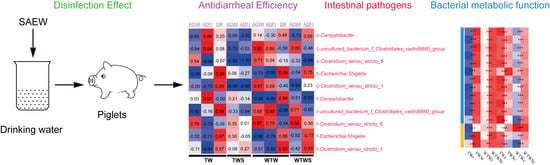
Effect of Slightly Acidic Electrolyzed Water on Growth, Diarrhea and Intestinal Bacteria of Newly Weaned Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Slightly Acidic Electrolyzed Water (SAEW) Preparation
2.2. Disinfection of Drinking Water
2.3. Animal Breeding and Experimental Design
2.4. Assessment of Intestinal Microbes
2.5. Statistical Analysis
3. Results
3.1. Disinfection Effect of the SAEW on Drinking Water
3.2. Improvement of SAEW on Water and Feed Consumption, Growth and Health Performance of Piglets
3.3. Effect of SAEW on Intestinal Bacterial Diversity
3.4. Effect of SAEW on Intestinal Pathogenic Bacteria
3.5. Effect of SAEW on Intestinal Bacterial Metabolic Functions
3.6. Relationship between Diarrhea Rate and Pathogenic Bacteria
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bügener, E.; Kump, A.W.; Casteel, M.; Klein, G. Benefits of neutral electrolyzed oxidizing water as a drinking water additive for broiler chickens. Poultry Sci. 2014, 93, 2320–2326. [Google Scholar] [CrossRef]
- Kamphues, J.; Schulz, I. Praxisrelevante Aspekte der Wasserversorgung von Nutz-und Liebhabertieren. Ubers. Tierernahrg. 2002, 30, 65–107. [Google Scholar]
- Kamphues, J.; Bohm, R.; Flachowsky, G.; Lahrssen-Wiederholt, M.; Meyer, U.; Schenkel, H. Empfehlungen zur Beurteilung der hygienischen Qualitat von Trankwasser fur Lebensmittel liefernde Tiere unter Berucksichtiung der gegebenen rechtlichen Rahmenbedingungen. Landbauforsch. Volkenrode 2007, 57, 255–272. [Google Scholar]
- Issa-Zacharia, A.; Kamitani, Y.; Morita, K.; Iwasaki, K. Sanitization potency of slightly acidic electrolyzed water against pure cultures of Escherichia coli and Staphylococcus aureus, in comparison with that of other food sanitizers. Food Control 2010, 21, 740–745. [Google Scholar] [CrossRef]
- Hao, X.; Shen, Z.; Wang, J.; Zhang, Q.; Li, B.; Wang, C.; Cao, W. In vitro inactivation of porcine reproductive and respiratory syndrome virus and pseudorabies virus by slightly acidic electrolyzed water. Vet. J. 2013, 197, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Guentzel, J.L.; Lam, K.L.; Callan, M.A.; Emmons, S.A.; Dunham, V.L. Reduction of bacteria on spinach, lettuce, and surfaces in food service areas using neutral electrolyzed oxidizing water. Food Microbiol. 2008, 25, 36–41. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y.-C.; Brackett, R.E. Efficacy of electrolyzed oxidizing (EO) and chemically modified water on different types of food-borne pathogens. Int. J. Food Microbiol. 2000, 61, 199–207. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y.-C.; Brackett, R.E. Roles of oxidation–reduction potential in electrolyzed oxidizing and chemically modified water for the inactivation of food-related pathogens. J. Food Prot. 2000, 63, 19–24. [Google Scholar] [CrossRef]
- Hao, X.; Li, B.; Zhang, Q.; Lin, B.; Ge, L.; Wang, C.; Cao, W. Disinfection efficiency of slightly acidic electrolyzed water in swine barns. J. Appl. Microbiol. 2013, 115, 703–710. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, X.; Cao, C.; Gu, Z.; Liu, Z.; Liu, L.; Lin, B. Feasibility study of the sterilization of pigskin used as wound dressings by neutral electrolyzed water. J. Trauma Acute Care Surg. 2012, 72, 1584–1587. [Google Scholar] [CrossRef]
- Inagaki, H.; Shibata, Y.; Obata, T.; Kawagoe, M.; Ikeda, K.; Sato, M.; Toida, K.; Kushima, H.; Matsuda, Y. Effects of slightly acidic electrolyzed drinking water on mice. Lab. Anim. 2011, 45, 283–285. [Google Scholar] [CrossRef]
