Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization Methods
2.3. Preparation of Spent Batteries
2.4. Leaching Process
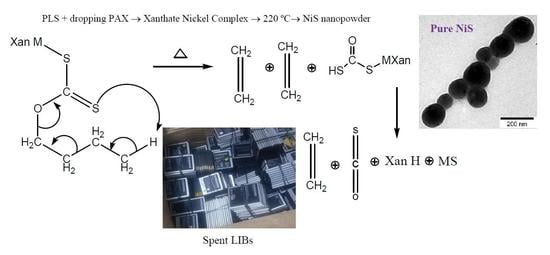
2.5. Fabrication of Nanoparticles
3. Results and Discussions
3.1. X-Ray Powder Diffraction (XRD)
3.2. Thermogravimetry & Differential Thermal Analysis (TGA-DTA)
3.3. Fourier-Transform Infrared (FTIR) Spectroscopy
3.4. Scanning Electron Microscopy (SEM) Energy Dispersive X-Ray Spectrum (EDS)
3.5. Transmission Electron Microscopy (TEM)
3.6. Photon Correlation Spectroscopy (PCS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciez, R.E.; Whitacre, J. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y. An overview of recycling and treatment of spent LiFePO4 batteries in China. Resour. Conserv. Recy. 2017, 127, 233–243. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.-M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Pranolo, Y.; Zhang, W.; Cheng, C. Recovery of metals from spent lithium-ion battery leach solutions with a mixed solvent extractant system. Hydrometallurgy 2010, 102, 37–42. [Google Scholar] [CrossRef]
- Lupi, C.; Pasquali, M. Electrolytic nickel recovery from lithium-ion batteries. Miner. Eng. 2003, 16, 537–542. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of end-of-life lithium ion batteries, Part I: Commercial processes. J. Sustain. Met. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Flett, D.S. Cobalt-nickel separation in hydrometallurgy: A review. Chem. Sustain. Dev. 2004, 12, 81–91. [Google Scholar]
- Armstrong, R.D.; Todd, M.; Atkinson, J.W.; Scott, K. Electroseparation of cobalt and nickel from a simulated wastewater. J. Appl. Electrochem. 1997, 27, 965–969. [Google Scholar] [CrossRef]
- Castillo, S.; Ansart, F.; Laberty-Robert, C.; Portal, J. Advances in the recovering of spent lithium battery compounds. J. Power Source 2002, 112, 247–254. [Google Scholar] [CrossRef]
- Miller, M.J.; Scheithauer, R.A. Method for Separation of Cobalt from Nickel. U.S. Patent 2,842,427, 8 July 1958. [Google Scholar]
- Liu, P.; Xiao, L.; Chen, Y.; Tang, Y.; Wu, J.; Chen, H. Recovering valuable metals from LiNixCoyMn1-x-yO2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching. J. Alloys Compd. 2019, 783, 743–752. [Google Scholar] [CrossRef]
- Chen, X.; Kang, D.; Cao, L.; Li, J.; Zhou, T.; Ma, H. Separation and recovery of valuable metals from spent lithium ion batteries: Simultaneous recovery of Li and Co in a single step. Sep. Purif. Technol. 2019, 210, 690–697. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Qu, L.; Feng, Y.; Li, J.; Liu, J.; Zhang, G.; Xie, W. Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manag. 2019, 88, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Zhou, Z.; Duan, J.; Li, X.; Lin, Y.; Meng, Q.; Pai, K.V.; et al. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J. Environ. Chem. Eng. 2019, 7, 102854. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Chien, S.-K.; Jhang, S.-R.; Lin, Y.-C. Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries. J. Taiwan Inst. Chem. E 2019, 100, 151–159. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Yuan, C.; Ni, S.; Li, L. Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Manag. 2019, 93, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Luo, X.; Lou, X.; Guo, Y.; Su, R.; Guan, J.; Li, Y.; Yuan, H.; Dai, J.; Jiao, Z. Efficient sulfuric acid-Vitamin C leaching system: Towards enhanced extraction of cobalt from spent lithium-ion batteries. J. Mater. Cycles Waste 2019, 21, 942–949. [Google Scholar] [CrossRef]
