3.2. Anthocyanin Adsorption
Figure 8 shows the adsorption results when all the materials were probed as ACy adsorbents at pH 3, as mentioned above, and the pH was chosen taking into consideration the stability of the ACy solutions [
1]. The results showed that the amount adsorbed by the samples was in the order of B3 > B4 > B2 > B1, suggesting that Na-bentonites have a higher ACy adsorption capacity than the Mg-Ca-Na-bentonite. This can be explained by considering that the adsorption mechanism reported for natural clays is cation exchange, and a monovalent cation, such as sodium, is more easily displaced than a divalent cation, such as calcium or magnesium. Thus, the flavylium cation present in the solution does not have sufficient strength to displace the divalent cations present in B1, decreasing its adsorption under these conditions.
Taking these results into account and to evaluate the pH required to carry out the kinetic and adsorption experiments, the sample B3 was chosen and the results of adsorption vs. pH are shown in
Figure 9. This figure shows that the highest adsorption capacity was obtained at pH 3. As the pH values increased, the adsorption decreased significantly. These results may be explained by the fact that the clay has a net negative structural charge throughout the pH range and that the main ACy species in solution is the flavylium cation, which implies that the amount adsorbed will be greater at the lowest mean pH. This behaviour is associated with the adsorption mechanism of the cationic exchange of the flavylium cation by the cations in the natural material, which is well known. As the pH of the media increases because the proportion of uncharged ACy molecules (quinoidal base) increases, the adsorption is gradually reduced. Taking these results into account, the pH selected for the kinetic studies was pH 3.
Considering that the kinetics studies showed similar results for the sodic bentonites,
Figure 10 shows the adsorption kinetics obtained for the B1 and B3 samples and their corresponding best fit. The results proved that the system quickly tends to equilibrium, with Na-bentonite reaching equilibrium after 1 h contact. Conversely, in the case of B1, the time necessary for the system to reach equilibrium was 4 h. Both results support the fact that the adsorption of ACys under these working conditions occurs by cation exchange because this type of mechanism has fast kinetics.
The experimental data obtained were fitted to the PFO and PSO models, and
Table 5 summarises the kinetic parameters calculated through nonlinear regression. As can be seen, the results exhibit a good fit for both models, but some slight differences can be observed. In the case of the B1 sample, the best fitting was obtained for the PFO model, suggesting that for this system, the adsorption process depends on the diffusion of ACy molecules from the bulk to the surface and their concentration [
36]. For the B3 sample, the best fit was noted for the PSO model, which is based on the adsorption capacity of the solid, and the controlling stage of the adsorption process is the change generated in the system by either a chemical or electrostatic process. The latter would be the case for the adsorption of the flavylium cation through cation exchange.
Figure 11 shows the adsorption isotherms of ACys on all minerals at pH 3 and 20 °C. According to the classification by Giles et al. (1974) [
49], the B1 isotherm can be classified as Type S (sigmoidal). This finding suggests that at the beginning of the adsorption process, the ACy molecule has low affinity for the clay surface. However, once the adsorbate is retained in the solid, there could be new adsorption sites for other molecules. Samples B2 and B4 show Type L (Langmuir) isotherms, which suggests that there is a considerable affinity of the ACy species in solution towards the adsorbent, reaching a maximum adsorption value under the conditions studied. Finally, the isotherm obtained for B3 can be classified as Type H (high affinity), showing that at low ACy concentrations, a significant increase in adsorption occurs, proving that the affinity of the adsorbate for the adsorbent is considerably higher than the other samples [
49,
50,
51]. Type L and Type H isotherms are associated with the adsorption of ionic solutes where there is no competition between the solute and the solvent for adsorption sites. These results support those previously proposed where the ACy species interacts electrostatically with the surface of the materials B2, B3, and B4 through a cationic exchange mechanism. In the case of sample B1, a higher Acy concentration is required to begin adsorption potentially because the one positive charge of the flavylium cation does not have sufficient strength to displace the divalent cations present in the interlayer of this material. Additionally, the latter is in agreement with the cooperative adsorption mechanism proposed for ACy adsorption on B1.
The experimental adsorption data were fitted to the Langmuir, Freundlich, and Sips models, and the corresponding fitting parameters are summarised in
Table 6. Subsequently, Langmuir separation factors (R
L) were calculated for all systems, resulting in values of 0.41, 0.12, 0.05, and 0.10 for B1, B2, B3, and B4, respectively [
38]. These values indicate a favorable adsorption process in all cases. Notably, the higher R
L value for B1 could be attributed to a more linear adsorption, potentially explained by the previously proposed cooperative adsorption mechanism.
