Fe-Bound Organic Carbon and Sorption of Aromatic Dissolved Organic Carbon in Surface Soil: Comparing a Forest, a Cropland, and a Pasture Soil in the Central Appalachian Region, West Virginia, U.S.A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Soil Sampling
2.3. Soil Physical and Chemical Analysis
2.4. Adsorption Experiments
2.5. Isotherm
2.6. Statistical Analysis
3. Results
3.1. The TOC, Fe-Bound OC, and Reactive Fe in Forest, Cropland, and Pasture Managed Soils
3.2. Amorphous and Exchangeable Fe and Extracted DOC
3.3. The Sorption of DOC to Three Land Management Practices Soils and Changes in ΔSUVA254
4. Discussion
4.1. Comparison of Three Forms of Fe Minerals and Associated Organic Carbon in Three Soils
4.2. Changes of DOC and Aromaticity of DOC during Sorption
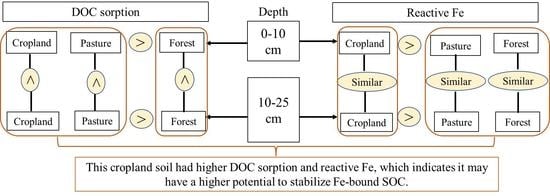
4.3. Implications for Land Use and Stabilization of DOC for Long-Term SOC Sequestration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, F.; Tong, X.; Deng, J.; Yang, G.; Chen, L.; Kang, D. Understanding soil carbon sequestration following the afforestation of former arable land by physical fractionation. Catena 2017, 150, 317–327. [Google Scholar] [CrossRef]
- Kritzberg, E.S.; Ekström, S.M. Increasing iron concentrations in surface waters—A factor behind brownification? Biogeosciences 2012, 9, 1465–1478. [Google Scholar] [CrossRef]
- Chorover, J.; Amistadi, M.K. Reaction of forest floor organic matter at goethite, birnessite and smectite surfaces. Geochim. Et Cosmochim. Acta 2001, 65, 95–109. [Google Scholar] [CrossRef]
- Kleber, M.; Mikutta, R.; Torn, M.; Jahn, R. Poorly crystalline mineral phases protect organic matter in acid subsoil horizons. Eur. J. Soil Sci. 2005, 56, 717–725. [Google Scholar] [CrossRef]
- Riedel, T.; Zak, D.; Biester, H.; Dittmar, T. Iron traps terrestrially derived dissolved organic matter at redox interfaces. Proc. Natl. Acad. Sci. USA 2013, 110, 10101–10105. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M. Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim. Cosmochim. Acta 2007, 71, 25–35. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M.; Kitayama, K.; Shirato, Y. Association of organic matter with iron and aluminum across a range of soils determined via selective dissolution techniques coupled with dissolved nitrogen analysis. Biogeochemistry 2013, 112, 95–109. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Rethemeyer, J.; Matzner, E. Stabilization of dissolved organic matter by sorption to the mineral soil. Soil Biol. Biochem. 2005, 37, 1319–1331. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org. Geochem. 2000, 31, 711–725. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Coward, E.; Ohno, T.; Sparks, D.L. Direct evidence for temporal molecular fractionation of dissolved organic matter at the iron oxyhydroxide interface. Environ. Sci. Technol. 2018, 53, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Eusterhues, K.; Rumpel, C.; Kleber, M.; Kögel-Knabner, I. Stabilisation of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation. Org. Geochem. 2003, 34, 1591–1600. [Google Scholar] [CrossRef]
- Oren, A.; Chefetz, B. Sorptive and desorptive fractionation of dissolved organic matter by mineral soil matrices. J. Environ. Qual. 2012, 41, 526–533. [Google Scholar] [CrossRef]
- Lützow, M.v.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Martens, D.A.; Reedy, T.E.; Lewis, D.T. Soil organic carbon content and composition of 130-year crop, pasture and forest land-use managements. Glob. Change Biol. 2004, 10, 65–78. [Google Scholar] [CrossRef]
