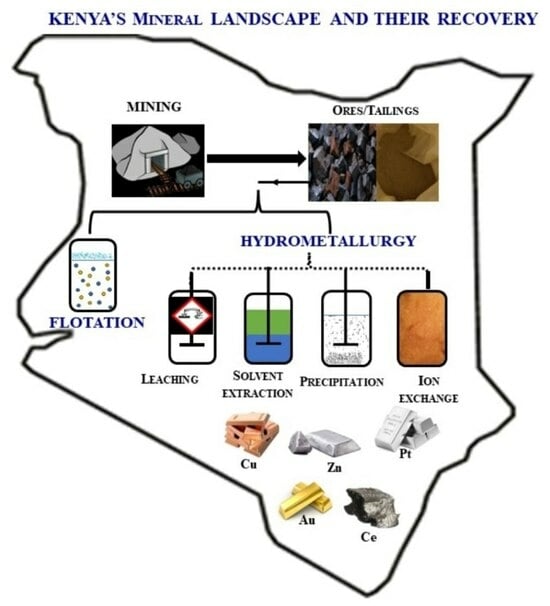
Kenya’s Mineral Landscape: A Review of the Mining Status and Potential Recovery of Strategic and Critical Metals through Hydrometallurgical and Flotation Techniques
Abstract
:1. Introduction
Strategic and Critical Metals
2. Mining Situation in Kenya
2.1. Environmental Impacts of Mining Activities
2.2. The Presence of Critical and Strategic Metals in the Mine Wastes
3. Recovery of the Strategic and Critical Metals from Various Sources
3.1. Leaching
3.2. Solvent Extraction of the Common Transition Metals and Uranium
3.3. Recovery of the Platinum Group Metals
3.4. Recovery of Rare Earth Elements
3.5. Precipitation
3.6. Ion Exchange
4. Recovery of Strategic and Critical Metals Using Flotation
5. Proposals and Prospects for Future Application of the Hydrometallurgical and Flotation Techniques
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haneklaus, N.; Sun, Y.; Bol, R.; Lottermoser, B.; Schnug, E. To extract, or not to extract uranium from phosphate rock, that is the question. Environ. Sci. Technol. 2017, 51, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Ascher, W. Why Governments Waste Natural Resources: Policy Failures in Developing Countries; JHU Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Hilson, G. The Africa Mining Vision: A manifesto for more inclusive extractive industry-led development? Can. J. Dev. Stud./Rev. Can. d’études Dév. 2020, 41, 417–431. [Google Scholar] [CrossRef]
- Kim, J.; Guillaume, B.; Chung, J.; Hwang, Y. Critical and precious materials consumption and requirement in wind energy system in the EU 27. Appl. Energy 2015, 139, 327–334. [Google Scholar] [CrossRef]
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- Moss, R.L.; Tzimas, E.; Kara, H.; Willis, P.; Kooroshy, J. Critical Metals in Strategic Energy Technologies: Assessing rare metals as supply-chain bottlenecks in low-carbon energy technologies. In JRC-Scientific and Strategic Reports, European Commission Joint Research Centre Institute for Energy and Transport; EU Publications: Luxembourg, 2011. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C.-c. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Smith, M.P.; Kynicky, J. From “strategic” tungsten to “green” neodymium: A century of critical metals at a glance. Ore Geol. Rev. 2015, 64, 455–458. [Google Scholar] [CrossRef]
- Žibret, G.; Lemiere, B.; Mendez, A.-M.; Cormio, C.; Sinnett, D.; Cleall, P.; Szabó, K.; Carvalho, M.T. National Mineral Waste Databases as an Information Source for Assessing Material Recovery Potential from Mine Waste, Tailings and Metallurgical Waste. Minerals 2020, 10, 446. [Google Scholar] [CrossRef]
- Kiprono, N.R.; Smolinski, T.R.; Rogowski, M.; Chmielewski, A.G. The State of Critical and Strategic Metals Recovery and the Role of Nuclear Techniques in the Separation Technologies Development: Review. Separations 2023, 10, 112. [Google Scholar] [CrossRef]
- Ayres, R.U.; Peiró, L.T. Material efficiency: Rare and critical metals. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20110563. [Google Scholar] [CrossRef]
- Purwadi, I.; van der Werff HM, A.; Lievens, C. Targeting rare earth element bearing mine tailings on Bangka Island, Indonesia, with Sentinel-2 MSI. Int. J. Appl. Earth Obs. Geoinf. 2020, 88, 102055. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Geochemical fractions of rare earth elements in soil around a mine tailing in Baotou, China. Sci. Rep. 2015, 5, 12483. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.A. Rare earth metals: A strategic concern. Miner. Econ. 2014, 27, 21–31. [Google Scholar] [CrossRef]
- Fleming, P.; Orrego, P.; Pinilla, F. Recovery of Rare Earth Elements Present in Mining Tails, by Leaching with Nitric and Hydrochloric Solutions. World J. Nucl. Sci. Technol. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Government of the Republic of Kenya. Mining and Mineral Policy. 2016. Available online: https://www.idlo.int/sites/default/files/pdfs/highlights/Kenya%20Mining%20Policy%20Popular%20Version-LowRes.pdf (accessed on 17 August 2023).
