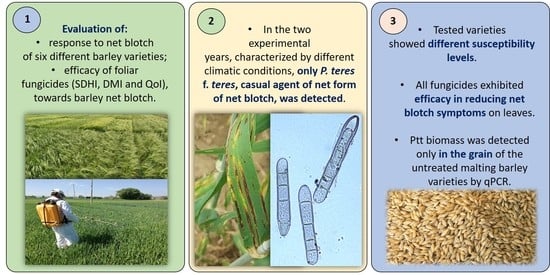
Management of Pyrenophora teres f. teres, the Causal Agent of Net Form Net Blotch of Barley, in A Two-Year Field Experiment in Central Italy
Abstract
:1. Introduction
2. Results
2.1. P. teres Identification
2.2. Weather Conditions Recorded in the Two Experimental Years
2.3. NFNB Visual Score and Production Parameters
2.4. P. teres f. teres Biomass in Barley Grains
2.5. Relationship between P. teres f. teres Biomass in the Grains and NFNB Symptoms on Leaves
3. Discussion
4. Materials and Methods
4.1. Field Experimental Design and Fungicide Application
4.2. Pathogen Isolation and Identification
4.3. Pyrenophora teres f. teres Biomass Quantification in Barley Grains
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). FAOSTAT. Statistic Division. Food and Agriculture Organization of the UN. Available online: http://faostat.fao.org (accessed on 15 September 2021).
- Clare, S.J.; Wyatt, N.A.; Bruggeman, R.S.; Friesen, T.L. Research advances in the Pyrenophora teres-barley interaction. Mol. Plant Pathol. 2020, 21, 272–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weibull, J.; Walther, U.; Sato, K.; Habekuss, A.; Kopahnke, D.; Proeseler, G. Diversity in resistance to biotic stresses. In Developments in Plant Genetics and Breeding, Diversity in Barley (Hordeum vulgare); von Bothmer, R., van Hintum, T., Knüpffer, H., Sato, K., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; Volume 7, pp. 143–178. [Google Scholar]
- Shoemaker, R.A. Nomenclature of Drechslera and Bipolaris, grass parasites segregated from “Helminthosporium”. Can. J. Bot. 1959, 37, 879–887. [Google Scholar] [CrossRef]
- Liu, Z.; Ellwood, S.R.; Oliver, R.P.; Friesen, T.L. Pyrenophora teres: Profile of an increasingly damaging barley pathogen. Mol. Plant Pathol. 2011, 12, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, D.; Attene, G.; Brown, A.H.D.; Nanni, L.; Maier, F.J.; Balmas, V.; Saba, E.; Schafer, W.; Papa, R. Phylogeny and evolution of mating-type genes from Pyrenophora teres, the causal agent of barley “net blotch” disease. Curr. Genet. 2007, 51, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Bogacki, M.P.; Keiper, F.J.; Oldach, K.H. Genetic structure of South Australian Pyrenophora teres populations as revealed by microsatellite analyses. Fungal Biol. 2010, 20, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Lehmensiek, A.; Bester-van der Merwe, A.E.; Sutherland, M.W.; Platz, G.; Kriel, W.M.; Potgieter, G.F.; Prins, R. Population structure of South African and Australian Pyrenophora teres isolates. Plant Pathol. 2010, 59, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Ellwood, S.R.; Syme, R.A.; Moffat, C.S.; Oliver, R.P. Evolution of three Pyrenophora cereal pathogens: Recent divergence, speciation and evolution of non-coding DNA. Fungal Genet. Biol. 2012, 49, 825–829. [Google Scholar] [CrossRef] [Green Version]
- Leisova-Svobodova, L.; Minarikova, V.; Matusinsky, P.; Hudocovicova, M.; Ondreickova, K.; Gubis, J. Genetic structure of Pyrenophora teres net and spot populations as revealed by microsatellite analysis. Fungal Biol. 2014, 118, 180–192. [Google Scholar] [CrossRef]
