Serological Survey of Aujeszky’s Disease in Wild Boar from Southeastern France
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
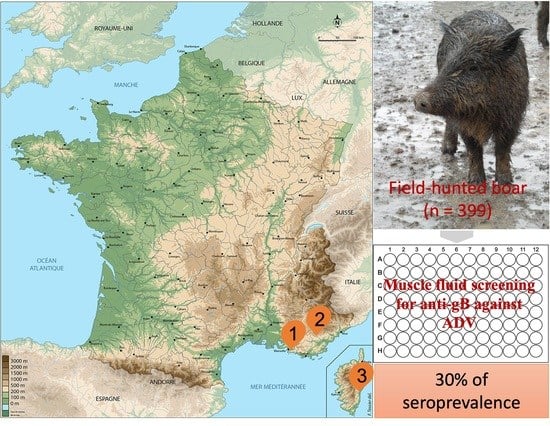
4.1. Study Area and Sampling
4.2. Anti-gB Antibodies Detection
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyssmann, E. Nochmals Zur Frage Des Vorkommens der Aujeszky’schen Krankheit, Pseudowut, in Der Schweiz. Schweiz. Arch. Tierheilk 1942, 84, 32–34. [Google Scholar]
- Hanson, R.P. The history of pseudorabies in the United States. J. Am. Vet. Med. Assoc. 1954, 124, 259–261. [Google Scholar]
- Köhler, M.; Köhler, W. Zentralblatt Für Bakteriologie—100 Years Ago: Aladár Aujeszky Detects a “New” Disease—Or: It Was the Cow and Not the Sow. Int. J. Med. Microbiol. 2003, 292, 423. [Google Scholar] [CrossRef]
- Aujeszky, A. Über Eine Neue Infektionskrankheit Bei Haustieren. Zbl. Bakt. 1. Abt. Orig. 1902, 32, 353. [Google Scholar]
- Mettenleiter, T.C. Aujeszky’s Disease and the Development of the Marker/DIVA Vaccination Concept. Pathogens 2020, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Elford, W.J.; Galloway, I.A. The Size of the Virus of Aujeszky’s Disease (“Pseudo-rabies”, “Infectious Bulbar Paralysis”, “Mad-itch”) by Ultrafiltration Analysis. Epidemiol. Infect. 1936, 36, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Sehl, J.; Teifke, J.P. Comparative Pathology of Pseudorabies in Different Naturally and Experimentally Infected Species—A Review. Pathogens 2020, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Jeffery-Smith, A.; Riddell, A. Herpesviruses. Medicine 2021, 45, 767–771. [Google Scholar] [CrossRef]
- Kimman, T.G.; Binkhorst, G.J.; Ingh, T.S.V.D.; Pol, J.M.; Gielkens, A.L.; E Roelvink, M. Aujeszky’s disease in horses fulfils Koch’s postulates. Vet. Rec. 1991, 128, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Delva, J.L.; Nauwynck, H.J.; Mettenleiter, T.C.; Favoreel, H.W. The Attenuated Pseudorabies Virus Vaccine Strain Bartha K61: A Brief Review on the Knowledge Gathered During 60 Years of Research. Pathogens 2020, 9, 897. [Google Scholar] [CrossRef]
- Müller, T.; Hahn, E.C.; Tottewitz, F.; Kramer, M.; Klupp, B.G.; Mettenleiter, T.C.; Freuling, C. Pseudorabies virus in wild swine: A global perspective. Arch. Virol. 2011, 156, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Auclert, L.Z.; Zhai, X.; Wong, G.; Zhang, C.; Zhu, H.; Xing, G.; Wang, S.; He, W.; Li, K.; et al. Interspecies Transmission, Genetic Diversity, and Evolutionary Dynamics of Pseudorabies Virus. J. Infect. Dis. 2018, 219, 1705–1715. [Google Scholar] [CrossRef]