- Vakylabad, A.B.; Schaffie, M.; Naseri, A.; Ranjbar, M.; Manafi, Z. A procedure for processing of pregnant leach solution (PLS) produced from a chalcopyrite-ore bio-heap: CuO Nano-powder fabrication. Hydrometallurgy 2016, 163, 24–32. [Google Scholar] [CrossRef]
- Bai, Y.; Blake Hawley, W.; Jafta, C.J.; Muralidharan, N.; Polzin, B.J.; Belharouak, I. Sustainable recycling of cathode scraps via Cyrene-based separation. Sustain. Mater. Technol. 2020, 25, e00202. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Li, J.; Essehli, R.; Belharouak, I. Sustainable direct recycling of lithium-ion batteries via solvent recovery of electrode materials. ChemSusChem 2020, 13, 5664–5670. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Walton, R.I. Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 2002, 31, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Man, R.W.; Brown, A.R.; Wolf, M.O. Mechanism of Formation of Palladium Nanoparticles: Lewis Base Assisted, Low-Temperature Preparation of Monodisperse Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 11350–11353. [Google Scholar] [CrossRef]
- Yu, H.; Gibbons, P.C.; Kelton, K.F.; Buhro, W.E. Heterogeneous seeded growth: A potentially general synthesis of monodisperse metallic nanoparticles. J. Am. Chem. Soc. 2001, 123, 9198–9199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wan, Z.; Yang, T.; Zhao, M.; Lv, X.; Wang, H.; Ren, X.; Mei, X. Preparation of Nickel Cobalt Sulfide Hollow Nanocolloids with Enhanced Electrochemical Property for Supercapacitors Application. Sci. Rep. UK 2016, 6, 25151. [Google Scholar] [CrossRef] [Green Version]
- RECHARGE-The-Batteries-Report-2018-April-18.pdf. Available online: https://www.storelio.com/files (accessed on 14 January 2021).
- Chen, X.; Liu, Q.; Bai, T.; Wang, W.; He, F.; Ye, M. Nickel and Cobalt Sulfide-Based Nanostructured Materials for Electrochemical Energy Storage Devices. Chem. Eng. J. 2020, 409, 127237. [Google Scholar] [CrossRef]
- Koohestani, B.; Khodadadi Darban, A.; Mokhtari, P.; Darezereshki, E.; Yilmaz, E.; Yilmaz, E. Influence of Hydrofluoric Acid Leaching and Roasting on Mineralogical Phase Transformation of Pyrite in Sulfidic Mine Tailings. Minerals 2020, 10, 513. [Google Scholar] [CrossRef]
- Akram, R.; Khan, M.D.; Zequine, C.; Zhao, C.; Gupta, R.K.; Akhtar, M.; Akhtar, J.; Malik, M.A.; Revaprasadu, N.; Bhatti, M.H. Cobalt sulfide nanoparticles: Synthesis, water splitting and supercapacitance studies. Mat. Sci. Semicon. Proc. 2020, 109, 104925. [Google Scholar] [CrossRef]
- Buchmaier, C.; Glänzer, M.; Torvisco, A.; Poelt, P.; Wewerka, K.; Kunert, B.; Gatterer, K.; Trimmel, G.; Rath, T. Nickel sulfide thin films and nanocrystals synthesized from nickel xanthate precursors. J. Mater. Sci. 2017, 52, 10898–10914. [Google Scholar] [CrossRef]
- Guan, B.; Li, Y.; Yin, B.; Liu, K.; Wang, D.; Zhang, H.; Cheng, C. Synthesis of hierarchical NiS microflowers for high performance asymmetric supercapacitor. Chem. Eng. J. 2017, 308, 1165–1173. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Lee, Y.-R. Binder-free electro-synthesis of highly ordered nickel oxide nanoparticles and its electrochemical performance. Electrochim. Acta 2018, 283, 1609–1617. [Google Scholar] [CrossRef]
- Sathiyaraj, E.; Thirumaran, S.; Ciattini, S.; Selvanayagm, S. Synthesis and characterization of Ni (II) complexes with functionalized dithiocarbamates: New single source precursors for nickel sulfide and nickel-iron sulfide nanoparticles. Inorg. Chim. Acta 2019, 498, 119162. [Google Scholar] [CrossRef]