- Syme, R.A.; Maritn, A.; Wyatt, N.A.; Lawrence, J.A.; Muria-Gonzalez, M.J.; Friesen, T.L.; Ellwood, S.R. Transposable element genomic fissuring in Pyrenophora teres is associated with genome expansion and dynamics of host-pathogen genetic interactions. Front. Genet. 2018, 9, 130. [Google Scholar] [CrossRef]
- Ohm, R.A.; Feau, N.; Henrissat, B.; Schoch, C.L.; Horwitz, B.A.; Barry, K.W.; Condon, B.J.; Copeland, A.C.; Dhillon, B.; Glaser, F.; et al. Diverse lifestyle and strategies of plant pathogenesis encoded in the genomes of eighteen Dothideomycetes fungi. PLoS Pathog. 2012, 8, e1003037. [Google Scholar] [CrossRef] [Green Version]
- Weiergang, I.; Jorgensen, H.J.L.; Moller, I.M.; Friis, P.; Smedegaard-Petersen, V. Correlation between sensitivity of barley to Pyrenophora teres toxins and susceptibility to the fungus. Physiol. Mol. Plant Pathol. 2002, 60, 121–129. [Google Scholar] [CrossRef]
- Muria-Gonzalez, M.J.; Zulak, K.G.; Allegaert, E.; Oliver, R.P.; Ellwood, S.R. Profile of the in vitro secretome of the barley net blotch fungus Pyrenophora teres f. teres. Physiol. Mol. Plant Pathol. 2020, 109, 101451. [Google Scholar] [CrossRef]
- Backes, A.; Guerriero, G.; Barka, E.A.; Jacquard, C. Pyrenophora teres: Taxonomy, morphology, interaction with barley, and mode of control. Front. Plant Sci. 2021, 12, 614951. [Google Scholar] [CrossRef] [PubMed]
- Steffenson, B. Net blotch. In Compendium of Barley Diseases, 2nd ed.; Mathre, D.E., Ed.; APS Press: St. Paul, MN, USA, 1997; pp. 28–31. [Google Scholar]
- Duczek, L.J.; Sutherland, K.A.; Reed, S.L.; Bailey, K.L.; Lafond, G.P. Survival of leaf spot pathogens on crop residues of wheat and barley in Saskatchewan. Can. J. Plant Pathol. 1999, 21, 165–173. [Google Scholar] [CrossRef]
- Jorgnesen, L.N.; Olsen, L.V. Control of tan spot (Drechslera tritici-repentis) using cultivar resistance, tillage methods and fungicides. Crop Prot. 2007, 26, 1606–1616. [Google Scholar] [CrossRef]
- Rusu, T.; Gus, P.; Bogdan, I.; Moraru, P.I.; Pop, A.I.; Clapa, D.; Marin, D.I.; Oroian, I.; Pop, L.I. Implications of minimum tillage systems on sustainability of agricultural production and soil conservation. J. Food Agric. Environ. 2009, 8, 335–338. [Google Scholar]
- Jalli, M. Sexual reproduction and soil tillage effects on virulence of Pyrenophora teres in Finland. Ann. Appl. Biol. 2011, 158, 95–105. [Google Scholar] [CrossRef]
- Akhavan, A.; Turkington, T.K.; Askarian, H.; Tekauz, A.; Xi, J.; Tucker, J.R.; Kutcher, H.R.; Strelkov, S.E. Virulence of Pyrenophora teres populations in western Canada. Can. J. Plant Pathol. 2016, 38, 183–196. [Google Scholar] [CrossRef]
- Heschele, E.E. The response of barleys to parasitic fungi Helmintosporium teres (Sacc.). Byul. Prikl. Bot. Genet. I Sel. 1928, 19, 371–384. (In Russian) [Google Scholar]
- Douiyssi, A.; Rasmusson, D.C.; Roelfs, A. Responses of barley cultivars and lines to isolates of Pyrenophora teres. Plant Dis. 1998, 82, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Novakazi, F.; Afanasenko, O.; Ansimova, A.; Platz, G.J.; Snowdon, R.; Kovaleva, O.; Zubkovich, A.; Ordon, F. Genetic analysis of a worldwide barley collection for resistance to net form of net blotch disease (Pyrenophora teres f. teres). Theor. Appl. Genet. 2019, 132, 2633–2650. [Google Scholar] [CrossRef] [PubMed]