- Ciarello, F.P.; Moreno, A.; Miragliotta, N.; Antonino, A.; Fiasconaro, M.; Purpari, G.; Amato, B.; Ippolito, D.; di Marco Lo Presti, V. Aujeszky’s disease in hunting dogs after the ingestion of wild boar raw meat in Sicily (Italy): Clinical, diagnostic and phylogenetic features. BMC Vet. Res. 2022, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- di Marco Lo Presti, V.; Moreno, A.; Castelli, A.; Ippolito, D.; Aliberti, A.; Amato, B.; Vitale, M.; Fiasconaro, M.; Ciarello, F.P. Retrieving Historical Cases of Aujeszky’s Disease in Sicily (Italy): Report of a Natural Outbreak Affecting Sheep, Goats, Dogs, Cats and Foxes and Considerations on Critical Issues and Perspectives in Light of the Recent EU Regulation 429/2016. Pathogens 2021, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Machalaba, C.M.; Jones, H.; Cáceres, P.; Popovic, M.; Olival, K.J.; Ben Jebara, K.; Karesh, W.B. Wildlife hosts for OIE-Listed diseases: Considerations regarding global wildlife trade and host–pathogen relationships. Vet. Med. Sci. 2017, 3, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Bulletin Hebdomadaire de Veille Sanitaire Internationale En Santé Animale Du 12/04/2022 Danger Sanitaire à Actualité Réduite; 2022, Volume 31. Available online: https://www.plateforme-esa.fr/bulletin-hebdomadaire-de-veille-sanitaire-internationale-en-sante-animale-du-12-04-2022 (accessed on 22 September 2022).
- Payne, A.; Rossi, S.; Lacour, S.; Vallée, I.; Garin-Bastuji, B.; Simon, G.; Hervé, S.; Pavio, N.; Richomme, C.; Dunoyer, C. Bilan Sanitaire Du Sanglier Vis-à-Vis de La Trichinellose, de La Maladie d’Aujeszky, de La Brucellose, de l’hépatite E et Des Virus Influenza Porcins En France. Bull. Épidémiologique St. Anim. Alim. 2011, 44, 2–8. [Google Scholar]
- Rossi, S.; Hars, J.; Garin-Bastuji, B.; le Potier, M.F.; Boireau, P.; Aubry, P.; Hattenberger, A.-M.; Louguet, Y.; Toma, B.; Boué, F. Résultats de l’enquête Nationale Sérologique Menée Chez Le Sanglier Sauvage (2000–2004). Bull. Epid. Santé Anim. Alim. 2008, 29, 5–7. [Google Scholar]
- Ministere de l’Agriculture, D.L.E.D.L.F. Note de Service DGAL/SDSPA/N2009-8289 Du 21 Octobre 2009, Précisions Sur Les Mesures de Police Sanitaire Vis-à-Vis de La Maladie d’Aujeszky En Application de l’arrêté Du 28 Janvier 2009. Available online: https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwikmoi3pLT6AhUI3nMBHdZ1CzwQFnoECBcQAQ&url=https%3A%2F%2Finfo.agriculture.gouv.fr%2Fgedei%2Fsite%2Fbo-agri%2Finstruction-N2009-8289%2Ftelechargement&usg=AOvVaw18XlS3kCMWAa3oVpe0a4kf (accessed on 22 September 2022).
