The Outcome of Surgical Treatment for the Neuropathic Diabetic Foot Lesions—A Single-Center Study
Abstract
:1. Introduction
1.1. The Clinical Diagnosis
- Charcot osteoarthropathy is the most typical lesion for diabetic peripheral neuropathy and occurs due to repeated, unnoticed microtraumas that lead to diffuse inflammation of the skeleton [11]. The collapse of the plantar arch is a consequence of this inflammation, with extensive changes in the foot biomechanics that will later require complex, multidisciplinary treatment: podiatry, surgery, orthopedics, and rheumatology [9,10].
- Hammertoe, which is often associated with hallux valgus, is seen in people wearing shoes that cause a foot malposition.
- Diabetic foot ulcerative lesions.
- Toe gangrene.
- Toe osteitis.
1.2. Paraclinical Tests
1.3. Prophylaxis of Lesions/Infections
1.4. The Treatment
- -
- -
- Treatment with negative pressure on wounds and diabetic foot ulcers is an effective adjunctive therapy. From the mechanism of action, it is based on the creation of an anti-inflammatory and pro-angiogenic effect, with the stimulation of the growth factors that will lead to the appearance of the granulation tissue [22,23,24].
- -
- Plastic surgery techniques cover defects in the foot’s soft tissue.
1.5. DNF Surgical Treatment
1.6. Objectives
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volmer-Thole, M.; Lobmann, R. Neuropathy and Diabetic Foot Syndrome. Int. J. Mol. Sci. 2016, 17, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caitlin, W.; Hicks, C.W.; Selvin, E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar]
- Boulton, A.J. The diabetic foot: Grand overview, epidemiology and pathogenesis. Diabet./Metab. Res. Rev. 2008, 24, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J. Diabetic neuropathy and foot complications. Handb. Clin. Neurol. 2014, 126, 97–107. [Google Scholar]
- Ziegler, D.; Keller, J.; Maier, C.; Pannek, J. Diabetic neuropathy. Exp. Clin. Endocrinol. Diabet. 2014, 122, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, G. Diabetic neuropathy. Handb. Clin. Neurol. 2013, 115, 579–589. [Google Scholar] [PubMed]
- Sandireddy, R.; Yerra, V.G.; Areti, A.; Komirishetty, P.; Kumar, A. Neuroinflammation and oxidative stress in diabetic neuropathy: Futuristic strategies based on these targets. Int. J. Endocrinol. 2014, 2014, 674987. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharm. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zenker, J.; Ziegler, D.; Chrast, R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013, 36, 439–449. [Google Scholar] [CrossRef]
- Feldman, E.L.; Nave, K.A.; Jensen, T.S.; Bennett, D.L.H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313, PMCID:PMC5400015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaroslawska, J.; Korytko, A.; Zglejc-Waszak, K.; Antonowski, T.; Pomianowski, A.S.; Wasowicz, K.; Wojtkiewicz, J.; Juranek, J.K. Peripheral Neuropathy Presents Similar Symptoms and Pathological Changes in Both High-Fat Diet and Pharmacologically Induced Pre- and Diabetic Mouse Models. Life 2021, 11, 1267, PMCID:PMC8618965. [Google Scholar] [CrossRef] [PubMed]
- Preguiça, I.; Alves, A.; Nunes, S.; Gomes, P.; Fernandes, R.; Viana, S.D.; Reis, F. Diet-Induced Rodent Models of Diabetic Peripheral Neuropathy, Retinopathy and Nephropathy. Nutrients 2020, 12, 250, PMCID:PMC7019796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broussard, K.C.; Powers, J.G. Wound dressings: Selecting the most appropriate type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef]
