Characteristics and Secondary Organic Aerosol Formation of Volatile Organic Compounds from Vehicle and Cooking Emissions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Sampling and Analysis
2.3. Estimation of Ozone and SOA Formation
3. Results
3.1. Emission Characteristics of VOCs from Vehicle and Cooking Emission
3.2. Characteristic Ratio of VOCs Produced by Vehicle and Cooking Emissions
3.3. Comparison of VOCs Produced by Vehicle and Cooking Emissions
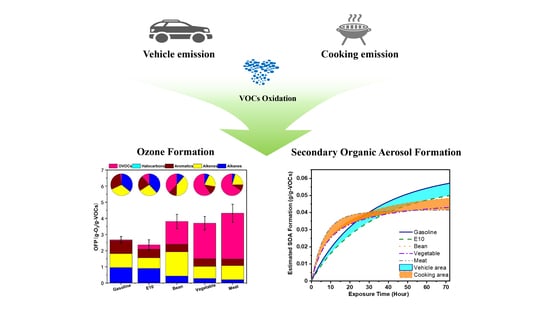
3.4. Ozone and Secondary Organic Aerosol Formation by Vehicle and Cooking Emissions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, W.; Zhou, M.; Cheng, Z.; Yan, N.; Huang, C.; Qiao, L.; Wang, H.; Liu, Y.; Lou, S.; Guo, S. Seasonal variation of aerosol compositions in Shanghai, China: Insights from particle aerosol mass spectrometer observations. Sci. Total Environ. 2021, 771, 144948. [Google Scholar] [CrossRef]
- Li, J.; Gao, W.; Cao, L.; Xiao, Y.; Zhang, Y.; Zhao, S.; Liu, Z.; Liu, Z.; Tang, G.; Ji, D.; et al. Significant changes in autumn and winter aerosol composition and sources in Beijing from 2012 to 2018: Effects of clean air actions. Environ. Pollut. 2021, 268, 115855. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Zamora, M.L.; Peng, J.; Shang, D.; Zheng, J.; Du, Z.; Wu, Z.; Shao, M.; Zeng, L.; et al. Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. USA 2014, 111, 17373–17378. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, Y. Changes in tropospheric ozone levels over the Three Representative Regions of China observed from space by the Tropospheric Emission Spectrometer (TES), 2005–2010. Chin. Sci. Bull. 2012, 57, 2865–2871. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013–2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Peng, J.; Wu, Z.; Zamora, M.L.; Shang, D.; Du, Z.; Zheng, J.; Fang, X.; Tang, R.; et al. Remarkable nucleation and growth of ultrafine particles from vehicular exhaust. Proc. Natl. Acad. Sci. USA 2020, 117, 3427–3432. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, S.; Yu, Y.; Shen, R.; Zhu, W.; Tang, R.; Tan, R.; Liu, K.; Song, K.; Zhang, W.; et al. Secondary aerosol formation from a Chinese gasoline vehicle: Impacts of fuel (E10, gasoline) and driving conditions (idling, cruising). Sci. Total Environ. 2021, 795, 148809. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Song, K.; Zhu, W.; Yu, Y.; Wang, H.; Shen, R.; Tan, R.; Lv, D.; Gong, Y.; Yu, X.; et al. A Closure Study of Secondary Organic Aerosol Estimation at an Urban Site of Yangtze River Delta, China. Atmosphere 2022, 13, 1679. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Zhu, R.; Zhang, R.; Zu, L.; Wang, Y.; Bao, X. On-road tailpipe emission characteristics and ozone formation potentials of VOCs from gasoline, diesel and liquefied petroleum gas fueled vehicles. Atmos. Environ. 2020, 223, 117294. [Google Scholar] [CrossRef]
- Hong-Li, W.; Sheng-Ao, J.; Sheng-Rong, L.; Qing-Yao, H.; Li, L.; Shi-Kang, T.; Cheng, H.; Li-Ping, Q.; Chang-Hong, C. Volatile organic compounds (VOCs) source profiles of on-road vehicle emissions in China. Sci. Total Environ. 2017, 607–608, 253–261. [Google Scholar] [CrossRef]
- Lv, D.; Lu, S.; Tan, X.; Shao, M.; Xie, S.; Wang, L. Source profiles, emission factors and associated contributions to secondary pollution of volatile organic compounds (VOCs) emitted from a local petroleum refinery in Shandong. Environ. Pollut. 2021, 274, 116589. [Google Scholar] [CrossRef]