- Le Potier, M.F.; Fournier, A.; Houdayer, C.; Hutet, E.; Auvigne, V.; Hery, D.; Sanaa, M.; Toma, B. Use of muscle exudates for the detection of anti-gE antibodies to Aujeszky’s disease virus. Vet. Rec. 1998, 143, 385–387. [Google Scholar] [CrossRef]
- Toma, B.; Agier, C.; Haddad, N.; Boué, F.; Terrier, M.-E.; Hars, J. Utilisation Comparée Du Sérum, Du Poumon et Du Muscle Pour Le Dépistage de La Maladie d’Aujeszky Chez Les Sangliers. Epidémiologie Et St. Anim. (Revue De l’AEEMA) 2004, 45, 25–31. [Google Scholar]
- The Center for Food Security and Public Health. Aujeszky’ s Disease Pseudorabies, Mad Itch.; 2020. Last Updated: January 2017. Available online: https://www.google.com/url?client=internal-element-cse&cx=014999493444943369949:2m026u-gsd8&q=https://www.cfsph.iastate.edu/Factsheets/pdfs/aujeszkys_disease.pdf&sa=U&ved=2ahUKEwi9nfbI37P6AhXQ_4UKHax_BOYQFnoECAkQAQ&usg=AOvVaw3WwJdjEEwXQVxqkSQq15Gm (accessed on 22 September 2022).
- Müller, A.; Melo, N.; González-Barrio, D.; Pinto, M.V.; Ruiz-Fons, F. Aujeszky’s disease in hunted wild boar (sus scrofa) in the iberian peninsula. J. Wildl. Dis. 2021, 57, 543–552. [Google Scholar] [CrossRef]
- Denzin, N.; Conraths, F.J.; Mettenleiter, T.C.; Freuling, C.M.; Müller, T. Monitoring of Pseudorabies in Wild Boar of Germany—A Spatiotemporal Analysis. Pathogens 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Vitale, N.; Prato, R.; Radaelli, M.C.; Zoppi, S.; Possidente, R.; Dondo, A.; Chiavacci, L.; Moreno Martin, A.M.; Masoero, L. Pseudorabies virus in North-West Italian wild boar (Sus scrofa) populations: Prevalence and risk factors to support a territorial risk-based surveillance. Vet. Ital. 2018, 54, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ministère de l’agriculture et de souveraineté alimentaire Tout Savoir Sur La Maladie d’Aujeszky. 29 April 2019 Santé animale 2019. Available online: https://agriculture.gouv.fr/questions-reponses-tout-savoir-sur-la-maladie-daujeszky (accessed on 22 September 2022).
- WOAH World Animal Health Information System: Follow-up Report 10, Evt_3985. Available online: https://wahis.woah.org/#/events?viewAll=true (accessed on 22 September 2022).
- The European Parliament and the Council of the European Union. Regulation (EU) 2016/429 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). Off. J. Eur. Union 2016, 429. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2016.084.01.0001.01.ENG (accessed on 22 September 2022).
- Ministère de l’Agriculture et de l’Alimentation Arrêté du 16 Octobre 2018 Relatif aux Mesures de Biosécurité Applicables dans les Exploitations Détenant des Suidés dans le Cadre de la Prévention de La Peste Porcine Africaine et des Autres Dangers Sanitaires Réglementés. Journal officiel électronique authentifié n° 0240 du 17 October 2018. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000037501487 (accessed on 22 September 2022).