- Burgess, J.; Frank, B.; Marshall, A.; Khalil, R.S.; Ponirakis, G.; Petropoulos, I.N.; Cuthbertson, D.J.; Malik, R.A.; Alam, U. Early Detection of Diabetic Peripheral Neuropathy: A Focus on Small Nerve Fibres. Diagnostics 2021, 11, 165, PMCID:PMC7911433. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; Jarl, G. Knee-High Devices Are Gold in Closing the Foot Ulcer Gap: A Review of Offloading Treatments to Heal Diabetic Foot Ulcers. Medicina 2021, 57, 941, PMCID:PMC8471745. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Deshpande, S.; O’Meara, S.; Speak, K. Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013, CD009099. [Google Scholar] [CrossRef]
- Jeffcoate, W.J.; Vileikyte, L.; Boyko, E.J.; Armstrong, D.G.; Boulton, A.J.M. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers. Diabetes Care 2018, 41, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Zhang, Y.; Bowers, D.T.; Liu, W.; Ma, M. Functional hydrogels for diabetic wound management. APL Bioeng. 2021, 5, 031503, PMCID:PMC8272650. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76, Correction in Theranostics 2021, 11, 10174–10175. [Google Scholar] [CrossRef]
- Bordianu, A.; Bobircă, F.; Pătraşcu, T. Skin Grafting in the Treatment of Diabetic Foot Soft Tissue Defects. Chirurgia 2018, 113, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072, PMCID:PMC8539411. [Google Scholar] [CrossRef] [PubMed]
- Bobircă, F.; Bobircă, A.; Bordianu, A.; Jauca, C.; Georgescu, D.; Radu, R.; Pătraşcu, T. Current Surgical Approach in the Pathology of the Arteriopathic Predominant Diabetic Foot. Chirurgia 2018, 113, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Storck, M.; Lawall, H.; Wozniak, G.; Mauckner, P.; Hochlenert, D.; Wetzel-Roth, W.; Sondern, K.; Hahn, M.; Rothenaicher, G. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: Results of the German DiaFu-RCT. BMJ Open 2020, 10, e026345, PMCID:PMC7202734. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Wetherhold, J.; Schulz, S.; Valerio, I. A Novel Use of Next-Generation Closed Incision Negative Pressure Wound Therapy After Major Limb Amputation and Amputation Revision. Cureus 2020, 12, e10393, PMCID:PMC7550024. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Butrico, L.; Sammarco, G.; de Franciscis, S.; Serra, R. The care of transmetatarsal amputation in diabetic foot gangrene. Int. Wound J. 2017, 14, 9–15, PMCID:PMC7949543. [Google Scholar] [CrossRef] [PubMed]
- Mandolfino, T.; Canciglia, A.; Salibra, M.; Ricciardello, D.; Cuticone, G. Functional outcomes of transmetatarsal amputation in the diabetic foot: Timing of revascularization, wound healing and ambulatory status. Updates Surg. 2016, 68, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Häller, T.V.; Uçkay, I.; Kaiser, D.; Berli, M.; Böni, T.; Waibel, F. Revision After Total Transmetatarsal Amputation. J. Foot Ankle Surg. 2019, 58, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Bik, P.M.; Heineman, K.; Levi, J.; Sansosti, L.E.; Meyr, A.J. The Effect of Remnant Metatarsal Parabola Structure on Transmetatarsal Amputation Primary Healing and Durability. J. Foot Ankle Surg. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.S.; Faulk, J. Lower Extremity Amputation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Rathnayake, A.; Saboo, A.; Malabu, U.H.; Falhammar, H. Lower extremity amputations and long-term outcomes in diabetic foot ulcers: A systematic review. World J. Diabetes 2020, 11, 391–399, PMCID:PMC7503503. [Google Scholar] [CrossRef] [PubMed]