- Mo, Z.; Shao, M.; Lu, S.; Qu, H.; Zhou, M.; Sun, J.; Gou, B. Process-specific emission characteristics of volatile organic compounds (VOCs) from petrochemical facilities in the Yangtze River Delta, China. Sci. Total Environ. 2015, 533, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Lu, S.; He, S.; Song, K.; Shao, M.; Xie, S.; Gong, Y. Research on accounting and detection of volatile organic compounds from a typical petroleum refinery in Hebei, North China. Chemosphere 2021, 281, 130653. [Google Scholar] [CrossRef]
- Qin, J.; Wang, X.; Yang, Y.; Qin, Y.; Shi, S.; Xu, P.; Chen, R.; Zhou, X.; Tan, J.; Wang, X. Source apportionment of VOCs in a typical medium-sized city in North China Plain and implications on control policy. J. Environ. Sci. 2021, 107, 26–37. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Han, L.; Tian, W.; Wang, C.; Li, Y.; Chen, J. Source apportionment of VOCs and ozone formation potential and transport in Chengdu, China. Atmos. Pollut. Res. 2023, 14, 101730. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, X.; Liu, Y.; Zhang, J.; Wang, H.; Sun, L.; Fang, T.; Mao, H.; Hu, J.; Wu, L.; et al. Source apportionment of VOCs based on photochemical loss in summer at a suburban site in Beijing. Atmos. Environ. 2023, 293, 119459. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Z.; Li, Z.; Sun, Y.; Wang, C.; Zhu, C.; Sun, L.; Yang, N.; Bai, G.; Fan, G.; et al. Characteristics, chemical transformation and source apportionment of volatile organic compounds (VOCs) during wintertime at a suburban site in a provincial capital city, east China. Atmos. Environ. 2023, 298, 119621. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Li, R.; Fu, H.; Duan, Y.; Gao, S.; Zhang, L.; Chen, J. Measurement report: Volatile organic compound characteristics of the different land-use types in Shanghai: Spatiotemporal variation, source apportionment and impact on secondary formations of ozone and aerosol. Atmos. Chem. Phys. 2023, 23, 2877–2900. [Google Scholar] [CrossRef]
- Wang, H.; Xiang, Z.; Wang, L.; Jing, S.; Lou, S.; Tao, S.; Liu, J.; Yu, M.; Li, L.; Lin, L.; et al. Emissions of volatile organic compounds (VOCs) from cooking and their speciation: A case study for Shanghai with implications for China. Sci. Total Environ. 2018, 621, 1300–1309. [Google Scholar] [CrossRef]
- He, W.-Q.; Shi, A.-J.; Shao, X.; Nie, L.; Wang, T.-Y.; Li, G.-H. Insights into the comprehensive characteristics of volatile organic compounds from multiple cooking emissions and aftertreatment control technologies application. Atmos. Environ. 2020, 240, 117646. [Google Scholar] [CrossRef]
- Yi, H.; Huang, Y.; Tang, X.; Zhao, S.; Xie, X.; Zhang, Y. Characteristics of non-methane hydrocarbons and benzene series emission from commonly cooking oil fumes. Atmos. Environ. 2019, 200, 208–220. [Google Scholar] [CrossRef]
- Zhang, D.-C.; Liu, J.-J.; Jia, L.-Z.; Wang, P.; Han, X. Speciation of VOCs in the cooking fumes from five edible oils and their corresponding health risk assessments. Atmos. Environ. 2019, 211, 6–17. [Google Scholar] [CrossRef]
- Ari, A.; Arı, P.E.; Yenisoy-Karakaş, S.; Gaga, E.O. Source characterization and risk assessment of occupational exposure to volatile organic compounds (VOCs) in a barbecue restaurant. Build. Environ. 2020, 174, 106791. [Google Scholar] [CrossRef]
- Huang, X.; Han, D.; Cheng, J.; Chen, X.; Zhou, Y.; Liao, H.; Dong, W.; Yuan, C. Characteristics and health risk assessment of volatile organic compounds (VOCs) in restaurants in Shanghai. Environ. Sci. Pollut. Res. 2020, 27, 490–499. [Google Scholar] [CrossRef]