In all cases, the Sips model exhibited the best fit, suggesting that the adsorption systems exhibit heterogeneity. This could be attributed to ACy being adsorbed at sites with varying energy on the surface of the minerals or the presence of different ACy species in the solution. The ACy adsorption capacities followed the order of B1 < B4 < B2 and B3. This finding is consistent with the earlier results, where the amount of adsorbed ACy on Mg-Ca-Na-bentonite was considerably lower than in the B3 sample, owing to the higher concentration of divalent cations in the former. Among the Na-bentonites, B3 had a comparable CEC values to the others, suggesting that the higher adsorption obtained may be due to other samples having higher contributions of divalent cations, such as calcium or magnesium, in their structure than B3, consequently reducing their adsorption capacities. The latter finding is made according to observations in the chemical analyses of bentonites. Additionally, the values obtained for parameter n, associated with system heterogeneity, indicated that B1 and B4 samples have values less than 1, indicating more homogeneous systems, while B2 and B3 exhibit higher heterogeneity. These results correlate with the adsorption capacities obtained for each material, suggesting that higher heterogeneity enhances ACy adsorption onto the samples under the studied conditions.
In order to investigate whether the adsorption of ACy on the bentonites was by cationic exchange in all cases, the adsorption complexes of ACy on the four samples were analysed. For this, adsorption was carried out under the same conditions as previously described. The supernatant was separated, and the solids were dried at room temperature. FTIR and XRD analyses were obtained for each of the samples.
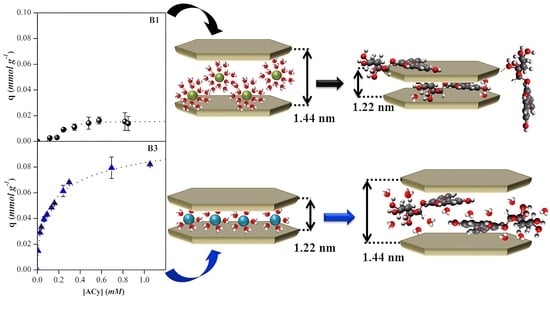
Figure 12 shows the diffractograms of the ACy–bentonite complexes obtained. For B2, B3, and B4, an increase of 1.2 to 1.4 nm in the basal spacing
d001 was observed. It is well known that sodium montmorillonites have a hydration layer of the sodium cation and are identified by the presence of a
d001 that appears in the vicinity of 1.26 nm, a value that includes the thickness of a TOT sheet (0.96 nm) plus the interlaminar space that will vary depending on the interlayer cation and its hydration [
52]. Because the laminar structure of the mineral is not modified during the adsorption process, the increase in the basal space demonstrates that the anthocyanin molecules were adsorbed in the interlaminar space of the sodium bentonites, which concurs with similar results reported for other ACy–clay systems [
9,
11,
12,
53]. Likewise, the width of the peaks obtained suggests a higher disorder in the interlayer of the complexes obtained than the natural material, which could be explained by the contribution made by different interlaminar spaces generated by different orientations of the ACy molecule adsorbed in the interlayer of the material. In the case of B1, it was observed that the basal spacing decreased from 1.46 nm, which is characteristic of bentonites with a predominance of divalent cations, to a value of 1.22 nm, which could indicate that the ACys were adsorbing in a position such that the smallest possible space is occupied. Alternatively, ACys are adsorbed outside the interlayer through other types of interactions. The latter could be because the ACy molecule adsorbs on the non-charged surface sites on B1, such as the siloxane surface, and through hydrophobic interactions, which would explain the cooperative adsorption mechanism proposed.