- Sierotzki, H.; Frey, R.; Wullschleger, J.; Palermo, S.; Karlin, S.; Godwin, J.; Gisi, U. Cytochrome b gene sequence and structure of Pyrenophora teres and P. tritici-repentis and implications for QoI resistance. Pest Manag. Sci. 2007, 63, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.A. Disease progression and yield loss in barley associated with net blotch, as influenced by fungicide seed treatment. Can. J. Plant Pathol. 1985, 7, 83–90. [Google Scholar] [CrossRef]
- Mair, W.J.; Deng, W.; Mullins, J.G.L.; West, S.; Wang, P.; Besharat, N.; Ellewood, S.R.; Oliver, R.P.; Lopez-Ruiz, F.J. Demethylase inhibitor fungicide resistance in Pyrenophora teres f. sp. teres associated with target site modification and inducible overexpression of Cyp51. Front. Microbiol. 2016, 7, 1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehfus, A.; Miessner, S.; Achenbach, J.; Strobel, D.; Bryson, R.; Stammler, G. Emergence of succinate dehydrogenase inhibitor resistance of Pyrenophora teres in Europe. Pest Manag. Sci. 2016, 72, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, M.; Rekanovic, E.; Milijasevic-Marcic, S.; Potocnik, I.; Todorovic, B.; Stepanovic, J. Field efficacy of different fungicide mixtures in control of net blotch on barley. Pestic. Fitomed. 2016, 31, 51–57. [Google Scholar] [CrossRef]
- Semar, M.; Strobel, D.; Koch, A.; Klappach, K.; Stammler, G. Field efficacy of pyraclostrobin against populations of Pyrenophora teres containing the F129L mutation in the cytochrome b gene. J. Plant Dis. Prot. 2007, 114, 117–119. [Google Scholar] [CrossRef]
- Lammari, H.I.; Rehfus, A.; Stammler, G.; Benslimane, H. Sensitivity of the Pyrenophora teres population in Algeria to Quinone outside inhibitors, succinate dehydrogenase inhibitors and demethylation inhibitors. Plant Pathol. J. 2020, 36, 218–230. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailiedes, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Jayasena, K.W.; van Burgel, A.; Tanaka, K.; Mejewski, J.; Loughman, R. Yield reduction in barley in relation to spot-type net blotch. Australas. Plant Pathol. 2007, 36, 429–433. [Google Scholar] [CrossRef]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian barley industry. Australas. Plant Pathol. 2010, 39, 85–96. [Google Scholar] [CrossRef]
- Jordan, V.W.L. Aetiology of barley net blotch caused by Pyrenopora teres and some effects on yield. Plant Pathol. 1981, 30, 77–87. [Google Scholar] [CrossRef]
- Khan, T.N. Relationship between net blotch (Drechslera teres) and losses in grain yield of barley in Western Australia. Aust. J. Agric. Res. 1987, 38, 671–679. [Google Scholar] [CrossRef]
- Martin, R.A.; Sanderson, J.B. Yield of barley in response to propioconazole. Can. J. Plant Pathol. 1988, 10, 66–72. [Google Scholar] [CrossRef]
- Arabi, M.I.E.; Berrault, G.; Sarrafi, A.; Albertini, L. Variation in the resistance of barley cultivars and the pathogenicity of Drechslera teres f. sp. maculata and D. teres f. sp. teres isolates from France. Plant Pathol. 1992, 41, 180–186. [Google Scholar]
