Controls on Lithium Incorporation and Isotopic Fractionation in Large Benthic Foraminifera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Experiments
2.2. Analytical Methods
2.2.1. Seawater Carbonate Parameters
2.2.2. Seawater Li Isotopes
2.2.3. Foraminifera Li Isotopes
| Treatment |
Experiment 1 (Normal Seawater) |
Experiment 2 1 (×5 Li-Enriched Seawater) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Size (µm) | δ⁷Li (‰) | Li/Ca (µmol/mol) | Size (µm) | δ⁷Li (‰) | Li/Ca (µmol/mol) | ||||
| light/dark | dark | light/dark | dark | light/dark | dark | light/dark | light/dark | light/dark | |
| Control | 569 ± 93 | 568 ± 104 | 26.0 ± 0.8 | 22.8 ± 1.5 | 14.3 ± 3.1 | 14.9 ± 8.3 | 693 ± 75 | 26.3 ± 1.4 | 55.7 ± 1.5 |
| High pH | 485 ± 87 | 445 ± 104 | 24.5 ± 1.4 | 24.4 ± 1.1 | 13.5 ± 2.8 | 16.6 ± 4.3 | 636 ± 88 | 24.1 ± 0.7 | 61.1 ± 2.6 |
| Low pH | 504 ± 110 | 512 ± 82 | 25.4 ± 0.9 | 23.1 ± 1.4 | 15.3 ± 1.8 | 12.0 ± 1.2 | 670 ± 121 | 23.6 ± 0.7 | 66.9 ± 3.3 |
| High DIC | 521 ± 88 | 471 ± 65 | 24.3 ± 1.3 | 24.5 ± 1.1 | 15.8 ± 2.1 | 19.4 ± 2.0 | 650 ± 130 | 25.7 ± 0.6 | 61.9 ± 2.6 |
| Low DIC | 476 ± 78 | 328 ± 61 | 25.0 ± 1.2 | - | 16.6 ± 3.8 | 27.6 ± 5.9 | 573 ± 113 | 24.1 ± 0.7 | 61.9 ± 2.6 |
2.2.4. Foraminifera Li Concentrations
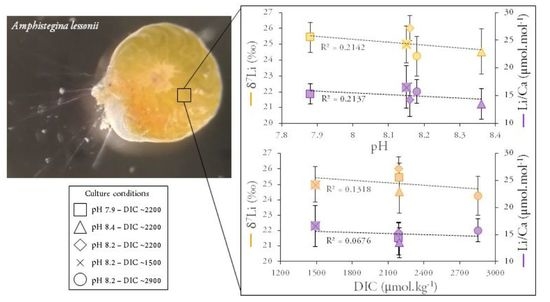
3. Results
4. Discussion
4.1. Comparison with Inorganic Calcite
4.2. Comparison with Biogenic Carbonates
4.2.1. Li/Ca Ratios
4.2.2. Li Isotopic Compositions
4.2.3. Effect of Light Intensity

5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berner, R.A.; Berner, E.K. Silicate Weathering and Climate. In Tectonic Uplift and Climate Change; Ruddiman, W.F., Ed.; Springer: Boston, MA, USA, 1997; pp. 353–365. [Google Scholar] [CrossRef]
- Chan, L.H.; Edmond, J.M.; Thompson, G.; Gillis, K. Lithium isotopic composition of submarine basalts: Implications for the lithium cycle in the oceans. Earth Planet. Sci. Lett. 1992, 108, 151–160. [Google Scholar] [CrossRef]
- Huh, Y.; Chan, L.H.; Zhang, L.; Edmond, J.M. Lithium and its isotopes in major world rivers: Implications for weathering and the oceanic budget. Geochim. Cosmochim. Acta 1998, 62, 2039–2051. [Google Scholar] [CrossRef]
- Pistiner, J.S.; Henderson, G.M. Lithium-isotope fractionation during continental weathering processes. Earth Planet. Sci. Lett. 2003, 214, 327–339. [Google Scholar] [CrossRef]
- Huh, Y.; Chan, L.H.; Edmond, J.M. Lithium isotopes as a probe of weathering processes: Orinoco River. Earth Planet. Sci. Lett. 2001, 194, 189–199. [Google Scholar] [CrossRef]
- Kısakűrek, B.; James, R.; Harris, N. Li and δ7Li in Himalayan rivers: Proxies for silicate weathering? Earth Planet. Sci. Lett. 2005, 237, 387–401. [Google Scholar] [CrossRef]
- Hathorne, E.C.; James, R.H. Temporal record of lithium in seawater: A tracer for silicate weathering? Earth Planet. Sci. Lett. 2006, 246, 393–406. [Google Scholar] [CrossRef]
- Misra, S.; Froelich, P.N. Lithium isotope history of Cenozoic seawater: Changes in silicate weathering and reverse weathering. Science 2012, 335, 818–823. [Google Scholar] [CrossRef] [Green Version]
- Wanner, C.; Sonnenthal, E.L.; Liu, X.M. Seawater δ7Li: A direct proxy for global CO2 consumption by continental silicate weathering? Chem. Geol. 2014, 381, 154–167. [Google Scholar] [CrossRef] [Green Version]
- Pogge von Strandmann, P.A.E.; Dellinger, M.; West, A.J. Lithium Isotopes: A Tracer of Past and Present Silicate Weathering; Cambridge University Press: Cambridge, UK, 2021; 75p. [Google Scholar] [CrossRef]
- Burton, K.W.; Vigier, N. Lithium Isotopes as Tracers in Marine and Terrestrial Environments. In Handbook of Environmental Isotope Geochemistry: Vol I; Baskaran, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 41–59. [Google Scholar] [CrossRef]
- Lécuyer, C. Seawater residence times of some elements of geochemical interest and the salinity of the oceans. Bull Soc. Géol. Fr. 2016, 187, 245–260. [Google Scholar] [CrossRef]