A possible explanation for the results obtained for the XRD analysis of the ACy–bentonite complexes derived from analysing the structure and dimensions of the ACy molecule is shown in
Figure 13. This figure shows that there is a fraction of the molecule composed of aromatic rings, which is flat (flavylium cation), while the glucoside substituents, due to their characteristic boat-shaped structure, generate a region of greater spatial distribution. Based on this finding, it could be inferred that, in the case of the sodium bentonites, the molecule gets into the interlaminar space in the conformation represented as B, where the aromatic rings of the flavylium cation in the ACy molecule remain parallel to the internal surface, resulting in a basal spacing that is the sum of the thickness of the ACy molecule (0.46 nm) and the TOT sheet (0.96 nm). The latter is in agreement with previous reports that suggest that molecules with aromatic rings can orient themselves in the interlayer of the clay mineral and interact through hydrophobic interactions with the siloxane surface of the tetrahedral sheet and with the previously proposed cation exchange mechanism [
11]. However, it can be seen that the maximum quantity of adsorbed ACys is low compared to the CEC of each of the bentonites. A possible explanation for this fact would be to consider the molecular size of the ACy. In addition, once the molecule is adsorbed, the neighbouring adsorption sites are blocked, preventing the entry of new ACy molecules into the structure. In the case of B1, and as mentioned above, the molecule may be adsorbed mostly on the external surface of siloxane. After that, it becomes an adsorption site for other molecules, favouring cooperative adsorption.
The FTIR spectra obtained for the ACy–bentonite complex show the same signals and are illustrated in
Figure 14. The absorption peaks at 3620, 3430, and 1030 cm
–1 are characteristic of montmorillonites and correspond to the stretching of the O-H bonds located between the tetrahedral and octahedral sheets of the mineral, the vibration of the O-H bonds of the water molecules, and the stretching of Si-O, respectively [
42,
54]. These results showed that the structure of the mineral did not suffer modifications following the adsorption of ACys on the surface (see
Figure 14A). Conversely, in
Figure 14B, the absorption observed at 2928 cm
–1 is associated with the scissor bending of the -CH
2- groups in the cyclohexanes, while the bands between 1736 cm
−1 and 1697 cm
–1 refer to the out-of-plane bending of the C–H bonds. These bands may appear superimposed on the broadband that appears around 1600 cm
−1 related to the hydration water of the clay mineral. Finally, the set of bands between 1585–1543 cm
−1 and 1508 cm
−1 are associated with the conjugated diene groups, and this may be due to the interaction of this fraction of the molecule with the natural material [
7,
55].
The last adsorption probe was to evaluate the material that showed the highest adsorption capacity at a pH value higher than 3, and pH 4.5 was chosen due to the instability of the Acy molecule.
Figure 15 shows the adsorption isotherms of ACys on B3 at pH 3 and 4.5. The isotherm obtained at pH 3 was previously analysed and classified as Type L according to the classification proposed by Giles et al. [
49] where the adsorption is the result of the cation exchange of the flavylium cation present in the solution. The isotherm obtained at pH 4.5 is a sigmoidal type isotherm (Type S), indicating cooperative adsorption, similar to that observed for B1 at pH 3. This could be explained by changes in the pH of the medium. Here, as the pH increases, the concentration of the flavylium cation decreases, and the neutral species (carbinol pseudobase) appear [
56,
57]. In this case, the adsorption generated by cation exchange will be considerably lower, and the species present could be adsorbing through other types of mechanisms, such as van der Waals type interactions or hydrogen bonding, among others, which is consistent with the cooperative adsorption previously proposed [
11,
58]
The experimental data were fitted to the Langmuir, Freundlich, and Sips models, with the best fits obtained for the Sips model (see
Table 7). The obtained R
L values were 0.05 and 0.27 for pH 3 and 4.5, respectively, indicating a favorable adsorption process. Similar to previous observations, the highest adsorption capacity and the n parameter value were obtained at pH 3, demonstrating that increased heterogeneity indeed leads to a higher ACy adsorption capacity. However, in this case, the high heterogeneity could be associated with different ACy species present in solution at pH 3 that are not present at pH 4.5 and could interact with the surface at different available sites [
56,
57]. The results presented include those obtained and shown previously.
The results reported by various authors in recent years for the ACys from red cabbage adsorption on different adsorbents are summarised in
Table 8 and compared with the results obtained at pH 3 in this study. As can be seen, there are many solids with the ability to adsorb ACys from red cabbage, with a notable scarcity of reports on natural clays. The adsorption of ACys onto diverse materials is primarily contingent upon the inherent characteristics of the adsorbent, encompassing its chemical composition, physical and textural properties, and operational conditions (pH, temperature, ionic strength, etc.). Notably, the results obtained for samples B2, B3, and B4 significantly surpass the majority of reported materials. Across all instances, the natural bentonites examined in this study emerge as exceptional adsorbents for ACys in aqueous environments.