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Mwaura, F. An audit of environmental impact assessments for mining projects in Kenya. J. S. Afr. Inst. Min. Metall. 2019, 119, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Opongo, E. Knowledge and Policy Gaps in Extractive Industries in Kenya. SSRN Electron. J. 2017, 12, 1–138. [Google Scholar] [CrossRef]
- Barmao, K.J.; Cherutoi, J.K.; Mitei, C.Y.; Were, M.L.L.; Kiprop, A.; Achieng’, O.G. Assessment of Fluoride and selected heavy metals in food chain around Fluorspar mining Plant, Kenya. Greener J. Environ. Manag. Public Saf. 2019, 8, 15–24. [Google Scholar] [CrossRef]
- Kipsang, R.B. Economic and Job Creation Economic and Job Creation Potential of Artisanal and Small-Scale Mining in Taita Taveta County, Kenya. Natural Resources Management for Sustainable Development in Kenya Extractive Industry, UNDP. 2014. Available online: https://www.jkuat.ac.ke/departments/mining/wp-content/uploads/2017/10/Small-Scale-Mining-n-Taita-Taveta-County-Kenya.pdf (accessed on 12 October 2023).
- Kaniu, I.; Iain, G.D.; Hudson, K.A. Radiological Mapping of the Alkaline Intrusive Complex of Jombo, South Coastal Kenya by In-Situ Gamma-Ray Spectrometry. 2016. Available online: https://meetingorganizer.copernicus.org/EGU2016/EGU2016-17917-1.pdf (accessed on 21 August 2023).
- African Natural Resources Centre. Rare Earth Elements (REE). Value Chain Analysis for Mineral Based Industrialization in Africa. 2021. Available online: https://www.afdb.org/fr/documents/rare-earth-elements-ree-value-chain-analysis-mineral-based-industrialization-africa (accessed on 21 August 2023).
- Odumo, B.O.; Nanos, N.; Carbonell, G.; Torrijos, M.; Patel, J.P.; Rodríguez Martín, J.A. Artisanal gold-mining in a rural environment: Land degradation in Kenya. Land Degrad. Dev. 2018, 29, 3285–3293. [Google Scholar] [CrossRef]
- Ogola, J.S.; Mitullah, W.V.; Omulo, M.A. Impact of Gold Mining on the Environment and Human health: A Case Study in the Migori Gold Belt, Kenya. Environ. Geochem. Health 2002, 24, 141–158. [Google Scholar] [CrossRef]
- Government of Kenya. Kenya Mining Investment Handbook. 2016. Available online: https://www.tralac.org/documents/resources/by-country/kenya/1928-kenya-mining-investment-handbook-2016/file.html (accessed on 21 August 2023).
- Bett, A.K.; Maranga, S.M. Considerations for Beneficiation of Low Grade Iron Ore for Steel Making in Kenya. In Proceedings of the Sustainable Research and Innovation Conference 2014, Nairobi, Kenya, 3–4 May 2012; pp. 263–267. Available online: https://sri.jkuat.ac.ke/jkuatsri/index.php/sri/article/view/481 (accessed on 12 October 2023).
- Bourdon, E.; Kalt, A.; Meisel, T.; Kaeser, B.; Bourdon, E.; Kalt, A.; Meisel, T.; Kaeser, B. Platinum-group element abundances in xenoliths from Marsabit Volcanic field (Kenya Rift). AGUFM 2006, 2006, V31D-0609. Available online: https://ui.adsabs.harvard.edu/abs/2006AGUFM.V31D0609B/abstract (accessed on 24 August 2023).