- Leifeld, J.; Bassin, S.; Fuhrer, J. Carbon stocks in Swiss agricultural soils predicted by land-use, soil characteristics, and altitude. Agric. Ecosyst. Environ. 2005, 105, 255–266. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Hübner, R.; Spörlein, P.; Geuß, U.; Hangen, E.; Reischl, A.; Schilling, B.; Lützow, M.; Kögel-Knabner, I. Carbon sequestration potential of soils in southeast Germany derived from stable soil organic carbon saturation. Glob. Chang. Biol. 2014, 20, 653–665. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gelinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [Green Version]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Prakash, V.; Kundu, S.; Srivastva, A.; Gupta, H.; Mitra, S. Long term effects of fertilization on carbon and nitrogen sequestration and aggregate associated carbon and nitrogen in the Indian sub-Himalayas. Nutr. Cycl. Agroecosyst. 2010, 86, 1–16. [Google Scholar] [CrossRef]
- Briske, D.D.; Derner, J.; Brown, J.; Fuhlendorf, S.D.; Teague, W.; Havstad, K.; Gillen, R.L.; Ash, A.J.; Willms, W. Rotational grazing on rangelands: Reconciliation of perception and experimental evidence. Rangel. Ecol. Manag. 2008, 61, 3–17. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-term manuring and fertilization effects on soil organic carbon pools under a wheat–maize cropping system in North China Plain. Plant Soil 2009, 314, 67–76. [Google Scholar] [CrossRef]
- Greenwood, K.; McKenzie, B. Grazing effects on soil physical properties and the consequences for pastures: A review. Aust. J. Exp. Agric. 2001, 41, 1231–1250. [Google Scholar] [CrossRef]
- Kalbitz, K.; Geyer, W.; Geyer, S. Spectroscopic properties of dissolved humic substances—a reflection of land use history in a fen area. Biogeochemistry 1999, 47, 219–238. [Google Scholar] [CrossRef]
- Huang, C.; Liu, S.; Li, R.; Sun, F.; Zhou, Y.; Yu, G. Spectroscopic evidence of the improvement of reactive iron mineral content in red soil by long-term application of swine manure. PLoS ONE 2016, 11, e0146364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Wang, P.; Huang, Q.; Yu, G.; Li, D.; Shen, Q.; Ran, W. The role of non-crystalline Fe in the increase of SOC after long-term organic manure application to the red soil of southern China. Eur. J. Soil Sci. 2013, 64, 797–804. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, W.; Deng, W.; He, X.; Yu, G. Impact of agricultural fertilization practices on organo-mineral associations in four long-term field experiments: Implications for soil C sequestration. Sci. Total Environ. 2019, 651, 591–600. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, H.; Li, Y.; Ma, Y.; Tang, H.; Ran, W.; Shen, Q. The role of poorly crystalline iron oxides in the stability of soil aggregate-associated organic carbon in a rice–wheat cropping system. Geoderma 2016, 279, 1–10. [Google Scholar] [CrossRef]
- Lovley, D.; Phillips, E. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 1987, 53, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Van Bodegom, P.M.; Van Reeven, J.; Van Der Gon, H.A.D. Prediction of reducible soil iron content from iron extraction data. Biogeochemistry 2003, 64, 231–245. [Google Scholar] [CrossRef]
- Kramer, M.G.; Sanderman, J.; Chadwick, O.A.; Chorover, J.; Vitousek, P.M. Long-term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Glob. Chang. Biol. 2012, 18, 2594–2605. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Chorover, J.; Goyne, K.W.; Brantley, S.L. Protection of mesopore-adsorbed organic matter from enzymatic degradation. Environ. Sci. Technol. 2004, 38, 4542–4548. [Google Scholar] [CrossRef]
- Takata, Y.; Tani, M.; Kato, T.; Koike, M. Effects of land use and long-term organic matter application on low-molecular-weight organic acids in an Andisol. J. Soil Sci. Environ. Manag. 2011, 2, 292–298. [Google Scholar]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2009, 2, 37–41. [Google Scholar] [CrossRef]