- Watson, R.D. Oral Administration of Electrolyzed Water for Treatment and Prevention of PEDv in Swine, Swine Herds and Swine Confinements. U.S. Patent US9283250, 15 March 2016. [Google Scholar]
- Bodas, R.; Bartolomé, D.J.; Tabernero De Paz, M.J.; Posado, R.; Garcí, J.J.; Rodríguez, L.; Olmedo, S.; Martín-Diana, A.B. Electrolyzed water as novel technology to improve hygiene of drinking water for dairy ewes. Res. Vet. Sci. 2013, 95, 1169–1170. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Hu, X.; Li, B. Experiment on bactericidal efficacy in drinking system using slightly acidic electrolyzed water in large-scale poultry houses. Trans. Chin. Soc. Agric. Eng. 2017, 33, 230–236. [Google Scholar] [CrossRef]
- Xiong, H.; Lin, S.; Chen, L.; Ouyang, K.; Wang, W. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Spreeuwenberg, M.A.M.; Verdonk, J.M.A.J.; Gaskins, H.R.; Verstegen, M.W.A. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.G.; Lee, J.-Y.; Jin, G.-D.; Park, J.; Choi, Y.H.; Chae, B.J.; Kim, E.B.; Choi, Y.-J. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16S rRNA sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 5903–5911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, R.; Tu, W.; Lu, Y.; Cai, X. Pyrodextrin enhances intestinal function through changing the intestinal microbiota composition and metabolism in early weaned piglets. Appl. Microbiol. Biotechnol. 2020, 104, 4141–4154. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhou, L.; Li, F.; Nan, X. The Application of Electrolyzed Water in Livestock Production. China Anim. Husb. Vet. Med. 2012, 2, 74–78. (In Chinese) [Google Scholar]
- Hsu, S.Y. Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. J. Food Eng. 2005, 66, 171–176. [Google Scholar] [CrossRef]
- da Silva, K.F.; Silva, B.A.N.; Eskinazi, S.; Jacob, D.V.; Araujo, W.A.G.; Tolentino, R.L.S.; Rebordões, F.I.G.; Gonçalves, M.F.; Lima, V.R.; Ataíde, I.Q. Influence of flavored drinking water on voluntary intake and performance of nursing and post-weaned piglets. Livest. Sci. 2020, 242, 104298. [Google Scholar] [CrossRef]
- Reiners, K.; Hessel, E.F.; Van den Weghe, H.F.A. The effect of heated mash on performance and feeding behavior of newly weaned piglets. J. Anim. Sci. 2008, 86, 3600–3607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelsmann, M.N.; Hansen, C.F.; Nielsen, M.N.; Kristensen, A.R.; Amdi, C. Glucose Injections at Birth, Warmth and Placing at a Nurse Sow Improve the Growth of IUGR Piglets. Animals 2019, 9, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Z.; Wang, S.; Gao, W.; Nan, S.; Zhu, S.; He, J.; Liu, D. Inactivation mechanism and apoptotic-like changes in aeromonas hydrophila induced by slightly acidic electrolyzed water in freshwater. Trans. ASAE 2018, 1, 305–314. [Google Scholar] [CrossRef]
- Higashimura, Y.; Baba, Y.; Inoue, R.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Hirai, Y.; Ushiroda, C.; Tanaka, Y.; Naito, Y. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med. Gas. Res. 2018, 8, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.S.; Tango, C.N.; Oh, D.H. Inactivation kinetics of slightly acidic electrolyzed water combined with benzalkonium chloride and mild heat treatment on vegetative cells, spores, and biofilms of Bacillus cereus. Food Res. Int. 2018, 115, 157–167. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Chi, M.; Sun, Y.; Zhang, J.; Jiang, S.; Cui, Z. Effects of co-digestion of cucumber residues to corn stover and pig manure ratio on methane production in solid state anaerobic digestion. Bioresour. Technol. 2018, 250, 328–336. [Google Scholar] [CrossRef]