- Henderson, P.; Pickford, M.; Williams, C.T. A geochemical study of rocks and spring waters at Kanam and Kanjera, Kenya, and the implications concerning element mobility and uptake. J. Afr. Earth Sci. 1987, 6, 221–227. [Google Scholar] [CrossRef]
- Compaore, W.F.; Dumoulin, A.; Rousseau DP, L. Gold Mine Impact on Soil Quality, Youga, Southern Burkina Faso, West Africa. Water Air Soil Pollut. 2019, 230, 207. [Google Scholar] [CrossRef]
- Ceniceros-Gómez, A.E.; Macías-Macías, K.Y.; de la Cruz-Moreno, J.E.; Gutiérrez-Ruiz, M.E.; Martínez-Jardines, L.G. Characterization of mining tailings in México for the possible recovery of strategic elements. J. S. Am. Earth Sci. 2018, 88, 72–79. [Google Scholar] [CrossRef]
- Antwi-Agyei, P.; Hogarh, J.N.; Foli, G. Trace elements contamination of soils around gold mine tailings dams at Obuasi, Ghana. Afr. J. Environ. Sci. Technol. 2009, 3, 353–359. Available online: https://www.ajol.info/index.php/ajest/article/view/56263 (accessed on 12 April 2023).
- Matinde, E.; Simate, G.S.; Ndlovu, S. Mining and metallurgical wastes: A review of recycling and re-use practices. J. S. Afr. Inst. Min. Metall. 2018, 118, 825–844. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Cukrowska, E.M.; Straker, C.J.; Weiersbye, I.M.; Tutu, H.; Rüde, T.R.; Freund, A.; Wolkersdorfer, C. Biosorption of heavy metals from gold-mine wastewaters by Penicillium simplicissimum immobilised on zeolite: Kinetic, equilibrium and thermodynamic study. In Mine Water–Managing the Challenges; Rüde, T., Freund, A., Wolkersdorfer, C., Eds.; International Mine Water Association: Aachen, Germany, 2011; pp. 271–272. [Google Scholar]
- Bridge, G. Contested terrain: Mining and the environment. Annu. Rev. Environ. Resour. 2004, 29, 205–259. [Google Scholar] [CrossRef]
- Oladipo, H.J.; Tajudeen, Y.A.; Taiwo, E.O.; Muili, A.O.; Yusuf, R.O.; Jimoh, S.A.; Oladipo, M.K.; Oladunjoye, I.O.; Egbewande, O.M.; Sodiq, Y.I.; et al. Global Environmental Health Impacts of Rare Earth Metals: Insights for Research and Policy Making in Africa. Challenges 2023, 14, 20. [Google Scholar] [CrossRef]
- Ndlovu, S.; Simate, G.S.; Matinde, E. Waste Production and Utilization in the Metal Extraction Industry. In Waste Production and Utilization in the Metal Extraction Industry; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–512. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Wawszczak, D.; Brykała, M. Possibility of uranium and rare metal recovery in the Polish copper mining industry. Hydrometallurgy 2016, 159, 12–18. [Google Scholar] [CrossRef]
- Kiegiel, K.; Gajda, D.; Zakrzewska-Kołtuniewicz, G. Recovery of uranium and other valuable metals from substrates and waste from copper and phosphate industries. Sep. Sci. Technol. 2020, 55, 2099–2107. [Google Scholar] [CrossRef]
- Tunsu, C.; Menard, Y.; Eriksen, D.Ø.; Ekberg, C.; Petranikova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Zhuang, W.Q.; Fitts, J.P.; Ajo-Franklin, C.M.; Maes, S.; Alvarez-Cohen, L.; Hennebel, T. Recovery of critical metals using biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Blengini, G.A.; Mathieux, F.; Mancini, L.; Nyberg, M.; Cavaco Viegas, H.; Salminen, J.; Garbarino, E.; Orveillion, G.; Saveyn, H. Recovery of Critical and Other Raw Materials from Mining Waste and Landfills: State of Play on Existing Practices; EU Publications: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Ngure, V.; Kinuthia, G. Health risk implications of lead, cadmium, zinc, and nickel for consumers of food items in Migori Gold mines, Kenya. J. Geochem. Explor. 2020, 209, 106430. [Google Scholar] [CrossRef]