- Ravindhranath, K.; Ramamoorty, M. Nickel Based Nano Particles as Adsorbents in Water Purification Methods-A Review. Orient. J. Chem. 2017, 33, 1603. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Tang, G.; Mei, J.; Liu, H. Construct hierarchical electrode with NixCo3-xS4 nanosheet coated on NiCo2O4 nanowire arrays grown on carbon fiber paper for high-performance asymmetric supercapacitors. J. Power Source 2017, 359, 262–269. [Google Scholar] [CrossRef]
- Mani, A.D.; Deepa, M.; Xanthopoulos, N.; Subrahmanyam, C. Novel one pot stoichiometric synthesis of nickel sulfide nanomaterials as counter electrodes for QDSSCs. Mater. Chem. Phys. 2014, 148, 395–402. [Google Scholar] [CrossRef]
- Ren, H.; Wang, J.; Cao, Y.; Luo, W.; Sun, Y. Nickel sulfide nanoparticle anchored reduced graphene oxide with improved lithium storage properties. Mater. Res. Bull. 2021, 133, 111047. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Qi, Y.; Han, Y.; Wang, R.; Fu, J.; Meng, F.; Yi, X.; Huang, J.; Shu, J. Cobalt and lithium leaching from waste lithium ion batteries by glycine. J. Power Source 2021, 482, 228942. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Lai, F.; Wang, H.; Huang, Y.; Zheng, F.; Wang, S.; Zhang, X.; Li, Q. Innovative Electrochemical Strategy to Recovery of Cathode and Efficient Lithium Leaching from Spent Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4767–4776. [Google Scholar] [CrossRef]
- Fan, E.; Yang, J.; Huang, Y.; Lin, J.; Arshad, F.; Wu, F.; Li, L.; Chen, R. Leaching Mechanisms of Recycling Valuable Metals from Spent Lithium-Ion Batteries by a Malonic Acid-Based Leaching System. ACS Appl. Energy Mater. 2020, 3, 8532–8542. [Google Scholar] [CrossRef]
- Refly, S.; Floweri, O.; Mayangsari, T.R.; Sumboja, A.; Santosa, S.P.; Ogi, T.; Iskandar, F. Regeneration of LiNi1/3Co1/3Mn1/3O2 Cathode Active Materials from End-of-Life Lithium-Ion Batteries through Ascorbic Acid Leaching and Oxalic Acid Coprecipitation Processes. ACS Sustain. Chem. Eng. 2020, 8, 16104–16114. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Farina, L.; Zanoni, R.; Altimari, P.; Cojocariu, I.; Rubino, A.; Navarra, M.A.; Panero, S.; Pagnanelli, S. Electrochemical synthesis of nanowire anodes from spent lithium ion batteries. Electrochim. Acta 2019, 319, 481–489. [Google Scholar] [CrossRef]
- Shin, S.-M.; Lee, D.-W.; Wang, J.-P. Fabrication of nickel nanosized powder from linio2 from spent lithium-ion battery. Metals 2018, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Ebin, B. Simple Preparation of Ni and NiO Nanoparticles Using Raffinate Solution Originated from Spent NiMH Battery Recycling. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2554–2563. [Google Scholar] [CrossRef] [Green Version]
- Tipman, N.R. Reactions of Potassium Ethyl Xanthate in Aqueous Solution; University of British Columbia: Vancouver, BC, Canada, 1970. [Google Scholar]
- Zhang, Y.-H.; Wu, L.-M.; Huang, P.-P.; Shen, Q.; Sun, Z.-X. Determination and application of the solubility product of metal xanthate in mineral flotation and heavy metal removal in wastewater treatment. Miner. Eng. 2018, 127, 67–73. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, F.; Song, S.; Tang, H.; Ding, S.; Sun, W.; Lei, S.; Xu, S. Recovering valuable metals from the leaching liquor of blended cathode material of spent lithium-ion battery. J. Environ. Chem. Eng. 2020, 8, 104358. [Google Scholar] [CrossRef]