- Zhao, Q.; Poulson, S.R.; Obrist, D.; Sumaila, S.; Dynes, J.J.; McBeth, J.M.; Yang, Y. Iron-bound organic carbon in forest soils: Quantification and characterization. Biogeosciences 2016, 13, 4777–4788. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, T.; Zhao, Q.; Guo, H.; Zhong, W.; Su, H.; Wu, Q. Soil organic carbon stabilization by iron in permafrost regions of the Qinghai-Tibet Plateau. Geophys. Res. Lett. 2016, 43, 10286–10294. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Pena-Yewtukhiw, E.M.; McDonald, L.M.; Skousen, J.; Sperow, M. Early C sequestration rate changes for reclaimed minesoils. Soil Sci. 2012, 177, 443–450. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Pena-Yewtukhiw, E.M.; McDonald, L.M.; Skousen, J.; Sperow, M. Land use effects on sample size requirements for soil organic carbon stock estimations. Soil Sci. 2011, 176, 110–114. [Google Scholar] [CrossRef]
- Yavitt, J. Carbon dynamics in Appalachian peatlands of west Virginia and western Maryland. Water Air Soil Pollut. 1994, 77, 271–290. [Google Scholar] [CrossRef]
- Lei, L.; Thompson, J.A.; McDonald, L.M. Assessment of dissolved organic carbon and iron effects on water color between a forest and pasture-dominated fine-scale catchment in a Central Appalachian region, West Virginia. Environ. Sci. Pollut. Res. 2020, 27, 29464–29474. [Google Scholar] [CrossRef] [PubMed]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Gosling, P.; Parsons, N.; Bending, G.D. What are the primary factors controlling the light fraction and particulate soil organic matter content of agricultural soils? Biol. Fertil. Soils 2013, 49, 1001–1014. [Google Scholar] [CrossRef]
- Luo, Z.; Feng, W.; Luo, Y.; Baldock, J.; Wang, E. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob. Chang. Biol. 2017, 23, 4430–4439. [Google Scholar] [CrossRef]
- Duval, M.E.; Galantini, J.A.; Martínez, J.M.; López, F.M.; Wall, L.G. Sensitivity of different soil quality indicators to assess sustainable land management: Influence of site features and seasonality. Soil Tillage Res. 2016, 159, 9–22. [Google Scholar] [CrossRef]
- Sainepo, B.M.; Gachene, C.K.; Karuma, A. Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag. 2018, 13, 4. [Google Scholar] [CrossRef]
- Kalambukattu, J.G.; Singh, R.; Patra, A.K.; Arunkumar, K. Soil carbon pools and carbon management index under different land use systems in the Central Himalayan region. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2013, 63, 200–205. [Google Scholar] [CrossRef]
- Nahrawi, H.; Husni, M.; Radziah, O. Labile carbon and carbon management index in peat planted with various crops. Commun. Soil Sci. Plant Anal. 2012, 43, 1647–1657. [Google Scholar] [CrossRef]
- Kellner, E.; Hubbart, J.; Stephan, K.; Morrissey, E.; Freedman, Z.; Kutta, E.; Kelly, C. Characterization of sub-watershed-scale stream chemistry regimes in an Appalachian mixed-land-use watershed. Environ. Monit. Assess. 2018, 190, 586. [Google Scholar] [CrossRef]
- Chevallier, T.; Woignier, T.; Toucet, J.; Blanchart, E. Organic carbon stabilization in the fractal pore structure of Andosols. Geoderma 2010, 159, 182–188. [Google Scholar] [CrossRef]
- Wiseman, C.; Püttmann, W. Interactions between mineral phases in the preservation of soil organic matter. Geoderma 2006, 134, 109–118. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Alva, A.; Sumner, M.; Miller, W. Relationship between ionic strength and electrical conductivity for soil solutions. Soil Sci. 1991, 152, 239–242. [Google Scholar] [CrossRef]