- Belardi, G.; Piga, L.; Quaresima, S.; Shehu, N. Application of physical separation methods for the upgrading of titanium dioxide contained in a fine waste. Int. J. Miner. Process. 1998, 53, 145–156. [Google Scholar] [CrossRef]
- Kyalo, M.N.; Munyerere, I.F.; Rop, B.; Maranga, S.M. Scouring abandoned mines in search for elusive metal (gold) in Kakamega’s Rosterman area-A case study in Kenya. In Proceedings of the Sustainable Research and Innovation Conference 2015, Nairobi, Kenya, 6–8 May 2015; pp. 362–366. [Google Scholar]
- Richardson, J.F.; Harker, J.H.; Backhurst, J.R. Coulson and Richardson’s Chemical Engineering, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2002; Volume 2. [Google Scholar]
- Bertuol, D.A.; Amado, F.R.; Cruz, E.D.; Tanabe, E.H. Metal recovery using supercritical carbon dioxide. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Supercritical Carbon Dioxide as Green Solvent; Elsevier: Amsterdam, The Netherlands, 2020; pp. 85–103. [Google Scholar] [CrossRef]
- Kumar, P.A.; Vengatasalam, R. Mineral Beneficiation by Heap Leaching Technique in Mining. Procedia Earth Planet. Sci. 2015, 11, 140–148. [Google Scholar] [CrossRef]
- Thenepalli, T.; Chilakala, R.; Habte, L.; Tuan, L.Q.; Kim, C.S. A brief note on the heap leaching technologies for the recovery of valuable metals. Sustainability 2019, 11, 3347. [Google Scholar] [CrossRef]
- Lommelen, R.; Vander Hoogerstraete, T.; Onghena, B.; Billard, I.; Binnemans, K. Model for Metal Extraction from Chloride Media with Basic Extractants: A Coordination Chemistry Approach. Inorg. Chem. 2019, 58, 12289–12301. [Google Scholar] [CrossRef] [PubMed]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Gajda, B.; Mariusz, B.B. The Effect of Tributyl Phosphate on the Extraction of Nickel(II) And Cobalt(II) ions with Di(2-Ethylhexyl)Phosphoric Acid. Physicochem. Probl. Miner. Process. 2007, 41, 145–152. [Google Scholar]
- Huang, Y.; Guo, H.; Zhang, C.; Liu, B.; Wang, L.; Peng, W.; Cao, Y.; Song, X.; Zhu, X. A novel method for the separation of zinc and cobalt from hazardous zinc–cobalt slag via an alkaline glycine solution. Sep. Purif. Technol. 2021, 273, 119009. [Google Scholar] [CrossRef]
- Choubey, P.K.; Dinkar, O.S.; Panda, R.; Kumari, A.; Jha, M.K.; Pathak, D.D. Selective extraction and separation of Li, Co and Mn from leach liquor of discarded lithium ion batteries (LIBs). Waste Manag. 2021, 121, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Lee, M.S.; Wang, L.Y.; Lee, M.S. Separation of Co(II) and Ni(II) from chloride leach solution of nickel laterite ore by solvent extraction with Cyanex 301. IJMP 2017, 166, 45–52. [Google Scholar] [CrossRef]
- Niobium, Tantalum, Vanadium and Zirconium Ore in Kenya. The Observatory of Economic Complexity. 2023. Available online: https://oec.world/en/profile/bilateral-product/niobium-tantalum-vanadium-and-zirconium-ore/reporter/ken (accessed on 12 October 2023).