- Lu, F.; Shen, B.; Li, S.; Liu, L.; Zhao, P.; Si, M. Exposure characteristics and risk assessment of VOCs from Chinese residential cooking. J. Environ. Manag. 2021, 289, 112535. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, W.; Hu, M.; Wang, H.; Chen, Z.; Shen, R.; Yu, Y.; Tan, R.; Guo, S. Secondary Organic Aerosol from Typical Chinese Domestic Cooking Emissions. Environ. Sci. Technol. Lett. 2021, 8, 24–31. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, W.; Hu, M.; Liu, K.; Wang, H.; Tang, R.; Shen, R.; Yu, Y.; Tan, R.; Song, K.; et al. Formation and evolution of secondary organic aerosols derived from urban-lifestyle sources: Vehicle exhaust and cooking emissions. Atmos. Chem. Phys. 2021, 21, 15221–15237. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, S.; Zhang, Z.; Wang, H.; Yu, Y.; Chen, Z.; Shen, R.; Tan, R.; Song, K.; Liu, K.; et al. Mass spectral characterization of secondary organic aerosol from urban cooking and vehicular sources. Atmos. Chem. Phys. 2021, 21, 15065–15079. [Google Scholar] [CrossRef]
- Carter, W.P. Development of the SAPRC-07 chemical mechanism. Atmos. Environ. 2010, 44, 5324–5335. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, W.; Shao, M.; Wang, M.; Chen, W.; Lu, S.; Zeng, L.; Hu, M. VOC emissions, evolutions and contributions to SOA formation at a receptor site in eastern China. Atmos. Chem. Phys. 2013, 13, 8815–8832. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Wang, T.; Song, K.; Tan, T.; Wan, Z.; Gao, Y.; Dong, H.; Chen, S.; Zeng, L.; et al. Elucidating the importance of semi-volatile organic compounds to secondary organic aerosol formation at a regional site during the EXPLORE-YRD campaign. Atmos. Environ. 2021, 246, 118043. [Google Scholar] [CrossRef]
- Lim, Y.B.; Ziemann, P.J. Effects of Molecular Structure on Aerosol Yields from OH Radical-Initiated Reactions of Linear, Branched, and Cyclic Alkanes in the Presence of NOx. Environ. Sci. Technol. 2009, 43, 2328–2334. [Google Scholar] [CrossRef]
- Chan, A.W.H.; Kautzman, K.; Chhabra, P.; Surratt, J.; Chan, M.; Crounse, J.; Kurten, A.; Wennberg, P.; Flagan, R.; Seinfeld, J. Secondary organic aerosol formation from photooxidation of naphthalene and alkylnaphthalenes: Implications for oxidation of intermediate volatility organic compounds (IVOCs). Atmos. Chem. Phys. 2009, 9, 3049–3060. [Google Scholar] [CrossRef]
- Ng, N.; Kroll, J.; Chan, A.; Chhabra, P.; Flagan, R.; Seinfeld, J. Secondary organic aerosol formation from m-xylene, toluene, and benzene. Atmos. Chem. Phys. 2007, 7, 3909–3922. [Google Scholar] [CrossRef]
- Zhang, X.; Cappa, C.; Jathar, S.; McVay, R.; Ensberg, J.; Kleeman, M.; Seinfeld, J. Influence of vapor wall loss in laboratory chambers on yields of secondary organic aerosol. Proc. Natl. Acad. Sci. USA 2014, 111, 5802–5807. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Zhang, X.; Jing, S.; Peng, Y.; Qiao, L.; Zhou, M.; Huang, D.; Wang, Q.; Li, X.; et al. Estimating Secondary Organic Aerosol Production from Toluene Photochemistry in a Megacity of China. Environ. Sci. Technol. 2019, 53, 8664–8671. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Gao, Y.; Zhou, M.; Jing, S.; Qiao, L.; Yuan, B.; Huang, D.; Huang, C.; Lou, S.; et al. Estimation of Secondary Organic Aerosol Formation During a Photochemical Smog Episode in Shanghai, China. J. Geophys. Res. Atmos. 2020, 125, e2019JD032033. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Yan, W.; Wu, N.; Bo, Y.; Zhang, Q.; He, K. Characteristics and sources of non-methane VOCs and their roles in SOA formation during autumn in a central Chinese city. Sci. Total Environ. 