- Makela, K. Leaf spot fungi on barley in Finland. Acta Agric. Fenn. 1972, 124, 22. [Google Scholar]
- Weller, J.A.; Rossnagel, B.G. Saskatchewan barley leaf disease survey. Can. Plant Dis. Surv. 1988, 68, 38. [Google Scholar]
- Liu, Z.H.; Friesen, T.L. Identification of Pyrenophora teres f. maculata, causal agent of spot type net blotch of barley in North Dakota. Plant Dis. 2010, 94, 480. [Google Scholar] [CrossRef]
- Marshall, J.M.; Kinzer, K.; Brueggeman, R.S. First report of Pyrenophora teres f. maculata the cause of spot form net blotch of barley in Idaho. Plant Dis. 2015, 99, 1860. [Google Scholar] [CrossRef]
- McLean, M.S.; Howlett, B.J.; Hollaway, G.J. Spot form of net blotch, caused by Pyrenopohra teres f. maculata, is the most prevalent foliar disease of barley in Victoria, Australia. Australas. Plant Pathol. 2010, 39, 46–49. [Google Scholar]
- Lammari, H.I.; Rehfus, A.; Stammler, G.; Fellahi, Z.E.A.; Benbelkacem, A.; Benslimane, H. Occurrence and frequency of spot form and net form of net blotch disease of barley in Algeria. J. Plant Dis. Prot. 2020, 127, 35–42. [Google Scholar] [CrossRef]
- Celik Oguz, A.; Olmez, F.; Karakaya, A. Mating type idiomorphs by Pyrenophora teres in Turkey. Zemdirbyste-Agriculture 2018, 105, 271–278. [Google Scholar] [CrossRef]
- Yitbarek, S.; Bekele, H.; Getaneh, W.; Dereje, T. Disease survey and loss assessment studies on barley. In Barley Research in Ethiopia, Past Work and Future Prospects; Gebre, H., van Leur, J., Eds.; IAR: Addis Ababa, Ethiopia, 1996; pp. 105–115. [Google Scholar]
- Serenius, M.; Mironenko, N.; Manninen, O. Genetic variation, occurrence of mating types and different forms of Pyrenophora teres causing net blotch of barley in Finland. Mycol. Res. 2005, 109, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Dokhanchi, H.; Arzanlou, M.; Abed-Ashtiani, F. Sexual recombination and genetic diversity in Iranian populations of Pyrenophora teres. J. Phytopathol. 2021, 69, 447–460. [Google Scholar] [CrossRef]
- Lhadj, W.A.; Boungab, K.; Assia, F.R.; Oguz, A.C.; Karakaya, A.; Olmez, F. Genetic diversity of Pyrenophora teres in Algeria. J. Plant Pathol. 2022, 104, 305–315. [Google Scholar] [CrossRef]
- Jordan, V.W.L.; Allen, E.C. Barley net blotch: Influence of straw disposal and cultivation methods on inoculum potential, and on incidence and severity of autumn disease. Plant Pathol. 1984, 33, 547–599. [Google Scholar] [CrossRef]
- Brown, M.P.; Steffenson, B.J.; Webster, R.K. Host range of Pyrenophora teres f. teres isolates from California. Plant Dis. 1993, 77, 942–947. [Google Scholar] [CrossRef]
- McLean, M.S.; Howlett, B.J.; Hollaway, G.J. Epidemiology and control of spot form of net blotch (Pyrenophora teres f. maculata) of barley: A review. Crop Pasture Sci. 2009, 60, 303–315. [Google Scholar] [CrossRef]
- Burlakoti, R.R.; Gyawali, S.; Chao, S.; Smith, K.P.; Horsley, R.D.; Cooper, B.; Muehlbauer, G.J.; Neate, S.M. Genome-wide association study of spot form of net blotch resistance in the upper Midwest barley breeding programs. Phytopathology 2017, 107, 100–108. [Google Scholar] [CrossRef]
