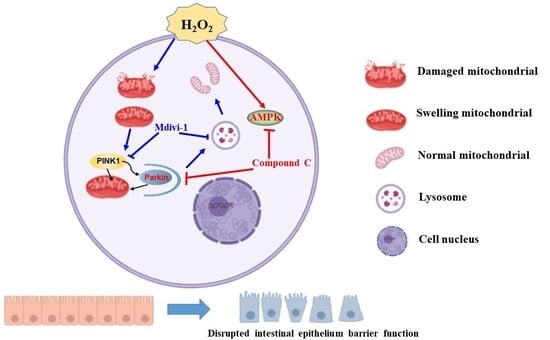
AMPK-PINK1/Parkin Mediated Mitophagy Is Necessary for Alleviating Oxidative Stress-Induced Intestinal Epithelial Barrier Damage and Mitochondrial Energy Metabolism Dysfunction in IPEC-J2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. MTT Measurement
2.3. Antioxidant Enzymes Activities and Malonaldehyde (MDA) Levels
2.4. Quantitative Real-Time PCR
2.5. Western Blot
2.6. IPEC-J2 Epithelial Barrier Function Analysis
2.7. MitoSOX
2.8. ATP Assay
2.9. Mitochondrial Membrane Potential
2.10. Transmission Electron Microscopy (TEM)
2.11. The Profiles of Mitochondrial Oxygen Consumption Rate (OCR)
2.12. Cell Transfection and RNA Knockdown
2.13. Measurement of Apoptosis and Necrosis
2.14. Statistical Analysis
3. Results
3.1. Effects of Different Concentrations of H2O2 Treatment on AMPK Signaling Pathway and Mitophagy-Related Protein Expression
3.2. Effects of Inhibited AMPK and Mitophagy on the Oxidative Stress Response of IPEC-J2 in H2O2-Induced Oxidative Stress Model
3.3. Effects of Inhibition of AMPK and Mitophagy on the IPEC-J2 Epithelial Barrier Function in H2O2-Oxidative Stress Model
3.4. Effects of Inhibition of AMPK and Mitophagy on the Mitochondrial Function and Ultrastructure of IPEC-J2 in H2O2-Induced Oxidative Stress Model
3.5. Effects of Inhibition of AMPK and Mitophagy on the Mitochondrial Metabolism of IPEC-J2 in H2O2-Induced Oxidative Stress Model
3.6. Effects of Inhibition of AMPK and Mitophagy on the Mitophagy Level of IPEC-J2 in H2O2-Induced Oxidative Stress Model
3.7. Effects of Knockdown of AMPK, PINK1 and Parkin in H2O2-Induced Oxidative Stress Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.; Blikslager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef] [Green Version]
- Wijtten, P.J.; Van Der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, Y.; Li, J.; Qi, M.; Tan, B. Serum biochemical parameters and amino acids metabolism are altered in piglets by early-weaning and proline and putrescine supplementations. Anim. Nutr. 2021, 7, 334–345. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning Induced Hepatic Oxidative Stress, Apoptosis, and Aminotransferases through MAPK Signaling Pathways in Piglets. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets2. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.T.; Wang, C.C.; Wu, H.; Zhang, Q.H.; Jiao, L.F.; Hu, C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets1. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.T.; Wu, H.; Wang, C.C.; Zhang, Q.H.; Jiao, L.F.; Lin, F.H.; Hu, C.H.H. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets1. J. Anim. Sci. 2018, 96, 1795–1805. [Google Scholar] [CrossRef]
- Li, M.; Yuan, D.; Liu, Y.; Jin, H.; Tan, B. Dietary Puerarin Supplementation Alleviates Oxidative Stress in the Small Intestines of Diquat-Challenged Piglets. Animals 2020, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Rath, E.; Moschetta, A.; Haller, D. Mitochondrial function—Gatekeeper of intestinal epithelial cell homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 497–516. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; Zhang, Y.; Ren, J. Mitophagy, Mitochondrial Dynamics, and Homeostasis in Cardiovascular Aging. Oxidative Med. Cell. Longev. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Baechler, B.L.; Bloemberg, D.; Quadrilatero, J. Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy 2019, 15, 1606–1619. [Google Scholar] [CrossRef]
- Wu, S.; Zou, M.-H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Laker, R.C.; Drake, J.C.; Wilson, R.J.; Lira, V.A.; Lewellen, B.M.; Ryall, K.A.; Fisher, C.C.; Zhang, M.; Saucerman, J.J.; Goodyear, L.J.; et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free. Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Xiao, K.; Cao, S.; Jiao, L.; Song, Z.; Lu, J.; Hu, C. TGF-β1 protects intestinal integrity and influences Smads and MAPK signal pathways in IPEC-J2 after TNF-α challenge. Innate Immun. 2017, 23, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.H.; Chung, Y.; Cheng, C.-T.; Ouyang, C.; Fu, Y.; Kuo, C.-Y.; Chi, K.K.; Sadeghi, M.; Chu, P.; Kung, H.J.; et al. Autophagic reliance promotes metabolic reprogramming in oncogenic KRAS-driven tumorigenesis. Autophagy 2018, 14, 1481–1498. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Xiao, H.; Li, F.; Zeng, L.; Yin, Y. The profiles of mitochondrial respiration and glycolysis using extracellular flux analysis in porcine enterocyte IPEC-J2. Anim. Nutr. 2015, 1, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxid. Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.; Li, Y.; Ren, W.; Xiao, H.; Chen, S.; Li, C.; Tan, B.; Ni, H.; Xiong, X.; et al. Toxicity assessment of hydrogen peroxide on Toll-like receptor system, apoptosis, and mitochondrial respiration in piglets and IPEC-J2 cells. Oncotarget 2017, 8, 3124–3131. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yin, J.; Ren, W.; Liu, T.; Cui, Z.; Huang, X.; Wu, L.; Kim, S.W.; Liu, G.; Wu, X.; et al. Dietary supplementation with l-glutamate and l-aspartate alleviates oxidative stress in weaned piglets challenged with hydrogen peroxide. Amino Acids 2016, 48, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Mol. 2018, 23, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.; Liang, Z.; Yi, J.; Chen, X.; Li, R.; Wu, Y.; Wu, J.; Sun, Z. Protective Effect of Koumine, an Alkaloid from Gelsemium Sempervirens, on Injury Induced by H2O2 in IPEC-J2 Cells. Int. J. Mol. Sci. 2019, 20, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, Y. AMPK and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 85–108. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free. Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Long, J.; Zhou, X.; Liu, Y.; Li, T.; Wu, X. Serine is required for the maintenance of redox balance and proliferation in the intestine under oxidative stress. FASEB J. 2020, 34, 4702–4717. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Linn, B.S.; Zhang, Y.; Ren, J. Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 2293–2302. [Google Scholar] [CrossRef]
- Barodia, S.K.; Creed, R.B.; Goldberg, M.S. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res. Bull. 2017, 133, 51–59. [Google Scholar] [CrossRef]
- Bader, V.; Winklhofer, K.F. PINK1 and Parkin: Team players in stress-induced mitophagy. Biol. Chem. 2020, 401, 891–899. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, Q.; Cao, B.; Yuan, Y.; Wen, S.; Liu, Z. Cadmium induces mitophagy via AMP-activated protein kinases activation in a PINK1/Parkin-dependent manner in PC12 cells. Cell Prolif. 2020, 53, 12817. [Google Scholar] [CrossRef]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.D.; Smith, B.K.; Green, A.E.; Ducommun, S.; Henriksen, T.I.; Rebalka, I.A.; Razi, A.; Sakamoto, K.; et al. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab. 2016, 24, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Luo, P.; Rao, W.; Dai, S.; Zhang, L.; Ma, W.; Pu, J.; Yu, Y.; Wang, J.; Fei, Z. Homer1a Attenuates Hydrogen Peroxide-Induced Oxidative Damage in HT-22 Cells through AMPK-Dependent Autophagy. Front. Neurosci. 2018, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Bankaitis, E.D.; Ha, A.; Kuo, C.J.; Magness, S.T. Reserve Stem Cells in Intestinal Homeostasis and Injury. Gastroenterology 2018, 155, 1348–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, F.; Sena, P.; Roncucci, L. Inflammatory pathways in the early steps of colorectal cancer development. World J. Gastroenterol. 2014, 20, 9716–9731. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M.; Madsen, K.; Spiller, R.; Meerveld, B.G.-V.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, S.; Liu, Q.; Shan, T.; Wang, Y. Metformin Protects against LPS-Induced Intestinal Barrier Dysfunction by Activating AMPK Pathway. Mol. Pharm. 2018, 15, 3272–3284. [Google Scholar] [CrossRef]
- Scharl, M.; Paul, G.; Barrett, K.E.; McCole, D.F. AMP-activated Protein Kinase Mediates the Interferon-γ-induced Decrease in Intestinal Epithelial Barrier Function. J. Biol. Chem. 2009, 284, 27952–27963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017, 11, 577–585. [Google Scholar] [CrossRef]
- Auciello, F.R.; Ross, F.A.; Ikematsu, N.; Hardie, D.G. Oxidative stress activates AMPK in cultured cells primarily by increasing cellular AMP and/or ADP. FEBS Lett. 2014, 588, 3361–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Y.; Xu, J.; Tian, F.; Hu, S.; Chen, Y.; Fu, Z. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal Res. 2019, 66, e12542. [Google Scholar] [CrossRef] [PubMed]
