Umuhengerin Neuroprotective Effects in Streptozotocin-Induced Alzheimer’s Disease Mouse Model via Targeting Nrf2 and NF-Kβ Signaling Cascades
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Purification of Umuhengerin
2.2. Animals
2.3. Materials
2.4. SAD Induction
2.5. Design of Experiment
2.6. Morris Water Maze (MWM) Test
2.7. Tissue Sampling
2.8. Estimation of Biochemical Parameters
2.9. Western Blot Analysis
2.10. Histopathological Examination
2.11. Statistical Analysis
3. Results
3.1. Characterization of Isolated Compounds
3.2. Effect of Umuhengerin on the Behavior of the Animals Receiving STZ during Morris Water Maze Task
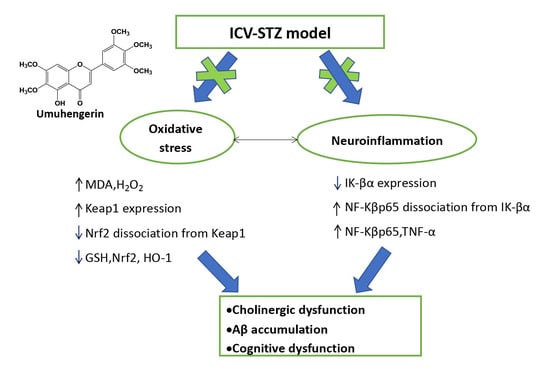
3.3. Effect of Umuhengerin on Oxidative Stress Associated with STZ Administration
3.4. Effect of Umuhengerin on Neuroinflammation Secondary to STZ Administration
3.5. Effect of Umuhengerin on the STZ-Mediated Increase in AChE Enzyme Activity and β-Secretase Protein Expression
3.6. Effect of Umuhengerin Administration on Mice Brain Histopathological Alterations Owing to STZ Administration
3.7. Effect of Umuhengerin Administration on Neuronal Survival Rate
3.8. Effect of Umuhengerin Administration on Amyloid Plaques Numbers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.; Ramalho, T.C. Future therapeutic perspectives into the alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef] [Green Version]
- Bedse, G.; Di Domenico, F.; Serviddio, G.; Cassano, T. Aberrant insulin signaling in Alzheimer’s disease: Current knowledge. Front. Neurosci. 2015, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Haque, R.; Uddin, S.N.; Hossain, A. Amyloid Beta (Aβ) and Oxidative Stress: Progression of Alzheimer’s Disease. AIBM 2018, 11, 555802. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R.; Zhai, S.; Zhang, Y.; Wang, D. Forsythoside B attenuates memory impairment and neuroinflammation via inhibition on NF-κB signaling in Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 305. [Google Scholar]
- Tamagno, E.; Guglielmotto, M.; Monteleone, D.; Vercelli, A.; Tabaton, M. Transcriptional and post-transcriptional regulation of β-secretase. IUBMB Life 2012, 64, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Sun, J.; Zhang, W.; Yu, J.; Zhuang, C. Transcription Factor NRF2 as a Promising Therapeutic Target for Alzheimer’s Disease. Free Radic. Biol. Med. 2020, 159, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Arora, R.B.; Kumar, K.; Deshmukh, R.R. FK506 attenuates intracerebroventricular streptozotocin-induced neurotoxicity in rats. Behav. Pharmacol. 2013, 24, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Atrahimovich, D.; Avni, D.; Khatib, S. Flavonoids-Macromolecules Interactions in Human Diseases with Focus on Alzheimer, Atherosclerosis and Cancer. Antioxidant 2021, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, H.M.; Hassan, N.A.; El-Halawany, A.M.; Mohamed, G.A.; Safo, M.K.; El-Bassossy, H.M. Major flavonoids from Psiadia punctulata produce vasodilation via activation of endothelial dependent NO signaling. J. Adv. Res. 2020, 24, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Zakaria, E.M.; El-Halawany, A.M.; Mohamed, G.A.; Safo, M.K.; El-Bassossy, H.M. Psiadia punctulata major flavonoids alleviate exaggerated vasoconstriction produced by advanced glycation end products. PLoS ONE 2019, 14, e0222101. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.N.; Martins, F.R.; Matheus, M.E.; Leitão, S.G.; Fernandes, P.D. Investigation of anti-inflammatory and antinociceptive activities of Lantana trifolia. J. Ethnopharmacol. 2005, 100, 254–259. [Google Scholar] [CrossRef]
- Sorial, M.E.; El Sayed, N.S.E.D. Protective effect of valproic acid in streptozotocin-induced sporadic Alzheimer’s disease mouse model: Possible involvement of the cholinergic system. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Eskandary, A.; Moazedi, A.A.; Zade, H.N.; Akhond, M.R. Effects of Donepezil Hydrochloride on Neuronal Response of Pyramidal Neurons of the CA1 Hippocampus in Rat Model of Alzheimer’s Disease. Basic Clin. Neurosci. 2019, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Joppa, M.; Carmouche, M.; Cullen, M.J.; Brown, B.; Murphy, B.; Grigoriadis, D.E.; Ling, N.; Foster, A.C. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J. Pharmacol. Exp. Ther. 2000, 293, 799–806. [Google Scholar]