- Tekauz, A. Characterization and distribution of pathogenic variation in Pyrenophora teres f. teres and P. teres f. maculata from western Canada. Can. J. Plant Pathol. 1990, 12, 141–148. [Google Scholar] [CrossRef]
- Wang, X.; Mace, E.S.; Platz, G.J.; Hunt, H.H.; Hickey, L.T.; Franckowiak, J.D.; Jordan, D.R. Spot form of net blotch resistance in barley under complex genetic control. Theor. Appl. Genet. 2015, 128, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhavan, A.; Strelkov, S.E.; Askarian, H.; Kher, S.V.; Fraser, M.; Kutcher, H.R.; Turkington, T.K. Sensitivity of western Canadian Pyrenophora teres f. teres and P. teres f. maculata isolates to propioconazole and pyraclostrobin. Can. J. Plant Pathol. 2017, 39, 11–24. [Google Scholar] [CrossRef]
- Marzani, Q.A. Fungicide Resistance and Efficacy for Control of Pyrenophora teres and Mycosphaerella graminicola on Barley and Wheat. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2011. [Google Scholar]
- Hobbelen, P.H.F.; Paveley, N.D.; van den Bosch, F. Delaying selection for fungicide insensitivity by mixing fungicides at low and high risk of resistance development: A modelling analysis. Phytopathology 2011, 101, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Hobbelen, P.H.F.; Paveley, N.D.; Oliver, R.P.; van den Bosch, F. The usefulness of fungicide mixtures and alternation for delaying the selection resistance in populations of Mycosphaerella graminicola on winter wheat: A modelling analysis. Phytopathology 2013, 103, 690–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbelen, P.H.F.; Paveley, N.D.; Oliver, R.P.; van den Bosch, F. The emergence of resistance to fungicides. PLoS ONE 2014, 9, e91910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bosch, F.; Paveley, N.; van den Berg, F.; Hobbelen, P.H.F.; Oliver, R. Mixtures as a fungicide resistance management tactic. Phytopathology 2014, 104, 1264–1273. [Google Scholar] [CrossRef]
- Hellin, P.; King, R.; Urban, M.; Hammond-Kosak, K.E.; Legreve, A. The adaptation of Fusarium culmorum to DMI fungicides is mediated by a major transcriptome modifications in response to azole fungicide, including the overexpression of a PDR transporter (FcABC1). Front. Microbiol. 2018, 9, 1385. [Google Scholar] [CrossRef]
- Tini, F.; Beccari, G.; Onofri, A.; Ciavatta, E.; Gardiner, D.M.; Covarelli, L. Fungicides may have differential efficacies towards the main causal agents of Fusarium head blight of wheat. Pest Manag. Sci. 2020, 76, 3738–3748. [Google Scholar] [CrossRef]
- Turkington, T.K.; O’Donovan, J.T.; Edney, M.J.; Juskiw, P.E.; McKenzie, R.H.; Harker, K.N.; Clayton, G.W.; Xi, K.; Lafond, G.P.; Irvine, R.B.; et al. Effect of crop residue, nitrogen rate and fungicide application on malting barley productivity, quality and foliar disease severity. Can. J. Plant Sci. 2012, 92, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Beccari, G.; Prodi, A.; Tini, F.; Bonciarelli, U.; Onofri, A.; Oueslati, S.; Limayma, M.; Covarelli, L. Changes in the Fusarium head blight complex of malting barley in a three-year field experiment in Italy. Toxins 2017, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- National Center for Biotechnology Information. Available online: http://blast.ncbi.nih.gov (accessed on 14 April 2021).
