 3, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernie, A.R.; Aharoni, A.; Willmitzer, L.; Stitt, M.; Tohge, T.; Kopka, J.; Carroll, A.J.; Saito, K.; Frase, P.D.; DeLuca, V. Recommendations for reporting metabolite data. Plant. Cell 2011, 23, 2477–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Cheng, L.; Zhong, G.-Y.; Liu, R.H. Antioxidant and antiproliferative activities of twenty-four Vitis vinifera grapes. PLoS ONE 2014, 9, e105146. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Singleton, V.L. Grape and Wine Phenolics: Background and Prospects. In Proceedings of the University of California, Davis, Grape and Wine Centenary Symposium, University of California, Davis, CA, USA, 1982; pp. 215–227. Available online: https://link.springer.com/chapter/10.1007/978-1-4615-5919-1_7 (accessed on 3 June 2021).
- Das, P.R.; Eun, J.B. Phenolic acids in tea and coffee and their health benefits. In Phenolic Acids: Properties, Food Sources and Health Effects; Flores, A., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2016; pp. 129–194. [Google Scholar]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Bohm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, Z.; Xie, C.; Gong, W.; Hu, Z.; Peng, Y. A novel mechanism of Gamma-aminobutyric acid (GABA) protecting human umbilical vein endothelial cells (HUVECs) against H2O2-induced oxidative injury. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 217, 68–75. [Google Scholar] [CrossRef]
- Cai, P.; Fang, S.Q.; Yang, X.L.; Wu, J.J.; Liu, Q.H.; Hong, H.; Wang, X.B.; Kong, L. Rational design and multi-biological profiling of novel donepezil-trolox hybrids against Alzheimer’s disease, with cholinergic, antioxidant, neuroprotective and cognition enhancing properties. ACS Chem. Neurosci. 2017, 8, 2496–2511. [Google Scholar] [CrossRef]
- Castro, I.A.; Rogero, M.M.; Junqueira, R.M.; Carrapeiro, M.M. Free radical scavenger and antioxidant capacity correlation of alpha-tocopherol and Trolox measured by three in vitro methodologies. Int. J. Food Sci. Nutr. 2006, 57, 75–82. [Google Scholar] [CrossRef]
- Kaur, M.; Asthir, B.; Mahajan, G. Variation in antioxidants, bioactive compounds and antioxidant capacity in germinated and ungerminated grains of ten rice cultivars. Rice Sci. 2017, 24, 349–359. [Google Scholar] [CrossRef]
- Ryan, E.M.; Duryee, M.J.; Hollins, A.; Dover, S.K.; Pirruccello, S.J.; Sayles, H.; Real, K.D.; Hunter, C.D.; Thiele, G.M.; Mikuls, T.R. Antioxidant properties of citric acid interfere with the uricase-based measurement of circulating uric acid. J. Pharm. Biomed. Anal. 2019, 164, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [Green Version]
- Uranga, J.G.; Podio, N.S.; Wunderlin, D.A.; Santiago, N.A. Theoretical and experimental study of the antioxidant behaviors of 5-O-Caffeoylquinic, quinic and caffeic acids based on electronic and structural properties. ChemistrySelect 2016, 1, 4113–4120. [Google Scholar] [CrossRef]
- Baratto, M.C.; Tattini, M.; Galardi, C.; Pinelli, P.; Romani, A.; Visioli, F.; Basosi, R.; Pogni, R. Antioxidant activity of galloyl quinic derivatives isolated from P. lentiscus leaves. Free Radic. Res. 2003, 37, 405–412. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seong, Y.H.; Bae, K.H. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Kobayashi, M.; Sudo, Y.; Watanabe, S.; Yasukawa, H.; Natori, D.; Hoshino, A.; Negishi, A.; Okita, N.; Komatsu, M.; et al. Trehalose protects against oxidative stress by regulating the Keap1–Nrf2 and autophagy pathways. Redox Biol. 2018, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiang, L.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Effects of dietary leucine on antioxidant activity and expression of antioxidant and mitochondrial-related genes in longissimus dorsi muscle and liver of piglets. Anim. Sci. J. 2019, 90, 990–998. [Google Scholar] [CrossRef]







