New Combined Depressant/Collectors System for the Separation of Powellite from Dolomite and the Interaction Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Minerals
2.2. Flotation Tests
2.3. Zeta Potential Measurements
2.4. XPS Detection
2.5. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Microflotation
3.2. Zeta Potential Measurements
3.3. XPS Measurements
3.4. Crystal Chemistry and Interaction Energy Analysis
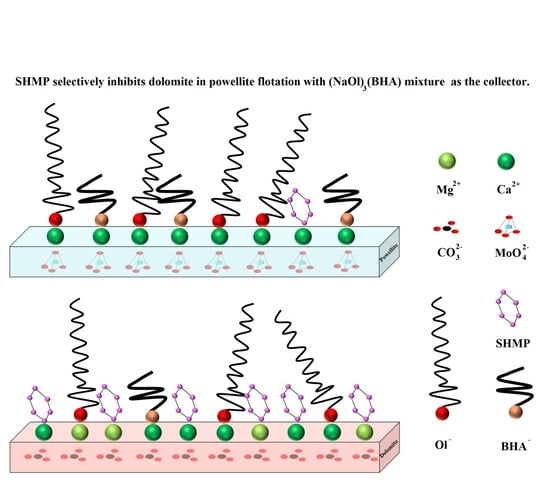
3.5. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.; Wang, L.; Zheng, Y.; Xiao, J. Role of calcium dioleate in the flotation of powellite particles using oleate. Miner. Eng. 2019, 138, 95–100. [Google Scholar] [CrossRef]
- Espiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A 2018, 546, 254–265. [Google Scholar] [CrossRef]
- Zhang, H.; Han, C.; Liu, W.; Hou, D.; Wei, D. The chain length and isomeric effects of monohydric alcohols on the flotation of magnesite and dolomite by sodium oleate. J. Mol. Liq. 2019, 276, 471–479. [Google Scholar] [CrossRef]
- Yang, F.; Sun, W.; Hu, Y.; Long, S. Cationic flotation of scheelite from calcite using quaternary ammonium salts as collector: Adsorption behavior and mechanism. Miner. Eng. 2015, 81, 18–28. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Cao, C.; Yue, T.; Meng, X.; Guo, Y.; Wang, J.; Gao, Z.; et al. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Miner. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R.Q.; Groppo, J.G. Flotation of monazite in the presence of calcite part I: Calcium ion effects on the adsorption of hydroxamic acid. Miner. Eng. 2017, 100, 40–48. [Google Scholar] [CrossRef]
- Espiritu, E.R.L.; Waters, K.E. Flotation studies of monazite and dolomite. Miner. Eng. 2018, 116, 101–106. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Ji, B.; Fan, R.; Liu, R.; Chen, P.; Sun, W.; Hu, Y. Selective flotation of cassiterite from calcite with salicylhydroxamic acid collector and carboxymethyl cellulose depressant. Minerals 2018, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Rai, B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J.; Cases, J.M. Molecular modeling of interactions of alkyl hydroxamates with calcium minerals. J. Colloid Interf. Sci. 2002, 256, 106–113. [Google Scholar]
- Yan, W.; Liu, C.; Ai, G.; Feng, Q.; Zhang, W. Flotation separation of scheelite from calcite using mixed collectors. Int. J. Miner. Process. 2017, 169, 106–110. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, Z.; Khoso, S.A.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Azizi, D.; Larachi, F. Surface interactions and flotation behavior of calcite, dolomite and ankerite with alkyl hydroxamic acid bearing collector and sodium silicate. Colloids Surf. A 2018, 537, 126–138. [Google Scholar] [CrossRef]
- Wang, Z. The adsorption of oleate on powellite and fluorapatite: A joint experimental and theoretical simulation study. Appl. Surf. Sci. 2017, 409, 65–70. [Google Scholar]
- Wang, Z.; Xu, L.; Wang, J.; Wang, L.; Xiao, J. A comparison study of adsorption of benzohydroxamic acid and amyl xanthate on smithsonite with dodecylamine as co-collector. Appl. Surf. Sci. 2017, 426, 1141–1147. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, J.; Sun, N.; Liu, R.; Sun, X.; Cao, X.; Wang, L.; Sun, W. Utilisation of propyl gallate as a novel selective collector for diaspore flotation. Miner. Eng. 2019, 131, 66–72. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Luo, D.; Zeng, Y. Use of ZnSO4 and SDD mixture as sphalerite depressant in copper flotation. Miner. Eng. 2018, 121, 31–38. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. The effect of the ionic strength of process water on the interaction of talc and CMC: Implications of recirculated water on floatable gangue depression. Minerals 2019, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Manono, M.; Corin, K.; Wiese, J. Inorganic electrolytes on the efficacy of a carboxymethyl cellulose as a coagulant for talc: Implications for talc depression in flotation. In Proceedings of the International Mine Water Association Congress, Perm, Russia, 15–19 July 2019. [Google Scholar]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Sun, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.; Sun, W.; Khoso, S.A.; Liu, R.; Zhang, X. Selective flotation of smithsonite from dolomite by using novel mixed collector system. Trans. Nonferr. Met. Soc. China 2019, 29, 1082–1089. [Google Scholar] [CrossRef]
- Abdalla, M.A.M.; Peng, H.; Younus, H.A.; Wu, D.; Abusin, L.; Shao, H. Effect of synthesized mustard soap on the scheelite surface during flotation. Colloid Surf. A 2018, 548, 108–116. [Google Scholar] [CrossRef]
- Mabudi, A.; Noaparast, M.; Gharabaghi, M.; Vasquez, V.R. Polystyrene nanoparticles as a flotation collector: A molecular dynamics study. J. Mol. Liq. 2019, 275, 554–566. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Sun, W. Adsorption of mixed DDA/NaOL surfactants at the air/water interface by molecular dynamics simulations. Chem. Eng. Sci. 2016, 155, 167–174. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Wang, Z.; Han, H.; Yang, Y.; Liu, R.; Hu, Y.; Tang, H.; Sun, W. Self-assembly of mixed dodecylamine–dodecanol molecules at the air/water interface based on large-scale molecular dynamics. J. Mol. Liq. 2019, 276, 867–874. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Liu, R.; Sun, W.; Xiao, J. Comparative studies of flotation and adsorption with cetyl pyridinium chloride on molybdite and fluorapatite. Int. J. Miner. Process. 2015, 143, 112–116. [Google Scholar] [CrossRef]
- Gallios, G.P.; Matis, K.A. Floatability of magnesium carbonates by sodium oleate in the presence of modifiers. Sep. Sci. Technol. 1989, 24, 129–143. [Google Scholar] [CrossRef]
- Matis, K.A.; Gallios, G.P. Anionic flotation of magnesium carbonates by modifiers. Int. J. Miner. Process. 1989, 25, 261–274. [Google Scholar] [CrossRef]
- Abarca, C.; Ali, M.M.; Pelton, R.H. Choosing mineral flotation collectors from large nanoparticle libraries. J. Colloid Interf. Sci. 2018, 516, 423–430. [Google Scholar] [CrossRef]
- Roy, S.; Datta, A.; Rehani, S. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures. Int. J. Miner. Process. 2015, 143, 43–49. [Google Scholar] [CrossRef]
- Taguta, J.; O’Connor, C.T.; McFadzean, B. Investigating the interaction of thiol collectors and collector mixtures with sulphide minerals using thermochemistry and microflotation. Miner. Eng. 2018, 119, 99–104. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Li, Z.; Nan, N.; Zhu, Y.; Li, Y. Froth flotation giant surfactants. Polymer 2019, 162, 58–62. [Google Scholar] [CrossRef]
- Peleka, E.N.; Gallios, G.P.; Matis, K.A. A perspective on flotation: A review. J. Chem. Technol. Biotechnol. 2018, 93, 615–623. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Yu, L.; Xu, Y.; Du, Y.; Zhang, C. Selective depression of titanaugite in the ilmenite flotation with carboxymethyl starch. Appl. Surf. Sci. 2018, 440, 955–962. [Google Scholar] [CrossRef]
- Araújo, A.C.A.; Lima, R.M.F. Influence of cations Ca2+, Mg2+ and Zn2+ on the flotation and surface charge of smithsonite and dolomite with sodium oleate and sodium silicate. Int. J. Miner. Process. 2017, 167, 35–41. [Google Scholar] [CrossRef]