- Poudel, B.; Ellwood, S.R.; Testa, A.C.; McLean, M.; Sutherland, M.W.; Martin, A. Rare Pyrenophora teres hybridization events revealed by development if sequence-specific PCR markers. Phytopathology 2017, 107, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Parry, D.W.; Nicholson, P. Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol. 1996, 45, 383–391. [Google Scholar] [CrossRef]
- Beccari, G.; Arellano, C.; Covarelli, L.; Tini, F.; Sulyok, M.; Cowger, C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int. J. Food Microbiol. 2019, 290, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Manes, J. Fusarium species, chemotype characterization and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Onofri, A.; Pannacci, E. Spreadsheet tools for biometry classes in crop science programs. CBCS 2014, 9, 43–53. [Google Scholar]





| Cultivar | Treatment | Grain Yield 2019 | Grain Yield 2020 | Protein Content 2019 | Protein Content 2020 | Specific Weight 2019 | Specific Weight 2020 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t ha−1 | SEa | MCPb | t ha−1 | SE | MCP | % | SE | MCP | % | SE | MCP | kg hL−1 | SE | MCP | kg hL−1 | SE | MCP | ||
| Quench | Untreated control | 10.5 | 0.10 | b | 7.7 | 0.33 | a | 10.0 | 0.26 | a | 12.1 | 0.40 | a | 66.0 | 0.06 | b | 67.4 | 0.15 | a |
| Aviator Xpro | 12.0 | 0.10 | a | 8.1 | 0.24 | a | 9.9 | 0.32 | a | 12.5 | 0.46 | a | 68.6 | 0.32 | a | 67.4 | 0.20 | a | |
| Elatus Era | 12.1 | 0.21 | a | 8.1 | 0.19 | a | 10.2 | 0.20 | a | 11.4 | 0.09 | a | 68.1 | 0.03 | a | 67.4 | 0.12 | a | |
| Priaxor | 11.6 | 0.25 | a | 7.6 | 0.54 | a | 10.2 | 0.29 | a | 11.6 | 0.25 | a | 68.6 | 0.30 | a | 66.9 | 0.35 | a | |
| Sunshine | Untreated control | 7.4 | 0.41 | a | 7.7 | 0.09 | b | 11.9 | 0.10 | a | 12.3 | 0.21 | a | 67.2 | 0.26 | b | 66.6 | 0.54 | a |
| Aviator Xpro | 8.1 | 0.58 | a | 8.1 | 0.09 | a | 11.7 | 0.17 | a | 11.8 | 0.15 | a | 68.8 | 0.25 | a | 67.2 | 0.32 | a | |
| Elatus Era | 8.1 | 0.46 | a | 8.1 | 0.15 | a | 12.0 | 0.07 | a | 12.2 | 0.23 | a | 68.5 | 0.17 | a | 67.6 | 0.23 | a | |
| Priaxor | 8.0 | 0.15 | a | 8.3 | 0.07 | a | 11.6 | 0.21 | a | 11.9 | 0.15 | a | 68.7 | 0.54 | a | 66.8 | 0.12 | a | |
| Atomo | Untreated control | 8.3 | 0.43 | a | 6.8 | 0.55 | a | 12.9 | 0.66 | a | 13.9 | 0.17 | a | 67.9 | 0.56 | a | 63.3 | 0.47 | a |
| Aviator Xpro | 8.7 | 0.57 | a | 7.1 | 0.64 | a | 12.8 | 0.43 | a | 14.0 | 0.03 | a | 68.2 | 0.38 | a | 62.8 | 0.31 | a | |
| Elatus Era | 8.6 | 0.38 | a | 7.3 | 0.44 | a | 13.5 | 0.30 | a | 14.1 | 0.26 | a | 66.9 | 1.47 | a | 62.4 | 0.81 | a | |
| Priaxor | 9.1 | 0.21 | a | 6.9 | 0.69 | a | 12.6 | 0.54 | a | 14.2 | 0.15 | a | 68.1 | 0.62 | a | 63.2 | 0.94 | a | |
| Capricorn | Untreated control | 10.7 | 0.36 | b | 8.4 | 0.07 | a | 11.9 | 0.10 | a | 12.5 | 0.03 | a | 64.2 | 0.21 | a | 63.6 | 0.10 | a |
| Aviator Xpro | 11.5 | 0.18 | a | 8.2 | 0.25 | a | 11.7 | 0.17 | a | 12.1 | 0.20 | a | 65.6 | 0.82 | a | 63.2 | 0.31 | a | |
| Elatus Era | 11.9 | 0.17 | a | 8.5 | 0.06 | a | 12.0 | 0.07 | a | 11.9 | 0.29 | a | 66.7 | 0.32 | a | 62.6 | 0.28 | a | |