- Moxey, P.W.; Gogalniceanu, P.; Hinchliffe, R.J.; Loftus, I.M.; Jones, K.J.; Thompson, M.M.; Holt, P.J. Lower extremity amputations—A review of global variability in incidence. Diabet. Med. 2011, 28, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Barcel, D.A.; Odum, S.; Rowe, T.; Sabatini, J.B.; Ford, S.E.; Davis, W.H.; Irwin, T.A. Mortality and Conversion Rates to Below-Knee or Above-Knee Amputation After Transmetatarsal Amputation. J. Am. Acad. Orthop. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Thorud, J.C.; Jupiter, D.C.; Lorenzana, J.; Nguyen, T.T.; Shibuya, N. Reoperation and Reamputation After Transmetatarsal Amputation: A Systematic Review and Meta-Analysis. J. Foot Ankle Surg. 2016, 55, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Kowal, M.; Paprocka Borowicz, M.; Starczewska, A.; Rutkowska-Kucharska, A. Biomechanical Parameters of Gait after Unilateral Above-knee Amputation. Current State of Research. Ortop. Traumatol. Rehabil. 2018, 20, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Demirdal, T.; Emir, B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab. Res. Rev. 2019, 35, e3165. [Google Scholar] [CrossRef] [PubMed]
- Pickwell, K.; Siersma, V.; Kars, M.; Apelqvist, J.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovská, A.; Jude, E.; Mauricio, D.; et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 2015, 38, 852–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgescu, D.E.; Mustăţea, P.; Mihalache, O.; Bobircă, F.; Agache, A.; Georgescu, T.F.; Chiriac, O.; Marin, V.; Doran, H.; Pătraşcu, T. Surgical Management of Diabetic Neuropathy Foot Complications. Chirurgia 2018, 113, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J. Lowering the risk of neuropathy, foot ulcers and amputations. Diabet. Med. 1998, 15 (Suppl. S4), S57–S59. [Google Scholar] [CrossRef] [PubMed]
- Bondar, A.; Popa, A.R.; Papanas, N.; Popoviciu, M.; Vesa, C.M.; Sabau, M.; Daina, C.; Stoica, R.A.; Katsiki, N.; Stoian, A.P. Diabetic neuropathy: A narrative review of risk factors, classification, screening and current pathogenic treatment options (Review). Exp. Ther. Med. 2021, 22, 690. [Google Scholar] [CrossRef]
- Sämann, A.; Tajiyeva, O.; Müller, N.; Tschauner, T.; Hoyer, H.; Wolf, G. and Müller, U.A. (2008), Prevalence of the diabetic foot syndrome at the primary care level in Germany: A cross-sectional study. Diabet. Med. 2008, 25, 557–563. [Google Scholar] [CrossRef]
- Serban, D.; Papanas, N.; Dascalu, A.M.; Kempler, P.; Raz, I.; Rizvi, A.A.; Rizzo, M.; Tudor, C.; Silviu Tudosie, M.; Tanasescu, D.; et al. Significance of Neutrophil to Lymphocyte Ratio (NLR) and Platelet Lymphocyte Ratio (PLR) in Diabetic Foot Ulcer and Potential New Therapeutic Targets. Int. J. Low. Extrem. Wounds 2021, 15347346211057742. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.; Papanas, N.; Dascalu, A.M.; Stana, D.; Nicolae, V.A.; Vancea, G.; Badiu, C.D.; Tanasescu, D.; Tudor, C.; Balasescu, S.A.; et al. Diabetic Retinopathy in Patients with Diabetic Foot Ulcer: A Systematic Review. Int. J. Low. Extrem. Wounds 2021, 20, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Hunt, N.A.; Ndip, A.; Lavery, D.C.; Van Houtum, W.; Boulton, A.J. Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care 2010, 33, 2365–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggers, P.W.; Gohdes, D.; Pugh, J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999, 56, 1524–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.H.R.; Cardoso, N.A.; Procópio, R.J.; Navarro, T.P.; Dardik, A.; de Loiola Cisneros, L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab. Syndr. 2017, 11 (Suppl. S2), S583–S587. [Google Scholar] [CrossRef]