2021, 782, 146802. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, D.; Seinfeld, J.H. Parameterization of the formation potential of secondary organic aerosols. Atmos. Environ. (1967) 1989, 23, 1733–1747. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, L.; Wei, N.; Wu, L.; Mao, H. The characteristics and source analysis of VOCs emissions at roadside: Assess the impact of ethanol-gasoline implementation. Atmos. Environ. 2021, 263, 118670. [Google Scholar] [CrossRef]
- Song, C.; Liu, Y.; Sun, L.; Zhang, Q.; Mao, H. Emissions of volatile organic compounds (VOCs) from gasoline- and liquified natural gas (LNG)-fueled vehicles in tunnel studies. Atmos. Environ. 2020, 234, 117626. [Google Scholar] [CrossRef]
- Cao, X.; Yao, Z.; Shen, X.; Ye, Y.; Jiang, X. On-road emission characteristics of VOCs from light-duty gasoline vehicles in Beijing, China. Atmos. Environ. 2016, 124, 146–155. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kaviti, A.K. Progress in regulated emissions of ethanol-gasoline blends from a spark ignition engine. Biofuels 2021, 12, 197–220. [Google Scholar] [CrossRef]
- Liang, X.; Chen, L.; Liu, M.; Lu, Q.; Lu, H.; Gao, B.; Zhao, W.; Sun, X.; Xu, J.; Ye, D. Carbonyls from commercial, canteen and residential cooking activities as crucial components of VOC emissions in China. Sci. Total Environ. 2022, 846, 157317. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, G.; Lang, J.; Wen, W.; Wang, X.; Yao, S. Characterization of volatile organic compounds from different cooking emissions. Atmos. Environ. 2016, 145, 299–307. [Google Scholar] [CrossRef]
- Mugica, V.; Vega, E.; Chow, J.; Reyes, E.; Sanchez, G.; Arriaga, J.; Egami, R.; Watson, J. Speciated non-methane organic compounds emissions from food cooking in Mexico. Atmos. Environ. 2001, 35, 1729–1734. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.; Roh, H.; Lee, C. Effect of bioethanol as an alternative fuel on the emissions reduction characteristics and combustion stability in a spark ignition engine. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2009, 223, 941–951. [Google Scholar] [CrossRef]
- Yao, Y.-C.; Tsai, J.-H.; Wang, I.; Tsai, H.-R. Investigating Criteria and Organic Air pollutant Emissions from Motorcycles by Using Various Ethanol-Gasoline Blends. Aerosol Air Qual. Res. 2017, 17, 167–175. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Jhang, S.-R.; Lin, S.-L.; Chen, K.-S. Comparative effect of fuel ethanol content on regulated and unregulated emissions from old model vehicles: An assessment and policy implications. Atmos. Pollut. Res. 2021, 12, 66–75. [Google Scholar] [CrossRef]
- Li, L.; Ge, Y.; Wang, M.; Peng, Z.; Song, Y.; Zhang, L.; Yuan, W. Exhaust and evaporative emissions from motorcycles fueled with ethanol gasoline blends. Sci. Total Environ. 2015, 502, 627–631. [Google Scholar] [CrossRef]
- Liang, X.J.; Li, X.Q. Research on Emissions of Benzene & Polycyclic Aromatic Hydrocarbons in Constant Volume Combustion Bomb. Adv. Mater. Res. 2012, 468–471, 2993–2997. [Google Scholar]
- Atamaleki, A.; Zarandi, S.M.; Massoudinejad, M.; Esrafili, A.; Khaneghah, A.M. Emission of BTEX compounds from the frying process: Quantification, environmental effects, and probabilistic health risk assessment. Environ. Res. 2022, 204, 112295. [Google Scholar] [CrossRef]
- Goicoechea, E.; Guillén, M.D. Volatile compounds generated in corn oil stored at room temperature. Presence of toxic compounds. Eur. J. Lipid Sci. Technol. 2014, 116, 395–406. [Google Scholar] [CrossRef]