- Caruso, C.; Dondo, A.; Cerutti, F.; Masoero, L.; Rosamilia, A.; Zoppi, S.; D’Errico, V.; Grattarola, C.; Acutis, P.L.; Peletto, S. Aujeszky’s Disease in Red Fox (Vulpes vulpes): Phylogenetic Analysis Unravels an Unexpected Epidemiologic Link. J. Wildl. Dis. 2014, 50, 707–710. [Google Scholar] [CrossRef]
- Moreno, A.; Chiapponi, C.; Sozzi, E.; Morelli, A.; Silenzi, V.; Gobbi, M.; Lavazza, A.; Paniccià, M. Detection of a gE-deleted Pseudorabies virus strain in an Italian red fox. Vet. Microbiol. 2020, 244, 108666. [Google Scholar] [CrossRef]
- Bitsch, V.; Knox, B. On pseudorabies in carnivores in Denmark. II. The blue fox (Alopex lagopus). Acta Vet. Scand. 1971, 12, 285–292. [Google Scholar] [CrossRef]
- Jin, H.-L.; Gao, S.-M.; Liu, Y.; Zhang, S.-F.; Hu, R.-L. Pseudorabies in farmed foxes fed pig offal in Shandong province, China. Arch. Virol. 2015, 161, 445–448. [Google Scholar] [CrossRef]
- Verpoest, S.; Cay, A.B.; Bertrand, O.; Saulmont, M.; De Regge, N. Isolation and characterization of pseudorabies virus from a wolf (Canis lupus) from Belgium. Eur. J. Wildl. Res. 2013, 60, 149–153. [Google Scholar] [CrossRef]
- Raymond, J.T.; Gillespie, R.G.; Woodruff, M.; Janovitz, E.B. Pseudorabies in Captive Coyotes. J. Wildl. Dis. 1997, 33, 916–918. [Google Scholar] [CrossRef]
- Zanin, E.; Capua, M.; Casaccia, C.; Zuin, A.; Moresco, A. Isolation and Characterization of Aujeszky’s Disease Virus in Captive Brown Bears from Italy. J. Wildl. Dis. 1997, 33, 632–634. [Google Scholar] [CrossRef]
- Schultze, A.E.; Maes, R.K.; Taylor, D.C. Pseudorabies and volvulus in a black bear. J. Am. Vet. Med. Assoc. 1986, 189, 1165–1166. [Google Scholar]
- Kirkpatrick, C.M.; Kanitz, C.L.; McCrocklin, S.M. Possible role of wild mammals in transmission of pseudorabies to swine. J. Wildl. Dis. 1980, 16, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Medkour, H.; Laidoudi, Y.; Marié, J.-L.; Fenollar, F.; Davoust, B.; Mediannikov, O. Molecular investigation of vector-borne pathogens in red foxes (VULPES VULPES) from southern france. J. Wildl. Dis. 2020, 56, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Teuffert, J.; Staubach, C.; Selhorst, T.; Depner, K.R. Long-Term Studies on Maternal Immunity for Aujeszky’s Disease and Classical Swine Fever in Wild Boar Piglets. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2005, 52, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Marcé, C.; Deblanc, C.; Oger, A.; Bourry, O.; Simon, G.; Rose, N.; le Potier, M.-F. Upholding of Aujeszky’s Disease-Free Status in 2014: Improvement of Detection in High Risk Pig Herds but Decrease in Field Player Vigilance. Bull. Épidémiol. St. Anim. Aliment. 2015, 71, 50–53. [Google Scholar]
- Toma, B.; Dufour, B. Transmission de La Maladie d’Aujeszky Des Sangliers Sauvages Aux Suidés Domestiques. Epidémiol et Santé Anim 2004, 45, 115–119. [Google Scholar]
- Deblanc, C.; Oger, A.; Simon, G.; Le Potier, M.-F. Genetic Diversity among Pseudorabies Viruses Isolated from Dogs in France from 2006 to 2018. Pathogens 2019, 8, 266. [Google Scholar] [CrossRef]
- Cano-Terriza, D.; Martínez, R.; Moreno, A.; Pérez-Marín, J.E.; Jiménez-Ruiz, S.; Paniagua, J.; Borge, C.; García-Bocanegra, I. Survey of Aujeszky’s Disease Virus in Hunting Dogs from Spain. EcoHealth 2019, 16, 351–355. [Google Scholar] [CrossRef]
- ProMED-International Society for Infectious Diseases PRO/AH/EDR: Pseudorabies (Aujeszky’s Disease)—France: (LP) Dog. Available online: https://actu.fr/faits-divers/attention-la-maladie-d-aujeszky-refait-surface-dans-les-hautes-pyrenees_48285202.html (accessed on 22 September 2022).