- Musuuza, J.; Sutherland, B.L.; Kurter, S.; Balasubramanian, P.; Bartels, C.M.; Brennan, M.B. A systematic review of multidisciplinary teams to reduce major amputations for patients with diabetic foot ulcers. J. Vasc. Surg. 2020, 71, 1433–1446.e3. [Google Scholar] [CrossRef] [PubMed]
- Lenselink, E.; Holloway, S.; Eefting, D. Outcomes after foot surgery in people with a diabetic foot ulcer and a 12-month follow-up. J. Wound Care 2017, 26, 218–227. [Google Scholar] [CrossRef]
- Ardeleanu, V.; Toma, A.; Pafili, K.; Papanas, N.; Motofei, I.; Diaconu, C.C.; Rizzo, M.; Stoian, A.P. Current Pharmacological Treatment of Painful Diabetic Neuropathy: A Narrative Review. Medicina 2020, 56, 25. [Google Scholar] [CrossRef] [Green Version]






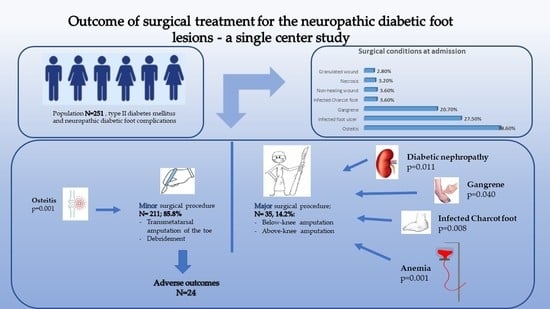
| All Subjects N = 251 | Patients Diagnosed with Osteitis N = 97 | Patients Diagnosed with Ulcer N = 69 | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | Value | Value | p-Value | OR (95% CI) | Value | p-Value | OR (95% CI) |
| Age at admission Mean ± SD ≤50 yo N (%) >50 and ≤70 yo N (%) >70 yo N (%) | 61.21 ± 10.7 40 (15.9%) 160 (63.7%) 51 (20.3%) | 61.08 ± 10.42 | 0.862 | 0.998 (0.975–1.022) | 60.40 ± 11.44 | 0.884 | 0.994 (0.969–1.020) |
| Male sex N (%) | 178 (70.9%) | 60 (61.9%) | 0.012 * | 0.496 (0.284–0.861) | 49 (71.1%) | 0.983 | 1.007 (0.547–1.854) |
| Urban residents N (%) | 148 (59.0%) | 56 (57.7%) | 0.753 | 0.920 (0.550–1.542) | 36 (52.2%) | 0.178 | 0.682 (0.390–1.192 |
| Diabetes duration (years) Mean ± SD | 11.56 ± 6.5 | 10.21 ± 5.49 | 0.015 * | 0.949 (0.909–0.991) | 10.83 ±5.72 | 0.419 | 0.978 (0.935–1.023) |
| Glucose level (mg/dL) Mean ± SD | 200.83 ± 102.0 | 179.29 ± 92.29 | 0.007 * | 0.997 (0.994–0.999) | 185.98 ± 91.39 | 0.215 | 0.998 (0.995–1.001) |
| Insulin-dependent Status N (%) | 113 (45.0%) | 37 (38.1%) | 0.082 | 1.580 (0.942–2.651) | 30 (43.5%) | 0.762 | 1.090 (0.624–1.905) |
| Retinopathy N (%) | 50 (19.9%) | 14 (14.4%) | 0.084 | 0.553 (0.281–1.089) | 16 (23.2%) | 0.425 | 1.314 (0.671–2.573) |
| Nephropathy N (%) | 43 (17.1%) | 14 (14.4%) | 0.368 | 0.727 (0.368–1.458) | 10 (14.5%) | 0.495 | 0.765 (0.355–1.651) |
| Cardiovascular diseases N (%) | 159 (63.3%) | 62 (63.9%) | 0.882 | 1.041 (0.614–1.765) | 39 (56.5%) | 0.167 | 0.672 (0.381–1.183) |
| Anemia N (%) | 153 (61.0%) | 47 (48.5%) | 0.001 * | 0.426 (0.252–0.719) | 38 (55.1%) | 0.239 | 0.714 (0407–1.253) |
| Leukocytosis N (%) | 89 (35.5%) | 26 (26.8%) | 0.023 * | 0.529 (0.304–0.919) | 24 (34.8%) | 0.890 | 0.960 (0.537–1.716) |
| Minor Surgical Procedure N = 211 (85.8%) | Major Surgical Procedure N= 35 (14.2%) | p Value | OR (95% CI) | |
|---|---|---|---|---|
| Diagnosis | ||||
| Gangrene | 40 (19.0%) | 12 (34.3%) | 0.040 * | 2.230 (1.024–4.857) |
| Osteitis | 92 (43.6%) | 5 (14.3%) | 0.001 * | 0.216 (0.080–0.577) |
| Ulcer | 61 (28.9%) | 8 (22.9%) | 0.460 | 0.729 (0.314–1.693) |
| Infected Charcot foot | 5 (2.4%) | 4 (11.4%) | 0.008 * | 5.316 (1.354–20.877) |
| Non-healing wound | 6 (2.8%) | 3 (8.6%) | 0.095 | 3.203 (0.763–13.453) |
| Necrosis (dry) | 5 (2.4%) | 3 (8.6%) | 0.055 | 3.863 (0.880–16.951) |
| Granulated wound | 2 (0.9%) | 0 | 0.563 | 0.857 (0.814–0.902) |
| Patient’s characteristics | ||||
| Insulin-dependent Status N (%) | 95 (45.0%) | 16 (45.7%) | 0.939 | 0.973(0.474–1.994) |