- Pelleymounter, M.A.; Joppa, M.; Ling, N.; Foster, A.C. Pharmacological evidence supporting a role for central corticotropin-releasing factor2 receptors in behavioral, but not endocrine, response to environmental stress. J. Pharmacol. Exp. Ther. 2002, 302, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnock, G.I. Study of the Central Corticotrophin-Releasing Factor System Using the 2-Deoxyglucose Method for Measurement of Local Cerebral Glucose Utilisation. Ph.D. Thesis, University of Bath, Bath, UK, 2007. [Google Scholar]
- Cunha, M.P.; Pazini, F.L.; Rosa, J.M.; Ramos-Hryb, A.B.; Oliveira, Á.; Kaster, M.P.; Rodrigues, A.L.S. Creatine, similarly to ketamine, affords antidepressant-like effects in the tail suspension test via adenosine A 1 and A 2A receptor activation. Purinergic Signal. 2015, 11, 215–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, N.O.A.; El Sayed, N.S.; El-Khatib, A.S. Targeting central β2 receptors ameliorates streptozotocin-induced neuroinflammation via inhibition of glycogen synthase kinase3 pathway in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 65–75. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Liu, L.; Li, S. Protective effects of evodiamine in experimental paradigm of Alzheimer’s disease. Cogn. Neurodyn. 2018, 12, 303–313. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, B.; Jaggi, A.S.; Singh, N. Attenuating effect of lisinopril and telmisartan in intracerebroventricular streptozotocin induced experimental dementia of Alzheimer’s disease type: Possible involvement of PPAR-γ agonistic property. J. Renin Angiotensin Aldosterone Syst. 2013, 14, 124–136. [Google Scholar] [CrossRef]
- Snowdon, D.A. Aging and Alzheimer’s disease: Lessons from the Nun Study. Gerontologist 1997, 37, 150–156. [Google Scholar] [CrossRef]
- West, M.J.; Coleman, P.D.; Flood, D.G.; Troncoso, J.C. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 1994, 344, 769–772. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Z.; Gu, Y.; Ji, Z.; Lin, Z.; Wang, S.; Pan, S.; Wu, Y. Glibenclamide prevents water diffusion abnormality in the brain after cardiac arrest in rats. Neurocrit. Care 2018, 29, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015, 6, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salkovic-Petrisic, M.; Osmanovic, J.; Grünblatt, E.; Riederer, P.; Hoyer, S. Modeling sporadic Alzheimer’s disease: The insulin resistant brain state generates multiple long-term morphobiological abnormalities including hyperphosphorylated tau protein and amyloid-β. J. Alzheimer’s Dis. 2009, 18, 729–750. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Kaur, A.; Singh, T.G. Counteracting role of nuclear factor erythroid 2-related factor 2 pathway in Alzheimer’s disease. Biomed. Pharmacother. 2020, 129, 110373. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Wang, Y.; Zhai, J.; Chen, G.; Hu, K. Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NF-κB and MAPK activation to reduce inflammation in LPS-activated RAW264. 7 cells. Biomed. Pharmacother. 2019, 109, 555–562. [Google Scholar] [CrossRef]
- El Halawany, A.M.; Sayed, N.S.E.; Abdallah, H.M.; El Dine, R.S. Protective effects of gingerol on streptozotocin-induced sporadic Alzheimer’s disease: Emphasis on inhibition of β-amyloid, COX-2, alpha-, beta-secretases and APH1a. Sci. Rep. 2017, 7, 2902. [Google Scholar]
- Chen, C.-H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirwi, A.; El Sayed, N.S.; Abdallah, H.M.; Ibrahim, S.R.M.; Mohamed, G.A.; El-Halawany, A.M.; Safo, M.K.; Abdel Rasheed, N.O. Umuhengerin Neuroprotective Effects in Streptozotocin-Induced Alzheimer’s Disease Mouse Model via Targeting Nrf2 and NF-Kβ Signaling Cascades. Antioxidants 2021, 10, 2011. https://doi.org/10.3390/antiox10122011
Sirwi A, El Sayed NS, Abdallah HM, Ibrahim SRM, Mohamed GA, El-Halawany AM, Safo MK, Abdel Rasheed NO. Umuhengerin Neuroprotective Effects in Streptozotocin-Induced Alzheimer’s Disease Mouse Model via Targeting Nrf2 and NF-Kβ Signaling Cascades. Antioxidants. 2021; 10(12):2011. https://doi.org/10.3390/antiox10122011
Chicago/Turabian StyleSirwi, Alaa, Nesrine S. El Sayed, Hossam M. Abdallah, Sabrin R. M. Ibrahim, Gamal A. Mohamed, Ali M. El-Halawany, Martin K. Safo, and Nora O. Abdel Rasheed. 2021. "Umuhengerin Neuroprotective Effects in Streptozotocin-Induced Alzheimer’s Disease Mouse Model via Targeting Nrf2 and NF-Kβ Signaling Cascades" Antioxidants 10, no. 12: 2011. https://doi.org/10.3390/antiox10122011