- Aaltonen, M.; Peng, C.; Wilson, B.P.; Lundström, M. Leaching of metals from spent lithium-ion batteries. Recycling 2017, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y. Extraction—Spectrophotometric determination of manganese (II) with xanthates. Anal. Chim. Acta 1982, 138, 419–424. [Google Scholar] [CrossRef]
- Pilipenko, A.; Ul’ko, N. Analytical Prverties of Xanthates (III). Zh. Analit. Khim 1955, 10, 299–304. [Google Scholar]
- Darezereshki, E.; Bakhtiari, F.; Alizadeh, M.; Vakylabad, B.A.; Ranjbar, M. Direct thermal decomposition synthesis and characterization of hematite (α-Fe2O3) nanoparticles. Mat. Sci. Semicon. Proc. 2012, 15, 91–97. [Google Scholar] [CrossRef]
- Darezereshki, E.; Khodadadi Darban, A.; Abdollahy, M.; Jamshidi-Zanjani, A.; Vakylabad, B.A.; Mohammadnejad, S. The leachability study of iron-oxides from mine tailings in a hybrid of sulfate-chloride lixiviant. J. Environ. Chem. Eng. 2018, 6, 5167–5176. [Google Scholar] [CrossRef]
- Darezereshki, E.; Ranjbar, M.; Bakhtiari, F. One-step synthesis of maghemite (γ-Fe2O3) nano-particles by wet chemical method. J. Alloys Compd. 2010, 502, 257–260. [Google Scholar] [CrossRef]
- Pradhan, N.; Katz, B.; Efrima, S. Synthesis of high-quality metal sulfide nanoparticles from alkyl xanthate single precursors in alkylamine solvents. J. Phys. Chem. B 2003, 107, 13843–13854. [Google Scholar] [CrossRef]
- Efrima, S.; Pradhan, N. Xanthates and related compounds as versatile agents in colloid science. C. R. Chim. 2003, 6, 1035–1045. [Google Scholar] [CrossRef]
- Kristl, M.; Dojer, B.; Gyergyek, S.; Kristl, J. Synthesis of nickel and cobalt sulfide nanoparticles using a low cost sonochemical method. Heliyon 2017, 3, e00273. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Component | Co | Mn | Ni | Li |
|---|---|---|---|---|
| PLS (mg/L) | 3186.03 | 4155 | 443.1 | 793.7 |
| Feed CM (mg/L) | 199,127 | 27.7 | 26,105 | 46,690 |
| hkl | Pos. (°2Th.) | d-spacing (Å) | Crystallite Size (Å) | Microstrain (%) | Crystallite Size Only (Å) | Microstrain Only (%) |
|---|---|---|---|---|---|---|
| 100 | 30.4666 | 2.93168 | 261.8913 | 0.522286 | 157.3711 | 0.931453 |
| 101 | 34.9458 | 2.56549 | 349.4471 | 0.357726 | 205.2646 | 0.624922 |
| 102 | 46.1364 | 1.96592 | 362.6012 | 0.264356 | 212.9156 | 0.461666 |
| 110 | 53.7784 | 1.70320 | 284.0213 | 0.280356 | 170.4897 | 0.499501 |
| 103 | 61.0836 | 1.51584 | 390.6485 | 0.190465 | 228.5735 | 0.331587 |
| 201 | 65.4320 | 1.42523 | 303.3146 | 0.221017 | 181.4979 | 0.392629 |
| 004 | 71.0005 | 1.32649 | 252.9233 | 0.240170 | 153.4484 | 0.432225 |
| 202 | 73.2689 | 1.29092 | 320.6339 | 0.190644 | 191.1957 | 0.33759 |
| Average crystallite size (Å) | 315.68515 | 187.5945625 | ||||
| Composition | Concentration of the Elements (%) |
|---|---|
| Ni | 63.98 |
| S | 34.95 |
| Co | 0.32 |
| Cu | 0.21 |
| Cd | 0.11 |
| Na | 0.02 |
| Li | 0.12 |
| Mn | 0.11 |
| Si | 0.04 |
| Cl | 0.10 |
| Al | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darezereshki, E.; Vakylabad, A.B.; Hassanzadeh, A.; Niedoba, T.; Surowiak, A.; Koohestani, B. Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries. Minerals 2021, 11, 419. https://doi.org/10.3390/min11040419
Darezereshki E, Vakylabad AB, Hassanzadeh A, Niedoba T, Surowiak A, Koohestani B. Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries. Minerals. 2021; 11(4):419. https://doi.org/10.3390/min11040419
Chicago/Turabian StyleDarezereshki, Esmaeel, Ali Behrad Vakylabad, Ahmad Hassanzadeh, Tomasz Niedoba, Agnieszka Surowiak, and Babak Koohestani. 2021. "Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries" Minerals 11, no. 4: 419. https://doi.org/10.3390/min11040419