- Houba, V.; Temminghoff, E.; Gaikhorst, G.; Van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Mayes, M.A.; Heal, K.R.; Brandt, C.C.; Phillips, J.R.; Jardine, P.M. Relation between soil order and sorption of dissolved organic carbon in temperate subsoils. Soil Sci. Soc. Am. J. 2012, 76, 1027–1037. [Google Scholar] [CrossRef]
- Jardine, P.M.; McCarthy, J.F.; Weber, N.L. Mechanisms of dissolved organic carbon adsorption on soil. Soil Sci. Soc. Am. J. 1989, 53, 1378–1385. [Google Scholar] [CrossRef]
- Sanderman, J.; Amundson, R. A comparative study of dissolved organic carbon transport and stabilization in California forest and grassland soils. Biogeochemistry 2008, 89, 309–327. [Google Scholar] [CrossRef]
- Gao, J.; Liang, C.; Shen, G.; Lv, J.; Wu, H. Spectral characteristics of dissolved organic matter in various agricultural soils throughout China. Chemosphere 2017, 176, 108–116. [Google Scholar] [CrossRef]
- Filep, T.; Zacháry, D.; Jakab, G.; Szalai, Z. Chemical composition of labile carbon fractions in Hungarian forest soils: Insight into biogeochemical coupling between DOM and POM. Geoderma 2022, 419, 115867. [Google Scholar] [CrossRef]
- Fernández-Romero, M.L.; Clark, J.M.; Collins, C.D.; Parras-Alcántara, L.; Lozano-García, B. Evaluation of optical techniques for characterising soil organic matter quality in agricultural soils. Soil Tillage Res. 2016, 155, 450–460. [Google Scholar] [CrossRef]
- Kothawala, D.; Moore, T.; Hendershot, W. Adsorption of dissolved organic carbon to mineral soils: A comparison of four isotherm approaches. Geoderma 2008, 148, 43–50. [Google Scholar] [CrossRef]
- Qi, J.-Y.; Zhao, X.; He, C.; Virk, A.L.; Jing, Z.-H.; Liu, Q.-Y.; Wang, X.; Kan, Z.-R.; Xiao, X.-P.; Zhang, H.-L. Effects of long-term tillage regimes on the vertical distribution of soil iron/aluminum oxides and carbon decomposition in rice paddies. Sci. Total Environ. 2021, 776, 145797. [Google Scholar] [CrossRef]
- Guggenberger, G.; Kaiser, K. Dissolved organic matter in soil: Challenging the paradigm of sorptive preservation. Geoderma 2003, 113, 293–310. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of Soil Organic Matter: Association with Minerals or Chemical Recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Zong, M.; Lin, C.; Li, S.; Li, H.; Duan, C.; Peng, C.; Guo, Y.; An, R. Tillage activates iron to prevent soil organic carbon loss following forest conversion to cornfields in tropical acidic red soils. Sci. Total Environ. 2021, 761, 143253. [Google Scholar] [CrossRef]
- Porras, R.C.; Pries, C.E.H.; McFarlane, K.J.; Hanson, P.J.; Torn, M.S. Association with pedogenic iron and aluminum: Effects on soil organic carbon storage and stability in four temperate forest soils. Biogeochemistry 2017, 133, 333–345. [Google Scholar] [CrossRef]
- Sharma, P.; Laor, Y.; Raviv, M.; Medina, S.; Saadi, I.; Krasnovsky, A.; Vager, M.; Levy, G.J.; Bar-Tal, A.; Borisover, M. Compositional characteristics of organic matter and its water-extractable components across a profile of organically managed soil. Geoderma 2017, 286, 73–82. [Google Scholar] [CrossRef]
- Coward, E.K.; Thompson, A.T.; Plante, A.F. Iron-mediated mineralogical control of organic matter accumulation in tropical soils. Geoderma 2017, 306, 206–216. [Google Scholar] [CrossRef]
- Chow, A.T.; Tanji, K.K.; Gao, S.; Dahlgren, R.A. Temperature, water content and wet–dry cycle effects on DOC production and carbon mineralization in agricultural peat soils. Soil Biol. Biochem. 2006, 38, 477–488. [Google Scholar] [CrossRef]
- Kaiser, K.; Zech, W. Release of natural organic matter sorbed to oxides and a subsoil. Soil Sci. Soc. Am. J. 1999, 63, 1157–1166. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G.; Zech, W. Sorption of DOM and DOM fractions to forest soils. Geoderma 1996, 74, 281–303. [Google Scholar] [CrossRef]