| Priaxor | 11.3 | 0.08 | ab | 8.1 | 0.23 | a | 11.6 | 0.21 | a | 11.8 | 0.52 | a | 65.7 | 0.73 | a | 63.2 | 0.18 | a | |
| Ketos | Untreated control | 9.1 | 0.53 | b | 6.9 | 0.68 | a | 11.3 | 0.35 | a | 12.3 | 0.13 | a | 66.3 | 0.00 | c | 61.9 | 1.32 | a |
| Aviator Xpro | 10.6 | 0.37 | a | 7.5 | 0.65 | a | 11.2 | 0.25 | a | 12.1 | 0.31 | a | 67.5 | 0.32 | ab | 62.1 | 1.41 | a | |
| Elatus Era | 10.9 | 0.05 | a | 7.7 | 0.53 | a | 11.5 | 0.22 | a | 12.0 | 0.17 | a | 68.0 | 0.18 | a | 63.3 | 1.24 | a | |
| Priaxor | 10.6 | 0.36 | a | 7.5 | 0.56 | a | 11.5 | 0.24 | a | 12.2 | 0.32 | a | 66.5 | 0.55 | bc | 61.9 | 0.95 | a | |
| Alimini | Untreated control | 10.1 | 0.04 | a | 7.6 | 0.08 | a | 12.3 | 0.17 | a | 12.2 | 0.03 | a | 64.2 | 0.52 | a | 60.2 | 0.29 | a |
| Aviator Xpro | 10.9 | 0.39 | a | 7.0 | 0.74 | a | 12.0 | 0.31 | a | 12.2 | 0.09 | a | 64.4 | 0.47 | a | 60.4 | 0.71 | a | |
| Elatus Era | 10.6 | 0.50 | a | 7.8 | 0.19 | a | 11.6 | 0.18 | A | 12.2 | 0.24 | a | 65.1 | 0.19 | a | 59.9 | 0.39 | a | |
| Priaxor | 10.6 | 0.18 | a | 7.6 | 0.09 | a | 11.9 | 0.18 | A | 12.1 | 0.12 | a | 64.4 | 0.44 | a | 60.5 | 0.66 | a | |
| Cultivar | Row | Type of Grain | Height of Vegetation | Harvesting Time | Typical Italian Cultivation Area | Main Destination |
|---|---|---|---|---|---|---|
| Quench | Two | Covered | Low | Early | Centre | Malting |
| Sunshine | Two | Covered | Low | Medium | All | Malting |
| Atomo | Two | Covered | Medium | Early | All | Feed |
| Capricorn | Two | Covered | Medium/High | Medium/Late | All | Feed |
| Ketos | Six | Covered | High | Medium | All | Food/Feed |
| Alimini | Six | Covered | High | Medium | North | Feed |
| Treatment | Active Ingredient | Active Ingredients Concentration (g L−1) | Mode of Action | Application Time | Dose (L ha−1) |
|---|---|---|---|---|---|
| Untreated control | - | - | - | - | - |
| Aviator Xpro | prothioconazole + bixafen | 150 + 75 | DMI a + SDHI b | BBCH 39 | 1 |
| Elatus Era | benzovindiflupyr + prothioconazole | 75 + 150 | SDHI + DMI | BBCH 39 | 1 |
| Priaxor | fluxapyroxad + pyraclostrobin | 75 + 150 | SDHI + QoI c | BBCH 39 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tini, F.; Covarelli, L.; Ricci, G.; Balducci, E.; Orfei, M.; Beccari, G. Management of Pyrenophora teres f. teres, the Causal Agent of Net Form Net Blotch of Barley, in A Two-Year Field Experiment in Central Italy. Pathogens 2022, 11, 291. https://doi.org/10.3390/pathogens11030291
Tini F, Covarelli L, Ricci G, Balducci E, Orfei M, Beccari G. Management of Pyrenophora teres f. teres, the Causal Agent of Net Form Net Blotch of Barley, in A Two-Year Field Experiment in Central Italy. Pathogens. 2022; 11(3):291. https://doi.org/10.3390/pathogens11030291
Chicago/Turabian StyleTini, Francesco, Lorenzo Covarelli, Giacomo Ricci, Emilio Balducci, Maurizio Orfei, and Giovanni Beccari. 2022. "Management of Pyrenophora teres f. teres, the Causal Agent of Net Form Net Blotch of Barley, in A Two-Year Field Experiment in Central Italy" Pathogens 11, no. 3: 291. https://doi.org/10.3390/pathogens11030291