- Jeffcoate, A.B.; Elliott, T.; Thomas, A.; Bouman, C. Precise/ Small Sample Size Determinations of Lithium Isotopic Compositions of Geological Reference Materials and Modern Seawater by MC-ICP-MS. Geostand. Geoanal. Res. 2004, 28, 161–172. [Google Scholar] [CrossRef]
- Millot, R.; Guerrot, C.; Vigier, N. Accurate and high-precision measurement of Lithium isotopes in two reference materials by MC-ICP-MS. Geostand. Geoanal. Res. 2004, 28, 153–159. [Google Scholar] [CrossRef]
- Ullmann, C.V.; Campbell, H.J.; Frei, R.; Hesselbo, S.P.; Pogge von Strandmann, P.A.E.; Korte, C. Partial diagenetic overprint of Late Jurassic belemnites from New Zealand: Implications for the preservation potential of δ7Li values in calcite fossils. Geochim. Cosmochim. Acta 2013, 120, 80–96. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Jenkyns, H.C.; Woodfine, R.G. Lithium isotope evidence for enhanced weathering during Oceanic Anoxic Event 2. Nat. Geosci. 2013, 6, 668–672. [Google Scholar] [CrossRef] [Green Version]
- Pogge von Strandmann, P.A.E.; Desrochers, A.; Murphy, M.J.; Finlay, A.J.; Selby, D.; Lenton, T.M. Global climate stabilisation by chemical weathering during the Hirnantian glaciation. Geochem. Perspect. Lett. 2017, 3, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Washington, K.E.; West, A.J.; Kalderon-Asael, B.; Katchinoff, J.A.R.; Stevenson, E.I.; Planavsky, N.J. Lithium isotope composition of modern and fossilized Cenozoic brachiopods. Geology 2020, 48, 1058–1061. [Google Scholar] [CrossRef]
- Kalderon-Asael, B.; Katchinoff, J.A.R.; Planavsky, N.J.; Hood, A.V.S.; Dellinger, M.; Bellefroid, E.J.; Jones, D.S.; Hofmann, A.; Ossa, F.O.; Macdonald, F.A.; et al. A lithium-isotope perspective on the evolution of carbon and silicon cycles. Nature 2021, 595, 394–398. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Vigier, N.; Meibom, A.; Blamart, D.; Reynaud, S.; Rodolfo-Metalpa, R.; Martin, S.; Gattuso, J.-P. Effect of environmental conditions and skeletal ultrastructure on the Li isotopic composition of scleractinian corals. Earth Planet. Sci. Lett. 2009, 286, 63–70. [Google Scholar] [CrossRef]
- Vigier, N.; Rollion-Bard, C.; Levenson, Y.; Erez, J. Lithium isotopes in foraminifera shells as a novel proxy for the ocean dissolved inorganic carbon (DIC). Comptes Rendus Geosci. 2015, 347, 43–51. [Google Scholar] [CrossRef]
- Roberts, J.; Kaczmarek, K.; Langer, G.; Skinner, L.C.; Bijma, J.; Bradbury, H.; Turchyn, A.V.; Lamy, F.; Misra, S. Lithium isotopic composition of benthic foraminifera: A new proxy for paleo-pH reconstruction. Geochim. Cosmochim. Acta 2018, 236, 336–350. [Google Scholar] [CrossRef]
- Dellinger, M.; West, A.J.; Paris, G.; Adkins, J.F.; Pogge von Strandmann, P.A.E.; Ullmann, C.V.; Eagle, R.A.; Freitas, P.; Bagard, M.-L.; Ries, J.B.; et al. The Li isotope composition of marine biogenic carbonates: Patterns and mechanisms. Geochim. Cosmochim. Acta 2018, 236, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Hillaire-Marcel, C.; de Vernal, A. Proxies in Late Cenozoic Paleoceanography, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; 863p. [Google Scholar]
- Košler, J.; Kučera, M.; Sylvester, P. Precise measurement of Li isotopes in planktonic foraminiferal tests by quadrupole ICPMS. Chem. Geol. 2001, 181, 169–179. [Google Scholar] [CrossRef]
- Hall, J.; Chan, L.H.; McDonough, W.; Turekian, K. Determination of the lithium isotopic composition of planktic foraminifera and its application as a paleo-seawater proxy. Mar. Geol. 2005, 217, 255–265. [Google Scholar] [CrossRef]
- Vigier, N.; Rollion-Bard, C.; Spezzaferri, S.; Brunet, F. In situ measurements of Li isotopes in foraminifera. Geochem. Geophys. Geosystems 2007, 8, Q01003. [Google Scholar] [CrossRef]