- Peng, H.; Zhang, C.; Hao, Z.; Jiang, S.; Guo, J.; Huang, H.; Li, B. Vanadium recovery by glycine precipitation. Environ. Chem. Lett. 2022, 20, 1569–1575. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, Y.; Li, H.; Zhou, Y. Selective separation and extraction of vanadium(IV) and manganese(II) from co-leaching solution of roasted stone coal and pyrolusite via solvent extraction. Ind. Eng. Chem. Res. 2013, 52, 13768–13776. [Google Scholar] [CrossRef]
- Pospiech, B. Synergistic Solvent Extraction and Transport of Zn(II) and Cu(II) across Polymer Inclusion Membranes with a Mixture of TOPO and Aliquat 336. Sep. Sci. Technol. 2014, 49, 1706–1712. [Google Scholar] [CrossRef]
- Rogowski, M.; Smolinski, T.; Pyszynska, M.; Brykala, M.; Chmielewski, A.G. Studies on hydrometallurgical processes using nuclear techniques to be applied in copper industry. II. Application of radiotracers in copper leaching from flotation tailings. Nukleonika 2018, 63, 131–137. [Google Scholar] [CrossRef]
- Smolinski, T.; Wawszczak, D.; Deptula, A.; Lada, W.; Olczak, T.; Rogowski, M.; Pyszynska, M.; Chmielewski, A.G. Solvent extraction of Cu, Mo, V, and U from leach solutions of copper ore and flotation tailings. J. Radioanal. Nucl. Chem. 2017, 314, 69–75. [Google Scholar] [CrossRef]
- Parent, M.; Cornelis, R.; Dams, R. Investigation of extraction and back-extraction behaviour of platinum (IV) with rubeanic acid in tributyl phosphate, with tributyl phosphate and with thenoyltrifuoroacetone in n-butyl alcohol-acetophenone by means of platinum-191 radiotracer for platinum-enrichment purposes. Anal. Chim. Acta 1993, 281, 153–160. [Google Scholar] [CrossRef]
- Abbass, M.K.; Jalhoom, M.G.; Kadhim, A.M. Extraction of Rare Earth Elements from Iraqi Phosphate Ore by Using of Tributyl Phosphate. Eng. Technol. J. 2020, 38, 240–245. [Google Scholar] [CrossRef]
- Hidayah, N.N.; Abidin, S.Z. The evolution of mineral processing in extraction of rare earth elements using solid-liquid extraction over liquid-liquid extraction: A review. Miner. Eng. 2017, 112, 103–113. [Google Scholar] [CrossRef]
- Fedorova, M.I.; Levina, A.V. Application of ionic liquid based on Aliquat 336 and D2EHPA in the extraction of transition metals. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1212, 012021. [Google Scholar] [CrossRef]
- Shen, L.; Chen, J.; Chen, L.; Liu, C.; Zhang, D.; Zhang, Y.; Su, W.; Deng, Y. Extraction of mid-heavy rare earth metal ions from sulphuric acid media by ionic liquid [A336][P507]. Hydrometallurgy 2016, 161, 152–159. [Google Scholar] [CrossRef]
- Gorzin, H.; Ghaemi, A.; Hemmati, A.; Maleki, A. Studies on effective interaction parameters in extraction of Pr and Nd using Aliquat 336 from NdFeB magnet-leaching solution: Multiple response optimizations by desirability function. J. Mol. Liq. 2021, 324, 115123. [Google Scholar] [CrossRef]
- Ferdowsi, A.; Yoozbashizadeh, H. Solvent Extraction of Rare Earth Elements from a Nitric Acid Leach Solution of Apatite by Mixtures of Tributyl Phosphate and Di-(2-ethylhexyl) Phosphoric Acid. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2017, 48, 3380–3387. [Google Scholar] [CrossRef]
- Jürjo, S.; Siinor, L.; Siimenson, C.; Paiste, P.; Lust, E. Two-Step Solvent Extraction of Radioactive Elements and Rare Earths from Estonian Phosphorite Ore Using Nitrated Aliquat 336 and Bis(2-ethylhexyl) Phosphate. Minerals 2021, 11, 388. [Google Scholar] [CrossRef]
- El-Nadi, Y.A. Solvent Extraction and Its Applications on Ore Processing and Recovery of Metals: Classical Approach. Sep. Purif. Rev. 2016, 46, 195–215. [Google Scholar] [CrossRef]
- Blais, J.F.; Djedidi, Z.; Cheikh, R.B.; Tyagi, R.D.; Mercier, G. Metals precipitation from effluents: Review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 135–149. [Google Scholar] [CrossRef]
- Nie, Z.R.; Ma, L.W.; Xi, X.L. “Complexation-precipitation” metal separation method system and its application in secondary resources. Rare Met. 2014, 33, 369–378. [Google Scholar] [CrossRef]
- Kononova, O.N.; Duba, E.V.; Shnaider, N.I.; Pozdnyakov, I.A. Ion exchange extraction of platinum (IV) and palladium (II) from hydrochloric acid solutions. Russ. J. Appl. Chem. 2017, 90, 1239–1245. [Google Scholar] [CrossRef]
