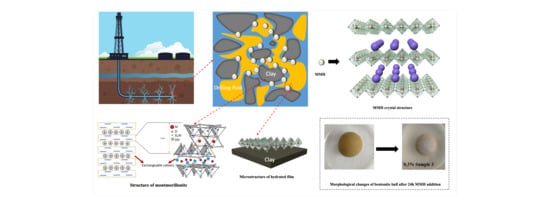
Preparation and Swelling Inhibition of Mixed Metal Hydroxide to Bentonite Clay
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of MMH
2.3. Inhibitory Evaluation
2.4. Drilling Fluid Evaluation
2.5. Particle Size Experiment
2.6. X-ray Diffraction
2.7. TGA and SEM
3. Results and Discussion
3.1. X-ray Diffraction Analysis
3.2. SEM Experiment
3.3. Linear Swelling Experiment
3.4. Mud Ball Experiment
3.5. Particle Size Measurement
3.6. X-ray Diffraction of Modified Samples
3.7. TGA Measurement
3.8. Performance in Drilling Fluid
3.9. Mechanism Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akkouche, A.; Benmounah, A.; Gueciouer, A.; Chalah, K. Valorization of mixed metal hydroxide on Algerian Na-Bentonite suspensions: Application to water-based drilling fluid. Egypt. J. Pet. 2020, 29, 127–131. [Google Scholar] [CrossRef]
- Rouahna, N.; Ouakouak, A.; Barkat, D.; Srasra, E. Zn-Al layered double hydroxide: Synthesis, characterization and application for orthophosphates ions adsorption in aqueous medium. Mater. Res. Express 2020, 7, 045502. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Graffin, P.; Tichit, D. Synthesis and characterization of sol–gel Mg/Al and Ni/Al layered double hydroxides and comparison with co-precipitated samples. Microporous Mesoporous Mater. 2000, 39, 229–247. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, Y.; Zhang, X.; Yuan, P.; He, H.P. Synthesis and characterization of layered double hydroxides with a high aspect ratio. J. Solid State Chemistr. 2006, 179, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Zhang, G.C.; Hou, W.G.; Sun, D.J.; Wang, G.T. Study on the Preparation and Structure of Positive Sol Composed of Mixed Metal Hydroxide. Colloid Polym. Sci. 1996, 274, 860–865. [Google Scholar] [CrossRef]
- Burba, J.L.; Holman, W.E.; Crabb, C.R. Laboratory and Field Evalutions of Novel Inorganic Drilling Fluid Additive. In Proceedings of the IADC/SPE Drilling Conference, Dallas, TX, USA, 28 February 1988; Volume 6, pp. 586–978. [Google Scholar] [CrossRef]
- Chen, S.Y.; Shi, Y.P.; Yang, X.Y.; Xie, K.Z.; Cai, J.H. Design and evaluation of a surfactant–mixed metal hydroxide-based drilling fluid for maintaining wellbore stability in coal measure strata. Energies 2019, 12, 1862. [Google Scholar] [CrossRef] [Green Version]
- Du, W.C.; Wang, X.Y.; Bi, T.F.; Liu, M.; Zhang, J.; Chen, G. Synthesis and inhibitive mechanism of a novel clay hydration inhibitor for water-based drilling fluids. Mater. Sci.-Medzg. 2021, 27, 128–142. [Google Scholar] [CrossRef]
- Fraser, L.J.; Reid, P.I.; Williamson, L.D.; Enriquez, F.P. Formation-damaging characteristics of mixed metal hydroxide drill-in fluids and a comparison with polymer-base fluids. SPE Drill. Completion 1999, 14, 178–184. [Google Scholar] [CrossRef]
- Kopka, H.; Beneke, K.; Lagaly, G. Anionic surfactants between double metal hydroxide layers. J. Colloid Interface Sci. 1988, 123, 427–436. [Google Scholar] [CrossRef]
- Du, W.C.; Slaný, M.; Wang, X.Y.; Chen, G.; Zhang, J. The inhibition property and mechanism of a novel low molecular weight zwitterionic copolymer for improving wellbore stability. Polymers 2020, 12, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Oyama, S.T. Review of the synthesis of layered double hydroxides: A thermodynamic approach. Quim. Nova 2016, 27, 601–614. [Google Scholar] [CrossRef]
- Xian, S.R.; Chen, S.J.; Du, W.C.; Song, Z.F.; Chen, G. Preparation and evaluation of ammonium-adipic solutions as inhibitors of shale rock Swelling. Minerals 2021, 11, 1013. [Google Scholar] [CrossRef]