| Male sex N (%) | 148 (79.1%) | 26 (74.3%) | 0.618 | 1.230 (0.545–2.774) |
| Urban residents N (%) | 124 (58.8%) | 20 (57.1%) | 0.857 | 0.935 (0.454–1.928) |
| Good outcome N (%) | 187 (88.6%) | 35 (100.0%) | 0.031 * | 0.842 (0.796–0.892) |
| Diabetes duration (yo) mean ± SD | 11.23 ± 6.4 | 12.77 ± 6.4 | 0.118 | 1.036 (0.983–1.092) |
| Glucose level (mg/dL) mean ± SD | 200.04 ± 101.1 | 208.70 ± 105.2 | 0.641 | 1.001 (0.997–1.004) |
| Anemia N (%) | 121 (57.3%) | 28 (80.0%) | 0.011 * | 2.975 (1.244–7.116) |
| Leukocytosis N (%) | 74 (35.1%) | 15 (42.9%) | 0.375 | 1.389 (0.671–2.872) |
| Cardiovascular diseases N (%) | 130 (61.6%) | 25 (71.4%) | 0.265 | 1.558 (0.711–3.412) |
| Retinopathy N (%) | 40 (19.0%) | 8 (22.9%) | 0.590 | 1.267 (0.536–2.996) |
| Nephropathy N (%) | 30 (14.4%) | 14 (37.1%) | 0.001 * | 3.565 (1.623–7.832) |
| Characteristics | Good Outcome N = 227 | Adverse Outcome N = 24 | p-Value | OR (95% CI) |
|---|---|---|---|---|
| Age at admission Mean ± SD | 61.24 ± 11.0 | 60.88 ± 7.7 | 0.927 | 1.007 (0.965–1.051) |
| Male sex N (%) | 162 (71.4%) | 16 (66.7%) | 0.630 | 0.802 (0.328–1.966) |
| Urban residents N (%) | 135 (59.4%) | 13 (54.2%) | 0.615 | 0.805 (0.346–1.876) |
| Diabetes duration (yo) Mean ± SD | 11.76 ± 6.5 | 9.71 ± 6.5 | 0.092 | 0.941 (0.868–1.019) |
| Insulin-dependent status N (%) | 102 (44.9%) | 11 (45.8%) | 0.933 | 0.965 (0.414–2.224) |
| Glucose level (mg/dL) Mean ± SD | 200.91 ± 101.8 | 200.08 ± 105.6 | 0.967 | 1.000 (0.996–1.005) |
| Retinopathy N (%) | 46 (20.3%) | 4 (16.7%) | 0.675 | 0.787 (0.256–2.415) |
| Nephropathy N (%) | 41 (18.1%) | 2 (8.3%) | 0.229 | 0.412 (0.093–1.824) |
| Anemia on admission N (%) | 134 (59.0%) | 19 (79.2%) | 0.054 | 2.637 (0.951–7.314) |
| Cardiovascular diseases N (%) | 147 (64.8%) | 12 (50.0%) | 0.154 | 0.544 (0.234–1.267) |
| Admission leukocytosis N (%) | 78 (34.4%) | 11 (45.8%) | 0.264 | 1.616 (0.692–3.776) |
| Diagnosis | ||||
| Gangrene N = 52 | 49 (21.6%) | 3 (12.5%) | 0.296 | 0.519 (0.149–1.812) |
| Osteitis N = 97 | 86 (37.9%) | 11 (45.8%) | 0.447 | 1.387 (0.595–3.235) |
| Ulcer N = 69 | 62 (27.3%) | 7 (29.2%) | 0.847 | 1.096 (0.434–2.770) |
| Infected Charcot foot N = 9 | 8 (3.5%) | 1 (4.2%) | 0.602 | 1.190 (0.142–9.943) |
| Non-healing wound N = 9 | 8 (3.5%) | 1 (4.2%) | 0.602 | 1.190 (0.142–9.943) |
| Necrosis (dry) N = 8 | 7 (3.1%) | 1 (4.2%) | 0.558 | 1.366 (0.161–11.601) |
| Granulated wound N = 7 | 7 (3.1%) | 0 | 0.383 | 0.902 (0.865–0.940) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobirca, F.; Smarandache, C.G.; Bobirca, A.; Alexandru, C.; Dumitrescu, D.; Stoian, A.P.; Bica, C.; Brinduse, L.A.; Musetescu, A.; Gheoca-Mutu, D.-E.; et al. The Outcome of Surgical Treatment for the Neuropathic Diabetic Foot Lesions—A Single-Center Study. Life 2022, 12, 1156. https://doi.org/10.3390/life12081156
Bobirca F, Smarandache CG, Bobirca A, Alexandru C, Dumitrescu D, Stoian AP, Bica C, Brinduse LA, Musetescu A, Gheoca-Mutu D-E, et al. The Outcome of Surgical Treatment for the Neuropathic Diabetic Foot Lesions—A Single-Center Study. Life. 2022; 12(8):1156. https://doi.org/10.3390/life12081156
Chicago/Turabian StyleBobirca, Florin, Catalin Gabriel Smarandache, Anca Bobirca, Cristina Alexandru, Dan Dumitrescu, Anca Pantea Stoian, Cristina Bica, Lacramioara Aurelia Brinduse, Anca Musetescu, Daniela-Elena Gheoca-Mutu, and et al. 2022. "The Outcome of Surgical Treatment for the Neuropathic Diabetic Foot Lesions—A Single-Center Study" Life 12, no. 8: 1156. https://doi.org/10.3390/life12081156