- Misra, S.; Froelich, P.N. Measurement of lithium isotope ratios by quadrupole-ICP-MS: Application to seawater and natural carbonates. J. Anal. At. Spectrom. 2009, 24, 1524–1533. [Google Scholar] [CrossRef]
- Marriott, C.S.; Henderson, G.; Belshaw, N.; Tudhope, A.W. Temperature dependence of δ7Li, δ44Ca and Li/Ca during growth of calcium carbonate. Earth Planet. Sci. Lett. 2004, 222, 615–624. [Google Scholar] [CrossRef]
- Marriott, C.S.; Henderson, G.M.; Crompton, R.; Staubwasser, M.; Shaw, S. Effect of mineralogy, salinity, and temperature on Li/Ca and Li isotope composition of calcium carbonate. Chem. Geol. 2004, 212, 5–15. [Google Scholar] [CrossRef]
- Füger, A.; Konrad, F.; Leis, A.; Dietzel, M.; Mavromatis, V. Effect of growth rate and pH on lithium incorporation in calcite. Geochim. Cosmochim. Acta 2019, 248, 14–24. [Google Scholar] [CrossRef]
- Füger, A.; Kuessner, M.; Rollion-Bard, C.; Leis, A.; Magna, T.; Dietzel, M.; Mavromatis, V. Effect of growth rate and pH on Li isotope fractionation during its incorporation in calcite. Geochim. Cosmochim. Acta 2022, 323, 276–290. [Google Scholar] [CrossRef]
- Day, C.C.; Pogge von Strandmann, P.A.E.; Mason, A.J. Lithium isotopes and partition coefficients in inorganic carbonates: Proxy calibration for weathering reconstruction. Geochim. Cosmochim. Acta 2021, 305, 243–262. [Google Scholar] [CrossRef]
- Hallock, P. Symbiont-bearing Foraminifera. In Modern Foraminifera; Sen Gupta, B.K., Ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 123–139. [Google Scholar] [CrossRef]
- BouDagher-Fadel, M.K. Biology and Evolutionary History of Larger Benthic Foraminifera. In Evolution and Geological Significance of Larger Benthic Foraminifera, 2nd ed.; UCL Press: London, UK, 2018; pp. 1–44. [Google Scholar] [CrossRef]
- Narayan, G.R.; Reymond, C.E.; Stuhr, M.; Doo, S.; Schmidt, C.; Mann, T.; Westphal, H. Response of large benthic foraminifera to climate and local changes: Implications for future carbonate production. Sedimentology 2021, 69, 121–161. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Langer, G.; Nehrke, G.; Horn, I.; Misra, S.; Janse, M.; Bijma, J. Boron incorporation in the foraminifer Amphistegina lessonii under a decoupled carbonate chemistry. Biogeosciences 2015, 12, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Pierrot, D.E.L.; Wallace, D.W.R. MS Excel Program Developed for CO2 System Calculations; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy: Oak Ridge, TN, USA, 2006. [Google Scholar] [CrossRef]
- Lueker, T.J.; Dickson, A.G.; Keeling, C.D. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: Validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem. 2000, 70, 105–119. [Google Scholar] [CrossRef]
- Dickson, A.G. Standard potential of the reaction: AgCl(s) + 1/2 H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodyn. 1990, 22, 113–127. [Google Scholar] [CrossRef]
- Uppström, L.R. The boron/chlorinity ratio of deep-sea water from the Pacific Ocean. Deep. Sea Res. Oceanogr. Abstr. 1974, 21, 161–162. [Google Scholar] [CrossRef]
- Orr, J.C.; Epitalon, J.M.; Gattuso, J.P. Comparison of ten packages that compute ocean carbonate chemistry. Biogeosciences 2015, 12, 1483–1510. [Google Scholar] [CrossRef] [Green Version]
- Pogge von Strandmann, P.A.E.; Fraser, W.T.; Hammond, S.J.; Tarbuck, G.; Wood, I.G.; Oelkers, E.H.; Murphy, M.J. Experimental determination of Li isotope behaviour during basalt weathering. Chem. Geol. 2019, 517, 34–43. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Elliott, T.; Marschall, H.R.; Coath, C.; Lai, Y.J.; Jeffcoate, A.B.; Ionov, D.A. Variations of Li and Mg isotope ratios in bulk chondrites and mantle xenoliths. Geochim. Cosmochim. Acta 2011, 75, 5247–5268. [Google Scholar] [CrossRef]
- Raitzsch, M. PULSE (Primary Utility for Laser Ablation Shiny-Assisted Data Evaluation). 2019. Available online: https://raitzsch.shinyapps.io/PULSE_NuAttom_web/. (accessed on 1 January 2023).