- Wauters, F.; Cornelissen, T.; Imberechts, D.; Martin, S.; Koentjoro, B.; Sue, C.; Vangheluwe, P.; Vandenberghe, W. LRRK2 mutations impair depolarization-induced mitophagy through inhibition of mitochondrial accumulation of RAB10. Autophagy 2020, 16, 203–222. [Google Scholar] [CrossRef]
- Heo, J.-M.; Ordureau, A.; Swarup, S.; Paulo, J.A.; Shen, K.; Sabatini, D.M.; Harper, J.W. RAB7A phosphorylation by TBK1 promotes mitophagy via the PINK-PARKIN pathway. Sci. Adv. 2018, 4, eaav0443. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Xu, Z.-X.; Ding, Z.; Lu, Y.; Yu, Q.; Werle, K.D.; Zhou, G.; Park, Y.-Y.; Peng, G.; Gambello, M.J.; et al. Myristoylation confers noncanonical AMPK functions in autophagy selectivity and mitochondrial surveillance. Nat. Commun. 2015, 6, 7926. [Google Scholar] [CrossRef]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Seabright, A.P.; Fine, N.H.F.; Barlow, J.P.; Lord, S.O.; Musa, I.; Gray, A.; Bryant, J.A.; Banzhaf, M.; Lavery, G.G.; Hardie, D.G.; et al. AMPK activation induces mitophagy and promotes mitochondrial fission while activating TBK1 in a PINK1-Parkin independent manner. FASEB J. 2020, 34, 6284–6301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Nie, J.; Wu, L.; Hu, Y.; Wen, Z.; Dong, L.; Zou, M.-H.; Chen, C.; Wang, D.W. AMPKα2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ. Res. 2018, 122, 712–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, L.; Hua, Y.; Nair, S.; Xu, X.; Ren, J. AMP-Activated Protein Kinase Deficiency Rescues Paraquat-Induced Cardiac Contractile Dysfunction Through an Autophagy-Dependent Mechanism. Toxicol. Sci. 2014, 142, 6–20. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Chang, L.; Luo, Y.; Zhou, Y.; Zhang, J. Mst1 inhibition attenuates non-alcoholic fatty liver disease via reversing Parkin-related mitophagy. Redox Biol. 2019, 21, 101120. [Google Scholar] [CrossRef]
- Feng, J.; Li, H.; Zhang, Y.; Wang, Q.; Zhao, S.; Meng, P.; Li, J. Mammalian STE20-Like Kinase 1 Deletion Alleviates Renal Ischaemia-Reperfusion Injury via Modulating Mitophagy and the AMPK-YAP Signalling Pathway. Cell. Physiol. Biochem. 2018, 51, 2359–2376. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Yu, B.; Mao, X.B.; Zheng, P.; He, J.; Chen, D.W. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animmal 2012, 6, 928–934. [Google Scholar] [CrossRef]
- Mao, X.B.; Lv, M.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Wang, Q.Y.; Chen, D.W. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J. Anim. Sci. Biotechnol. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Liu, M.; Ren, W.; Duan, J.; Yang, G.; Zhao, Y.; Fang, R.; Chen, L.; Li, T.; Yin, Y. Effects of Dietary Supplementation with Glutamate and Aspartate on Diquat-Induced Oxidative Stress in Piglets. PLoS ONE 2015, 10, e0122893. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Yu, B.; He, J.; Yu, J.; Mao, X.; Luo, Y.; Luo, J.; Huang, Z.; Tian, G.; Zeng, Q.; et al. Arginine metabolism and its protective effects on intestinal health and functions in weaned piglets under oxidative stress induced by diquat. Br. J. Nutr. 2017, 117, 1495–1502. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Cao, S.; Shen, Z.; Hong, Q.; Feng, J.; Peng, Y.; Hu, C. Effects of dietary tributyrin on intestinal mucosa development, mitochondrial function and AMPK-mTOR pathway in weaned pigs. J. Anim. Sci. Biotechnol. 2019, 10, 93. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Xiao, H.; Li, X.; Zhu, J.; Gao, J.; Wang, L.; Hu, C. AMPK-PINK1/Parkin Mediated Mitophagy Is Necessary for Alleviating Oxidative Stress-Induced Intestinal Epithelial Barrier Damage and Mitochondrial Energy Metabolism Dysfunction in IPEC-J2. Antioxidants 2021, 10, 2010. https://doi.org/10.3390/antiox10122010
Cao S, Xiao H, Li X, Zhu J, Gao J, Wang L, Hu C. AMPK-PINK1/Parkin Mediated Mitophagy Is Necessary for Alleviating Oxidative Stress-Induced Intestinal Epithelial Barrier Damage and Mitochondrial Energy Metabolism Dysfunction in IPEC-J2. Antioxidants. 2021; 10(12):2010. https://doi.org/10.3390/antiox10122010
Chicago/Turabian StyleCao, Shuting, Hao Xiao, Xin Li, Jiang Zhu, Jingchun Gao, Li Wang, and Caihong Hu. 2021. "AMPK-PINK1/Parkin Mediated Mitophagy Is Necessary for Alleviating Oxidative Stress-Induced Intestinal Epithelial Barrier Damage and Mitochondrial Energy Metabolism Dysfunction in IPEC-J2" Antioxidants 10, no. 12: 2010. https://doi.org/10.3390/antiox10122010