- de Lange, K.; Haddad, N.; Agier, C.; Le Potier, M.F.; Le Vée, M.; Amar, P.; Toma, B. Specificity of three ELISA-gE kits for screening pig meat for antibodies to Aujeszky’s disease. Vet. Rec. 2003, 153, 621–624. [Google Scholar] [CrossRef]
- Weiler, U.; Claus, R.; Schnoebelen-Combes, S.; Louveau, I. Influence of age and genotype on endocrine parameters and growth performance: A comparative study in Wild boars, Meishan and Large White boars. Livest. Prod. Sci. 1998, 54, 21–31. [Google Scholar] [CrossRef]
- Vidal, N.P.; Manful, C.F.; Pham, T.H.; Stewart, P.; Keough, D.; Thomas, R. The use of XLSTAT in conducting principal component analysis (PCA) when evaluating the relationships between sensory and quality attributes in grilled foods. MethodsX 2020, 7, 100835. [Google Scholar] [CrossRef] [PubMed]
| Demography | ELISA Results | Statistics | |||||
|---|---|---|---|---|---|---|---|
| Period | Parameters | Tested | Positive | Seroprevalence (%) | Binomial 95% CI | Wald’ chi² | p-Value |
| 2019–20 | Sites | ||||||
| Canjuers | 219 | 64 | 29.2 | [23.3; 35.7] | |||
| Carpiagne | 20 | 0 | 0 | 6.65 | 0.01 | ||
| Solenzara | 13 | 10 | 76.9 | [46.2; 94.9] | 70.50 | <0.0001 | |
| Gender | |||||||
| Female | 166 | 48 | 28.9 | [22.2; 36.5] | |||
| Male | 86 | 26 | 30.2 | [20.8; 41.1] | 1.23 | 0.267 | |
| Age (Weeks) | |||||||
| [≤20] | 61 | 13 | 21.3 | [11.9; 33.7] | |||
| [20–35] | 180 | 57 | 31.7 | [25.0; 39.0] | 6.16 | 0.013 | |
| [35–45] | 11 | 4 | 36.4 | [10.9; 69.2] | 19.76 | <0.0001 | |
| [≥45] | 0 | ||||||
| 2021–22 | Sites | ||||||
| Canjuers | 87 | 37 | 42.5 | [32.0; 53.6] | |||
| Carpiagne | 35 | 0 | 0 | 11.79 | 0.0001 | ||
| Solenzara | 25 | 10 | 40 | [21.1; 61.3] | 3.05 | 0.081 | |
| Gender | |||||||
| Female | 81 | 26 | 32.1 | [22.2; 43.4] | |||
| Male | 66 | 21 | 31.8 | [20.9; 44.4] | 1.72 | 0.190 | |
| Age (Weeks) | |||||||
| [≤20] | 27 | 7 | 25.9 | [11.1; 46.3] | |||
| [20–35] | 55 | 13 | 23.6 | [13.2; 37.0] | 4.36 | 0.037 | |
| [35–45] | 46 | 17 | 37.0 | [23.2; 52.5] | 12.48 | 0.0004 | |
| [≥45] | 19 | 10 | 52.6 | [28.9; 75.6] | 11.85 | 0.0005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laidoudi, Y.; Davoust, B.; Watier-Grillot, S.; Oger, A.; Le Potier, M.-F.; Deblanc, C. Serological Survey of Aujeszky’s Disease in Wild Boar from Southeastern France. Pathogens 2022, 11, 1107. https://doi.org/10.3390/pathogens11101107
Laidoudi Y, Davoust B, Watier-Grillot S, Oger A, Le Potier M-F, Deblanc C. Serological Survey of Aujeszky’s Disease in Wild Boar from Southeastern France. Pathogens. 2022; 11(10):1107. https://doi.org/10.3390/pathogens11101107
Chicago/Turabian StyleLaidoudi, Younes, Bernard Davoust, Stéphanie Watier-Grillot, Aurélie Oger, Marie-Frédérique Le Potier, and Céline Deblanc. 2022. "Serological Survey of Aujeszky’s Disease in Wild Boar from Southeastern France" Pathogens 11, no. 10: 1107. https://doi.org/10.3390/pathogens11101107