- Rumpel, C.; Baumann, K.; Remusat, L.; Dignac, M.-F.; Barré, P.; Deldicque, D.; Glasser, G.; Lieberwirth, I.; Chabbi, A. Nanoscale evidence of contrasted processes for root-derived organic matter stabilization by mineral interactions depending on soil depth. Soil Biol. Biochem. 2015, 85, 82–88. [Google Scholar] [CrossRef]
- Scott, E.E.; Rothstein, D.E. The dynamic exchange of dissolved organic matter percolating through six diverse soils. Soil Biol. Biochem. 2014, 69, 83–92. [Google Scholar] [CrossRef]
- Sowers, T.D.; Holden, K.L.; Coward, E.K.; Sparks, D.L. Dissolved organic matter sorption and molecular fractionation by naturally-occurring bacteriogenic iron (oxyhydr) oxides. Environ. Sci. Technol. 2019, 53, 4295–4304. [Google Scholar] [CrossRef]
- Leinemann, T.; Preusser, S.; Mikutta, R.; Kalbitz, K.; Cerli, C.; Höschen, C.; Mueller, C.; Kandeler, E.; Guggenberger, G. Multiple exchange processes on mineral surfaces control the transport of dissolved organic matter through soil profiles. Soil Biol. Biochem. 2018, 118, 79–90. [Google Scholar] [CrossRef]
- Adhikari, D.; Yang, Y. Selective stabilization of aliphatic organic carbon by iron oxide. Sci. Rep. 2015, 5, 11214. [Google Scholar] [CrossRef]
- Kalbitz, K.; Kaiser, K. Contribution of dissolved organic matter to carbon storage in forest mineral soils. J. Plant Nutr. Soil Sci. 2008, 171, 52–60. [Google Scholar] [CrossRef]
- Zhao, Q.; Callister, S.J.; Thompson, A.M.; Kukkadapu, R.K.; Tfaily, M.M.; Bramer, L.M.; Qafoku, N.P.; Bell, S.L.; Hobbie, S.E.; Seabloom, E.W.; et al. Strong mineralogic control of soil organic matter composition in response to nutrient addition across diverse grassland sites. Sci. Total Environ. 2020, 736, 137839. [Google Scholar] [CrossRef]
- Du, Y.; Ramirez, C.E.; Jaffé, R. Fractionation of dissolved organic matter by co-precipitation with iron: Effects of composition. Environ. Processes 2018, 5, 5–21. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Anesio, A.M.; Granéli, W.; Aiken, G.R.; Kieber, D.J.; Mopper, K. Effect of humic substance photodegradation on bacterial growth and respiration in lake water. Appl. Environ. Microbiol. 2005, 71, 6267–6275. [Google Scholar] [CrossRef] [PubMed]
- Hobley, E.; Baldock, J.; Hua, Q.; Wilson, B. Land-use contrasts reveal instability of subsoil organic carbon. Glob. Change Biol. 2017, 23, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Kalbitz, K. Properties of organic matter in soil solution in a German fen area as dependent on land use and depth. Geoderma 2001, 104, 203–214. [Google Scholar] [CrossRef]
- Petrone, K.C.; Richards, J.S.; Grierson, P.F. Bioavailability and composition of dissolved organic carbon and nitrogen in a near coastal catchment of south-western Australia. Biogeochemistry 2009, 92, 27–40. [Google Scholar] [CrossRef]
- Václavík, T.; Lautenbach, S.; Kuemmerle, T.; Seppelt, R. Mapping global land system archetypes. Glob. Environ. Chang. 2013, 23, 1637–1647. [Google Scholar] [CrossRef]
- Fithian, R.W. A history and evaluation of the plantations at the West Virginia University farm woods. Ph.D. Thesis, West Virginia University, Morgantown, WV, USA, 1979. [Google Scholar]
- Kettler, T.; Doran, J.W.; Gilbert, T. Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef]
- Wolf, A.; Beegle, D. Recommended Soil Testing Procedures for the Northeastern United States, 3rd ed.; The Northeast Coordinating Committee for Soil Testing: Amherst, MA, USA, 2011; pp. 39–48. [Google Scholar]
- Zinn, Y.L.; Lal, R.; Resck, D.V. Texture and organic carbon relations described by a profile pedotransfer function for Brazilian Cerrado soils. Geoderma 2005, 127, 168–173. [Google Scholar] [CrossRef]