- Huang, Y.; Ling, Z.; Lee, S.; Ho, S.; Cao, J.; Blake, D.; Cheng, Y.; Lai, S.; Ho, K.; Gao, Y.; et al. Characterization of volatile organic compounds at a roadside environment in Hong Kong: An investigation of influences after air pollution control strategies. Atmos. Environ. 2015, 122, 809–818. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Q.; Wang, J.; Zhou, C.; Zhang, Y.; Pan, Z. Emission characteristics and ozone formation potentials of VOCs from gasoline passenger cars at different driving modes. Atmos. Pollut. Res. 2018, 9, 804–813. [Google Scholar] [CrossRef]
- Tibaquirá, J.E.; Huertas, J.; Ospina, S.; Quirama, L.; Niño, J. The Effect of Using Ethanol-Gasoline Blends on the Mechanical, Energy and Environmental Performance of In-Use Vehicles. Energies 2018, 11, 221. [Google Scholar] [CrossRef]
- Yao, Y.-C.; Tsai, J.-H.; Chou, H.-H. Air Pollutant Emission Abatement using Application of Various Ethanol-gasoline Blends in High-mileage Vehicles. Aerosol Air Qual. Res. 2011, 11, 547–559. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, Y.; Wang, X.; Thomas, D.; Su, S.; Li, H. An assessment of how bio-E10 will impact the vehicle-related ozone contamination in China. Energy Rep. 2020, 6, 572–581. [Google Scholar] [CrossRef]
- Tang, R.; Lu, Q.; Guo, S.; Wang, H.; Song, K.; Yu, Y.; Tan, R.; Liu, K.; Shen, R.; Chen, S.; et al. Measurement report: Distinct emissions and volatility distribution of intermediate-volatility organic compounds from on-road Chinese gasoline vehicles: Implication of high secondary organic aerosol formation potential. Atmos. Chem. Phys. 2021, 21, 2569–2583. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, S.; Wang, H.; Shen, R.; Zhu, W.; Tan, R.; Song, K.; Zhang, Z.; Li, S.; Chen, Y.; et al. Importance of Semivolatile/Intermediate-Volatility Organic Compounds to Secondary Organic Aerosol Formation from Chinese Domestic Cooking Emissions. Environ. Sci. Technol. Lett. 2022, 9, 507–512. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Song, K.; Gong, Y.; Lv, D.; Wan, Z.; Li, T.; Zhang, C.; Lu, S.; Chen, S.; et al. Secondary Organic Aerosol Formation from Semi-Volatile and Intermediate Volatility Organic Compounds in the Fall in Beijing. Atmosphere 2023, 14, 94. [Google Scholar] [CrossRef]
- Song, K.; Guo, S.; Gong, Y.; Lv, D.; Wan, Z.; Zhang, Y.; Fu, Z.; Hu, K.; Lu, S. Non-target scanning of organics from cooking emissions using comprehensive two-dimensional gas chromatography-mass spectrometer (GC×GC-MS). Appl. Geochem. 2023, 151, 105601. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, R.; Guo, S.; Lu, S.; Wang, H.; Zhu, W.; Yu, Y.; Tang, R.; Shen, R.; Song, K.; Lv, D.; et al. Characteristics and Secondary Organic Aerosol Formation of Volatile Organic Compounds from Vehicle and Cooking Emissions. Atmosphere 2023, 14, 806. https://doi.org/10.3390/atmos14050806
Tan R, Guo S, Lu S, Wang H, Zhu W, Yu Y, Tang R, Shen R, Song K, Lv D, et al. Characteristics and Secondary Organic Aerosol Formation of Volatile Organic Compounds from Vehicle and Cooking Emissions. Atmosphere. 2023; 14(5):806. https://doi.org/10.3390/atmos14050806
Chicago/Turabian StyleTan, Rui, Song Guo, Sihua Lu, Hui Wang, Wenfei Zhu, Ying Yu, Rongzhi Tang, Ruizhe Shen, Kai Song, Daqi Lv, and et al. 2023. "Characteristics and Secondary Organic Aerosol Formation of Volatile Organic Compounds from Vehicle and Cooking Emissions" Atmosphere 14, no. 5: 806. https://doi.org/10.3390/atmos14050806