- Multani, R.S.; Williams, H.; Johnson, B.; Li, R.; Waters, K.E. The effect of superstructure on the zeta potential, xanthate adsorption, and flotation response of pyrrhotite. Colloids Surf. A 2018, 551, 108–116. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of calcium minerals reactivity on fatty acids adsorption and flotation. Colloid Surf. A 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Zhang, N.; Ejtemaei, M.; Nguyen, A.V.; Zhou, C. XPS analysis of the surface chemistry of sulfuric acid-treated kaolinite and diaspore minerals with flotation reagents. Miner. Eng. 2019, 136, 1–7. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interf. Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, J. Effects of particle size and particle interactions on scheelite flotation. Trans. Nonferr. Met. Soc. China 2014, 24, 3682–3687. [Google Scholar] [CrossRef]









| Mineral | Elements Content (wt. %) | ||||||
|---|---|---|---|---|---|---|---|
| Mo | CaO | MgO | SiO2 | WO3 | Fe2O3 | Al2O3 | |
| Dolomite | - | 30.01 | 21.22 | 2.16 | - | 0.39 | 0.22 |
| Powellite | 47.04 | 27.34 | - | 1.18 | 0.09 | 0.27 | 0.24 |
| P2O5 | MnO | ZnO | SO3 | BaO | TiO2 | SrO | |
| Dolomite | 4.47 | 4.59 | 0.94 | 1.24 | 0.68 | - | 0.16 |
| Powellite | 3.68 | 2.77 | 0.049 | 0.68 | 2.28 | 1.08 | 1.25 |
| Index | Minerals | Mo | Ca | Mg | C | O | P | N |
|---|---|---|---|---|---|---|---|---|
| Percentage (at. %) | Dolomite | - | 8.42 | 6.79 | 39.30 | 45.49 | - | - |
| Dolomite/SHMP | - | 7.07 | 5.94 | 40.87 | 43.44 | 2.68 | - | |
| Dolomite/BHA | - | 7.04 | 5.90 | 41.96 | 44.28 | - | 0.82 | |
| Dolomite/NaOl | - | 6.97 | 5.85 | 42.24 | 44.94 | - | - | |
| Powellite | 11.38 | 12.46 | - | 20.23 | 55.93 | - | - | |
| Powellite/SHMP | 11.14 | 11.73 | 23.46 | 53.48 | 0.19 | - | ||
| Powellite/BHA | 10.37 | 10.15 | - | 33.37 | 43.88 | - | 2.23 | |
| Powellite/NaOl | 9.85 | 10.24 | 34.67 | 45.24 | - | - | ||
| Binding Energy (eV) | Dolomite | - | 347.05 | 1303.88 | 285.36 | 531.52 | - | - |
| Dolomite/SHMP | - | 346.60 (−0.45) | 1303.45 (−0.43) | 284.89 (−0.47) | 532.38 (0.86) | 130.64 | - | |
| Dolomite/BHA | - | 346.68 (−0.37) | 1303.52 (−0.36) | 285.00 (−0.36) | 532.26 (0.74) | - | 399.46 | |
| Dolomite/NaOl | - | 346.47 (−0.58) | 1302.96 (−0.56) | 284.78 (−0.58) | 532.45 (0.93) | - | - | |
| Powellite | 228.37 | 348.26 | - | 284.92 | 532.05 | - | - | |
| Powellite/SHMP | 228.35 (−0.02) | 348.12 (−0.14) | - | 284.80 (−0.12) | 532.13 (0.08) | 130.72 | - | |
| Powellite/BHA | 228.34 (−0.03) | 347.78 (−0.48) | - | 284.41 (−0.51) | 532.93 (0.88) | - | 399.49 | |
| Powellite/NaOl | 228.35 (−0.02) | 347.74 (−0.52) | - | 284.39 (−0.53) | 532.92 (0.87) | - | - |
| Mineral | Characteristic Parameters | ||
|---|---|---|---|
| O–O/Å | Ca(Mg)–O/Å | D(Ca + Mg)/(μmol·m−2) | |
| Dolomite | 2.222 | 2.381 (2.087) | 8.22 |
| Powellite | 2.848 | 2.465 | 6.58 |
| NaOl | 2.615 | - | - |
| BHA | 3.006 | - | - |
| Mineral | Adsorption Energy (△E, kJ/mol) | |||
|---|---|---|---|---|
| NaOl | BHA | SHMP | NaOl/BHA | |
| Dolomite | −216.87 | −98.15 | −196.53 | –235.76 |
| Powellite | −203.52 | −174.64 | –47.16 | −387.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Ding, W.; Wang, Z.; Peng, Y. New Combined Depressant/Collectors System for the Separation of Powellite from Dolomite and the Interaction Mechanism. Minerals 2020, 10, 291. https://doi.org/10.3390/min10030291
Qian Y, Ding W, Wang Z, Peng Y. New Combined Depressant/Collectors System for the Separation of Powellite from Dolomite and the Interaction Mechanism. Minerals. 2020; 10(3):291. https://doi.org/10.3390/min10030291
Chicago/Turabian StyleQian, Yunlou, Wei Ding, Zhen Wang, and Yang Peng. 2020. "New Combined Depressant/Collectors System for the Separation of Powellite from Dolomite and the Interaction Mechanism" Minerals 10, no. 3: 291. https://doi.org/10.3390/min10030291