- Cummins, P.M.; Dowling, O.; O’Connor, B.F. Ion-exchange chromatography: Basic principles and application to the partial purification of soluble mammalian prolyl oligopeptidase. Methods Mol. Biol. 2011, 681, 215–228. [Google Scholar] [CrossRef]
- Rodriguez-Freire, L.; Gonzalez-Estrella, J.; Li, G. Technologies for fractionation of wastewater and resource recovery. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 329–354. [Google Scholar] [CrossRef]
- Zewail, T.M.; Yousef, N.S. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex. Eng. J. 2015, 54, 83–90. [Google Scholar] [CrossRef]
- Walkowiak, W. Mechanism of Selective Ion Flotation. 1. Selective Flotation of Transition Metal Cations. Sep. Sci. Technol. 1991, 26, 559–568. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Önal, G.; Bulut, G.; Gül, A.; Kangal, O.; Perek, K.T.; Arslan, F. Flotation of Aladağ oxide lead–zinc ores. Miner. Eng. 2005, 18, 279–282. [Google Scholar] [CrossRef]
- Crundwell, F.K.; du Preez, N.B.; Knights BD, H. Production of cobalt from copper-cobalt ores on the African Copperbelt—An overview. Miner. Eng. 2020, 156, 106450. [Google Scholar] [CrossRef]
- Kar, B.; Sahoo, H.; Rath, S.S.; Das, B. Investigations on different starches as depressants for iron ore flotation. Miner. Eng. 2013, 49, 1–6. [Google Scholar] [CrossRef]
- Lutandula, M.S.; Maloba, B. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Zuo, Z.; Feng, Y.; Dong, X.; Luo, S.; Ren, D.; Wang, W.; Wu, Y.; Yu, Q.; Lin, H.; Lin, X. Advances in recovery of valuable metals and waste heat from copper slag. Fuel Process. Technol. 2022, 235, 107361. [Google Scholar] [CrossRef]
- Bulut, G.; Perek, K.T.; Gui, A.; Arslan, F.; Onal, G. Recovery of metal values from copper slags by flotation and roasting with pyrite. Min. Metall. Explor. 2007, 14, 13–18. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Forrest, K.; Yan, D.; Dunne, R. Optimisation of gold recovery by selective gold flotation for copper-gold-pyrite ores. Miner. Eng. 2001, 14, 227–241. [Google Scholar] [CrossRef]
- Acarkan, N.; Bulut, G.; Gül, A.; Kangal, O.; Karakaş, F.; Kökkiliç, O.; Önal, G. The effect of collector’s type on gold and silver flotation in a complex ore. Sep. Sci. Technol. 2011, 46, 283–289. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of selective flotation recovery of rare earth oxides from hematite and quartz using hydroxamic acid as a collector. Adv. Powder Technol. 2018, 29, 1886–1899. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Rudolph, M.; Leistner, T.; Gutzmer, J.; Peuker, U.A.; Chelgani, S.C.; Rudolph, M.; Leistner, T.; Gutzmer, J.; Peuker, U.A. A review of rare earth minerals flotation: Monazite and xenotime. IJMST 2015, 25, 877–883. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Johnson, B.; Addai-Mensah, J.; Skinner, W. Recovery of Rare Earth Elements Minerals in Complex Low-Grade Saprolite Ore by Froth Flotation. Minerals 2022, 12, 1138. [Google Scholar] [CrossRef]
- Li, R.; Marion, C.; Espiritu ER, L.; Multani, R.; Sun, X.; Waters, K.E. Investigating the use of an ionic liquid for rare earth mineral flotation. J. Rare Earths 2021, 39, 866–874. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiprono, N.R.; Smoliński, T.; Rogowski, M.; Herdzik-Koniecko, I.; Sudlitz, M.; Chmielewski, A.G. Kenya’s Mineral Landscape: A Review of the Mining Status and Potential Recovery of Strategic and Critical Metals through Hydrometallurgical and Flotation Techniques. Minerals 2024, 14, 21. https://doi.org/10.3390/min14010021
Kiprono NR, Smoliński T, Rogowski M, Herdzik-Koniecko I, Sudlitz M, Chmielewski AG. Kenya’s Mineral Landscape: A Review of the Mining Status and Potential Recovery of Strategic and Critical Metals through Hydrometallurgical and Flotation Techniques. Minerals. 2024; 14(1):21. https://doi.org/10.3390/min14010021
Chicago/Turabian StyleKiprono, Nelson R., Tomasz Smoliński, Marcin Rogowski, Irena Herdzik-Koniecko, Marcin Sudlitz, and Andrzej G. Chmielewski. 2024. "Kenya’s Mineral Landscape: A Review of the Mining Status and Potential Recovery of Strategic and Critical Metals through Hydrometallurgical and Flotation Techniques" Minerals 14, no. 1: 21. https://doi.org/10.3390/min14010021