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Milner Garcia, S.; Burckel, P.; Angiolini, L.; Jurikova, H.; Tomašových, A.; Henkel, D. Assessing the biomineralization processes in the shell layers of modern brachiopods from oxygen isotopic composition and elemental ratios: Implications for their use as paleoenvironmental proxies. Chem. Geol. 2019, 524, 49–66. [Google Scholar] [CrossRef]
- Auclair, A.C.; Joachimski, M.M.; Lécuyer, C. Deciphering kinetic, metabolic and environmental controls on stable isotope fractionations between seawater and the shell of Terebratalia transversa (Brachiopoda). Chem. Geol. 2003, 202, 59–78. [Google Scholar] [CrossRef]
- Langer, G.; Sadekov, A.; Thoms, S.; Mewes, A.; Nehrke, G.; Greaves, M.; Misra, S.; Bijma, J.; Elderfield, H. Li partitioning in the benthic foraminifera Amphistegina lessonii. Geochem. Geophys. Geosystems 2015, 16, 4275–4279. [Google Scholar] [CrossRef] [Green Version]
- Delaney, M.L.; Bé, W.H.A.; Boyle, E.A. Li, Sr, Mg, and Na in foraminiferal calcite shells from laboratory culture, sediment traps, and sediment cores. Geochim. Cosmochim. Acta 1985, 49, 1327–1341. [Google Scholar] [CrossRef]
- Evans, D.; Erez, J.; Oron, S.; Müller, W. Mg/Ca-temperature and seawater-test chemistry relationships in the shallow-dwelling large benthic foraminifera Operculina ammonoides. Geochim. Cosmochim. Acta 2015, 148, 325–342. [Google Scholar] [CrossRef] [Green Version]
- Hauzer, H.; Evans, D.; Müller, W.; Rosenthal, Y.; Erez, J. Salinity effect on trace element incorporation in cultured chells of the large benthic foraminifer Operculina ammonoides. Paleoceanogr. Paleoclimatol. 2021, 36, e2021PA004218. [Google Scholar] [CrossRef]
- Delaney, M.L.; Popp, B.N.; Lepzelter, C.G.; Anderson, T.F. Lithium-to-calcium ratios in Modern, Cenozoic, and Paleozoic articulate brachiopod shells. Paleoceanography 1989, 4, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Quigg, A. Micronutrients. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 211–231. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Burton, K.W.; Opfergelt, S.; Eiríksdóttir, E.S.; Murphy, M.J.; Einarsson, A.; Gislason, S.R. The effect of hydrothermal spring weathering processes and primary productivity on lithium isotopes: Lake Myvatn, Iceland. Chem. Geol. 2016, 445, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Köhler-Rink, S.; Kühl, M. Microsensor studies of photosynthesis and respiration in larger symbiotic foraminifera. I The physico-chemical microenvironment of Marginopora vertebralis, Amphistegina lobifera and Amphisorus hemprichii. Mar. Biol. 2000, 137, 473–486. [Google Scholar] [CrossRef]
- Glas, M.S.; Fabricius, K.E.; de Beer, D.; Uthicke, S. The O2, pH and Ca2+ microenvironment of benthic foraminifera in a high CO2 world. PLoS ONE 2012, 7, e50010. [Google Scholar] [CrossRef] [Green Version]