- Zhang, R.J.; Gao, L.; Du, W.C.; Hu, W.M.; Duan, W.G.; Gu, X.F.; Zhang, J.; Chen, G. The Application of Ferric Chloride-Lignin Sulfonate as Shale Inhibitor in Water-Based Drilling Fluid. Molecules 2019, 24, 4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Yan, J.; Li, L.L.; Zhang, J.; Gu, X.F.; Song, H. Preparation and performance of amine-tartaric salt as potential clay swelling inhibitor. Appl. Clay Sci. 2017, 138, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Baruah, B.; Mishra, M.; Bhattacharjee, C.R.; Nihalani, M.C.; Mishra, S.K.; Baruah, S.D.; Phukan, P.; Goswamee, R.J. The effect of particle size of clay on the viscosity build up property of mixed metal hydroxides (MMH) in the low solid-drilling mud compositions. Appl. Clay Sci. 2013, 80, 169–175. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.B.; Kwon, S.; Salmeron, M. Internal and external atomic steps in graphite exhibit dramatically different physical and chemical properties. ACS Nano 2015, 9, 3814–3819. [Google Scholar] [CrossRef]
- Reichle, W.T.; Kang, S.Y.; Everhardt, D.S. The nature of the thermal decomposition of a catalytically active anionic clay mineral. Cheminform 1986, 101, 352–359. [Google Scholar] [CrossRef]
- Satoshi, M.; Hisashi, S.; Yasuji, M.; Masahiko, M. Interfacial effect between graphite and iron substrate on basal plane orientation and lubricity of graphite. Tribol. Int. 2020, 151, 341–346. [Google Scholar] [CrossRef]
- Liu, Q.N.; Bai, Y.; Dong, S.B.; Li, J.L.; Song, Z.F.; Zhang, J.; Chen, G. Preparation and the foaming activity study of hydroxymethyl cetyltrimethyl ammonium chloride. Tenside Surfactants Deterg. 2021, 58, 153–160. [Google Scholar] [CrossRef]
- Jing, Y.T.; Zhang, J.; Du, W.C.; Hu, W.M.; Xie, J.; Qu, C.T.; Chen, G. Preparation and evaluation of ammonium-succinic salts as shale swelling inhibitor and its application in water-based drilling fluid. Russ. J. Phys. Chem. B 2021, 15, 102–108. [Google Scholar] [CrossRef]
- Ni, W.J.; Wang, W.L.; Wang, Q.C.; Du, W.C.; Chen, G. Modification and application of waste shaddock peel as a green additive for water-based drilling fluid. J. Biobased Mater. Bioenergy 2021, 15, 380–384. [Google Scholar] [CrossRef]
- Liu, X.X.; Gao, L.; Wang, Q.C.; Du, W.C.; Gu, X.F.; Chen, G.; Zhang, J. Evaluation and application of PEG as lubricant in water-based drilling fluid for horizontal well in Sulige Gas Field. Polym. Int. 2021, 70, 83–89. [Google Scholar] [CrossRef]
- Du, W.C.; Wang, X.Y.; Liu, M.; Bi, T.F.; Song, S.X.; Zhang, J.; Chen, G. Synthesis and performance of AM/SSS/THDAB as clay hydration dispersion inhibitor. Polímeros 2019, 29, e2019053. [Google Scholar] [CrossRef]
- Segad, M.; Jonsson, B.; Akesson, T.; Cabane, B. Ca/Na montmorillonite: Structure, forces and swelling properties. Langmuir 2010, 26, 5782–5790. [Google Scholar] [CrossRef]












| Clay | Average Particle Size/μm | Median Particle Size/μm |
|---|---|---|
| Original bentonite | 34.62 | 30.18 |
| Bentonite + water | 16.92 | 11.33 |
| Bentonite + MMH-1 | 22.74 | 15.16 |
| Bentonite +MMH-2 | 35.75 | 24.54 |
| Bentonite +MMH-3 | 36.86 | 24.59 |
| MMH Dosage (%) | Temperature (°C) | PV (mPa·s) | YP (Pa) | AV (mPa·s) | YP/PV (Pa/mPa·s) | FL (mL, 7.5 min) |
|---|---|---|---|---|---|---|
| 0.0 | 30 | 3.00 | 0.00 | 3.00 | 0.00 | 16.5 |
| 0.3 | 3.25 | 1.00 | 4.25 | 0.31 | 16.2 | |
| 0.0 | 150 | 3.00 | 0.25 | 3.25 | 0.08 | 18.4 |
| 0.3 | 3.25 | 0.75 | 4.00 | 0.21 | 21.5 | |
| 0.0 | 180 | 3.00 | 0.00 | 3.00 | 0.00 | 20.1 |
| 0.3 | 4.00 | 0.75 | 4.75 | 0.18 | 23.3 | |
| 0.0 | 190 | 3.25 | 0.25 | 3.50 | 0.08 | 22.3 |
| 0.3 | 3.50 | 1.00 | 4.50 | 0.29 | 24.2 | |
| 0.0 | 200 | 3.00 | 0.00 | 3.00 | 0.00 | 24.5 |
| 0.3 | 4.00 | 1.00 | 5.00 | 0.25 | 15.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wang, Q.; Wei, Y.; Wei, W.; Du, W.; Zhang, J.; Chen, G.; Slaný, M. Preparation and Swelling Inhibition of Mixed Metal Hydroxide to Bentonite Clay. Minerals 2022, 12, 459. https://doi.org/10.3390/min12040459
Zhang B, Wang Q, Wei Y, Wei W, Du W, Zhang J, Chen G, Slaný M. Preparation and Swelling Inhibition of Mixed Metal Hydroxide to Bentonite Clay. Minerals. 2022; 12(4):459. https://doi.org/10.3390/min12040459
Chicago/Turabian StyleZhang, Bowen, Qingchen Wang, Yan Wei, Wei Wei, Weichao Du, Jie Zhang, Gang Chen, and Michal Slaný. 2022. "Preparation and Swelling Inhibition of Mixed Metal Hydroxide to Bentonite Clay" Minerals 12, no. 4: 459. https://doi.org/10.3390/min12040459