- Hassink, J. The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Franzluebbers, A.; Arshad, M. Particulate organic carbon content and potential mineralization as affected by tillage and texture. Soil Sci. Soc. Am. J. 1997, 61, 1382–1386. [Google Scholar] [CrossRef]
- Hassink, J. Effects of soil texture and grassland management on soil organic C and N and rates of C and N mineralization. Soil Biol. Biochem. 1994, 26, 1221–1231. [Google Scholar] [CrossRef]
- Matus, F.J.; Lusk, C.H.; Maire, C.R. Effects of soil texture, carbon input rates, and litter quality on free organic matter and nitrogen mineralization in Chilean rain forest and agricultural soils. Commun. Soil Sci. Plant Anal. 2007, 39, 187–201. [Google Scholar] [CrossRef]
- Franzluebbers, A.; Haney, R.; Hons, F.; Zuberer, D. Active fractions of organic matter in soils with different texture. Soil Biol. Biochem. 1996, 28, 1367–1372. [Google Scholar] [CrossRef]



| Land Type | Elevation (m) | Slope (%) | Topographic Position | Parent Materials |
|---|---|---|---|---|
| Forest | 303.5–338.7 | 25–35 | Backslope | loamy residuum weathered from limestone, sandstone, and shale |
| Pasture | 324.4–341.8 | 25–35 | Backslope | loamy residuum weathered from limestone, sandstone, and shale |
| Cropland | 289.0–292.4 | 3–5 | Flood plain | loamy alluvium derived from limestone, sandstone, and shale |
| Land Type | Depth(cm) | Fe | Released DOC | SUVA254 of Released DOC |
|---|---|---|---|---|
| (g kg−1) | (g kg−1) | (L m−1 mg−1 per g Soil) | ||
| HF | 0–10 | 1.38 ± 0.04 b | 0.63 ± 0.04 a | 36.89 ± 1.5 bc |
| 10–25 | 0.52 ± 0.01 e | 0.44 ± 0.02 b | 33.16 ± 1.1 c | |
| CM | 0–10 | 1.53 ± 0.02 a | 0.42 ± 0.02 b | 45.38 ± 3.2 ab |
| 10–25 | 0.81 ± 0.002 d | 0.30 ± 0.02 c | 35.03 ± 2.3 c | |
| CP | 0–10 | 1.23 ± 0.02 c | 0.57 ± 0.01 a | 48.16 ± 1.4 a |
| 10–25 | 0.47 ± 0.01 e | 0.30 ± 0.01 c | 32.04 ± 1.4 c | |
| HF | 0–10 | 0.12 ± 0.006 d | 0.37 ± 0.02 ab | 15.36 ± 0.18 d |
| 10–25 | 0.07 ± 0.004 e | 0.25 ± 0.01 cd | 13.50 ± 0.2 d | |
| CM | 0–10 | 0.24 ± 0.009 a | 0.28 ± 0.01 bc | 28.93 ± 1.0 a |
| 10–25 | 0.15 ± 0.002 c | 0.16 ± 0.01 d | 22.68 ± 0.7 b | |
| CP | 0–10 | 0.18 ± 0.004 b | 0.42 ± 0.03 a | 27.99 ± 2.6 a |
| 10–25 | 0.06 ± 0.009 e | 0.22 ± 0.02 cd | 15.67 ± 2.5 d |
| Land type | Depth | Equation | Adjusted r2 | p |
|---|---|---|---|---|
| Forest | 0–10 cm | ΔSUVA254 = −0.014 qe + 1.10 | 0.93 | 0.005 |
| 10–25 cm | ΔSUVA254 = −0.016 qe + 0.71 | 0.86 | 0.014 | |
| Cropland | 0–10 cm | ΔSUVA254 = −0.002 qe + 0.18 | 0.68 | 0.053 |
| 10–25 cm | ΔSUVA254 = 0.014 qe − 0.76 | 0.78 | 0.031 | |
| Pasture | 0–10 cm | ΔSUVA254 = −0.006 qe + 0.61 | 0.77 | 0.031 |
| 10–25 cm | ΔSUVA254 = 0.012 qe − 0.49 | 0.89 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, L.; Holásková, I.; Thompson, J.A.; McDonald, L.M. Fe-Bound Organic Carbon and Sorption of Aromatic Dissolved Organic Carbon in Surface Soil: Comparing a Forest, a Cropland, and a Pasture Soil in the Central Appalachian Region, West Virginia, U.S.A. Environments 2022, 9, 113. https://doi.org/10.3390/environments9090113
Lei L, Holásková I, Thompson JA, McDonald LM. Fe-Bound Organic Carbon and Sorption of Aromatic Dissolved Organic Carbon in Surface Soil: Comparing a Forest, a Cropland, and a Pasture Soil in the Central Appalachian Region, West Virginia, U.S.A. Environments. 2022; 9(9):113. https://doi.org/10.3390/environments9090113
Chicago/Turabian StyleLei, Lili, Ida Holásková, James A. Thompson, and Louis M. McDonald. 2022. "Fe-Bound Organic Carbon and Sorption of Aromatic Dissolved Organic Carbon in Surface Soil: Comparing a Forest, a Cropland, and a Pasture Soil in the Central Appalachian Region, West Virginia, U.S.A" Environments 9, no. 9: 113. https://doi.org/10.3390/environments9090113