- Charrieau, L.M.; Nagai, Y.; Kimoto, K.; Dissard, D.; Below, B.; Fujita, K.; Toyofuku, T. The coral reef-dwelling Peneroplis spp. shows calcification recovery to ocean acidification conditions. Sci. Rep. 2022, 12, 63–73. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Erez, J.; Revsbech, P.; Cohen, Y. Symbiotic photosynthesis in a planktonic foraminiferan, Globigerinoides sacculifer (Brady), studied with microelectrodes1. Limnol. Oceanogr. 1985, 30, 1253–1267. [Google Scholar] [CrossRef]
- de Nooijer, L.J.; Toyofuku, T.; Oguri, K.; Nomaki, H.; Kitazato, H. Intracellular pH distribution in foraminifera determined by the fluorescent probe HPTS. Limnol. Oceanogr. Methods 2008, 6, 610–618. [Google Scholar] [CrossRef]
- de Nooijer, L.J.; Toyofuku, T.; Kitazato, H. Foraminifera promote calcification by elevating their intracellular pH. Proc. Natl. Acad. Sci. USA 2009, 106, 15374–15378. [Google Scholar] [CrossRef] [Green Version]
- Rollion-Bard, C.; Erez, J. Intra-shell boron isotope ratios in the symbiont-bearing benthic foraminifera Amphistegina lobifera: Implications for δ11B vital effects and paleo-pH reconstructions. Geochim. Cosmochim. Acta 2010, 74, 1530–1536. [Google Scholar] [CrossRef]
- Gaspers, N.; Magna, T.; Jurikova, H.; Henkel, D.; Eisenhauer, A.; Azmy, K.; Tomašových, A. Lithium elemental and isotope systematics of modern and cultured brachiopods: Implications for seawater evolution. Chem. Geol. 2021, 586, 120566. [Google Scholar] [CrossRef]




| Treatment |
Experiment 1 (Normal Seawater) |
Experiment 2 (×5 Li-Enriched Seawater) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alkalinity at 25 °C (μeq kg−1) | pH (pH NBS scale) | DIC (μmol·kg−1) | pCO2 (μatm) | Ωcal | Alkalinity at 25 °C (μeq kg−1) | pH (pH NBS scale) | DIC (μmol·kg−1) | pCO2 (μatm) | Ωcal | |
| Control | 2501 ± 9 | 8.16 ± 0.01 | 2188 ± 10 | 471 | 5.33 | 2468 ± 7 | 8.10 ± 0.01 | 2189 ± 18 | 549 | 4.67 |
| High pH | 2657 ± 10 | 8.36 ± 0.01 | 2194 ± 20 | 282 | 8.07 | 2607 ± 17 | 8.22 ± 0.02 | 2235 ± 27 | 415 | 6.14 |
| Low pH | 2368 ± 14 | 7.88 ± 0.03 | 2191 ± 10 | 945 | 2.96 | 2356 ± 8 | 7.90 ± 0.01 | 2167 ± 20 | 889 | 3.02 |
| High DIC | 3248 ± 8 | 8.18 ± 0.01 | 2854 ± 12 | 584 | 7.26 | 3009 ± 8 | 8.10 ± 0.01 | 2580 ± 42 | 672 | 5.72 |
| Low DIC | 1575 ± 11 | 8.15 ± 0.01 | 1489 ± 83 | 299 | 3.26 | 1658 ± 13 | 8.05 ± 0.03 | 1397 ± 42 | 415 | 2.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charrieau, L.M.; Rollion-Bard, C.; Terbrueggen, A.; Wilson, D.J.; Pogge von Strandmann, P.A.E.; Misra, S.; Bijma, J. Controls on Lithium Incorporation and Isotopic Fractionation in Large Benthic Foraminifera. Minerals 2023, 13, 127. https://doi.org/10.3390/min13010127
Charrieau LM, Rollion-Bard C, Terbrueggen A, Wilson DJ, Pogge von Strandmann PAE, Misra S, Bijma J. Controls on Lithium Incorporation and Isotopic Fractionation in Large Benthic Foraminifera. Minerals. 2023; 13(1):127. https://doi.org/10.3390/min13010127
Chicago/Turabian StyleCharrieau, Laurie M., Claire Rollion-Bard, Anja Terbrueggen, David J. Wilson, Philip A. E. Pogge von Strandmann, Sambuddha Misra, and Jelle Bijma. 2023. "Controls on Lithium Incorporation and Isotopic Fractionation in Large Benthic Foraminifera" Minerals 13, no. 1: 127. https://doi.org/10.3390/min13010127