| No. | Compounds | VIP | Chemical Class | FS | V | RIP-SF | RIP-S |
|---|---|---|---|---|---|---|---|
| 1 | Malic acid | 11.16 | Dicarboxylic acid | YES | YES | YES | YES |
| 2 | Malonic acid | 7.35 | Dicarboxylic acid | YES | YES | YES | YES |
| 3 | Tartaric acid | 7.34 | Carboxylic acid | YES | YES | YES | YES |
| 4 | Oxalic acid | 7.33 | Dicarboxylic acid | YES | YES | YES | YES |
| 5 | Trehalose | 3.04 | Disaccharide | YES | YES | YES | YES |
| 6 | Catechin | 2.27 | Flavonoid | YES | YES | YES | YES |
| 7 | Gallic acid | 2.06 | Phenolic acid | YES | YES | YES | YES |
| 8 | L-arginine | 1.97 | Essential amino acid | YES | YES | YES | YES |
| 9 | Citric acid | 1.65 | Tricarboxylic acid | YES | YES | YES | YES |
| 10 | Oxalacetic acid | 1.63 | Oxodicarboxylic acid | YES | YES | YES | YES |
| 11 | Quinic acid | 1.53 | Cyclohexanecarboxylic acid | YES | YES | YES | YES |
| 12 | 4-Acetamidobutanoic acid | 1.04 | Gamma amino acids | YES | YES | YES | YES |
| 13 | Glutamine | 0.98 | Amino acid | YES | YES | YES | YES |
| 14 | Dihydrouracil | 0.95 | Pyrimidones | YES | YES | YES | YES |
| 15 | 2-Furanmethanol | 0.86 | Heteroaromatic compound | YES | YES | YES | YES |
| 16 | Pyroglutamic acid | 0.82 | Alpha amino acids | YES | YES | YES | YES |
| 17 | Serine | 0.81 | Amino acid | YES | YES | YES | YES |
| 18 | Ornithine | 0.79 | Amino acid | YES | YES | YES | YES |
| 19 | Proline | 0.79 | Amino acid | YES | YES | YES | YES |
| 20 | Glycerophosphocholine | 0.75 | Glycerophosphocholine | YES | YES | YES | YES |
| 21 | Epicatechin-3-gallate | 0.71 | Flavan-3-ol | YES | YES | YES | YES |
| 22 | L-leucine | 0.67 | Essential amino acid | YES | YES | YES | YES |
| 23 | Fumaric acid | 0.61 | Organic compound | YES | YES | YES | YES |
| 24 | 7-Methylxanthine | 0.55 | Purines | YES | YES | YES | YES |
| 25 | Ellagic acid | 0.53 | Hydrolyzable tannin | YES | YES | YES | YES |
| 26 | Hydroxypropionic acid | 0.52 | Alpha hydroxy acid | YES | YES | YES | YES |
| 27 | Tiglic acid | 0.51 | Monocarboxylic unsaturated organic acid | YES | YES | YES | YES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, A.G.; Das, P.R.; Ismail, A.; Gajjar, P.; Balasubramani, S.P.; Sheikh, M.B.; Tsolova, V.; Sherif, S.M.; El-Sharkawy, I. Untargeted Metabolomics and Antioxidant Capacities of Muscadine Grape Genotypes during Berry Development. Antioxidants 2021, 10, 914. https://doi.org/10.3390/antiox10060914
Darwish AG, Das PR, Ismail A, Gajjar P, Balasubramani SP, Sheikh MB, Tsolova V, Sherif SM, El-Sharkawy I. Untargeted Metabolomics and Antioxidant Capacities of Muscadine Grape Genotypes during Berry Development. Antioxidants. 2021; 10(6):914. https://doi.org/10.3390/antiox10060914
Chicago/Turabian StyleDarwish, Ahmed G., Protiva Rani Das, Ahmed Ismail, Pranavkumar Gajjar, Subramani Paranthaman Balasubramani, Mehboob B. Sheikh, Violeta Tsolova, Sherif M. Sherif, and Islam El-Sharkawy. 2021. "Untargeted Metabolomics and Antioxidant Capacities of Muscadine Grape Genotypes during Berry Development" Antioxidants 10, no. 6: 914. https://doi.org/10.3390/antiox10060914