- Le Huërou-Luron, I.; Bouzerzour, K.; Ferret-Bernard, S.; Ménard, O.; Le Normand, L.; Perrier, C.; Le Bourgot, C.; Jardin, J.; Bourlieu, C.; Carton, T.; et al. A mixture of milk and vegetable lipids in infant formula changes gut digestion, mucosal immunity and microbiota composition in neonatal piglets. Eur. J. Nutr. 2018, 57, 463–476. [Google Scholar] [CrossRef]
- Monica, M.M.; Rosaria, D.A.; Ilaria, S.; Paolo, T.; Sara, D.F.; Luisa, C.; Maurizio, M.; Paolo, B.; Bruno, B. A novel strategy to select Bifidobacterium strains and prebiotics as natural growth promoters in newly weaned pigs. Livest. Sci. 2009, 122, 248–258. [Google Scholar] [CrossRef]
- Ji, Y.; Guo, Q.; Yin, Y.; Blachier, F.; Kong, X. Dietary proline supplementation alters colonic luminal microbiota and bacterial metabolite composition between days 45 and 70 of pregnancy in Huanjiang mini-pigs. J. Anim. Sci. Biotechnol. 2018, 9, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Badalato, N.; Guillot, A.; Sabarly, V.; Dubois, M.; Pourette, N.; Pontoire, B.; Robert, P.; Bridier, A.; Monnet, V.; Sousa, D.Z.; et al. Whole ProteomeAnalyses on Ruminiclostridium cellulolyticum Show a Modulation of the Cellulolysis Machineryin Response to Cellulosic Materials with Subtle Differences in Chemical and Structural Properties. PLoS ONE 2017, 12, e0170524. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Xie, Q.; Liu, L.; Kong, P.; Sheng, J.; Xiang, H. Metabolic adaptation to the aqueous leaf extract of Moringa oleifera Lam.-supplemented diet is related to the modulation of gut microbiota in mice. Appl. Microbiol. Biotechnol. 2017, 101, 5115–5130. [Google Scholar] [CrossRef]
- Faden, H. The Role of Faecalibacterium, Roseburia and Butyrate in Inflammatory Bowel Disease. Dig. Dis. 2022, 40, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Reed, L. Dealing Out Piglet Diarrhea. Natl. Hog Farmer 2013, 58, 30. [Google Scholar]
- Regon, M.; Pathak, D.C.; Tamuli, S.M.; Baruah, G.K. Serotyping of Escherichia coli isolated from piglet diarrhoea. Vet. World 2014, 7, 614–616. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, V.L.A.; Bersano, J.G.; Carvalho, A.F.; Catroxo, M.H.B.; Chiebao, D.P.; Gregori, F.; Miyashiro, S.; Nassar, A.F.C.; Oliveira, T.M.F.S.; Ogata, R.A.; et al. Case-control study of pathogens involved in piglet diarrhea. BMC Res. Notes 2016, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhu, Y.; Wei, H.; Chen, Y.; Shang, H. Study on the Diversity and Function of Gut Microbiota in Pigs Following Long-Term Antibiotic and Antibiotic-Free Breeding. Curr. Microbiol. 2020, 77, 4114–4128. [Google Scholar] [CrossRef] [PubMed]





| Groups | Total Bacteria (CFU/mL) | Coliforms (CFU/100 mL) | pH Value |
|---|---|---|---|
| TW (tap water) | 215 ± 2.50 b | 110 ± 0 b | 6.89 ± 0.01 |
| TWS (TW + 3% SAEW) | 25 ± 1.32 d | 0 c | 6.80 ± 0 |
| WTW (warm tap water, 30 °C) | >300 a | 120 ± 10 a | 6.85 ± 0.02 |
| WTWS (WTW + 3% SAEW) | 90 ± 3.12 c | 0 c | 6.85 ± 0.01 |
| Groups | ADG (kg/d/Pig) | FCR | DR (%) |
|---|---|---|---|
| TW (tap water) | 0.44 ± 0.04 | 1.74 ± 0.09 | 1.35 ± 0.58 a |
| TWS (TW + 3% SAEW) | 0.49 ± 0.05 | 1.68 ± 0.12 | 0 c |
| WTW (warm tap water, 30 °C) | 0.45 ± 0.05 | 1.73 ± 0.10 | 0.51 ± 0 b |
| WTWS (WTW + 3% SAEW) | 0.43 ± 0.02 | 1.68 ± 0.03 | 0 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Xie, D.; Jiang, D.; Zhu, L.; Shen, L.; Gan, M.; Bai, L. Effect of Slightly Acidic Electrolyzed Water on Growth, Diarrhea and Intestinal Bacteria of Newly Weaned Piglets. Genes 2023, 14, 1398. https://doi.org/10.3390/genes14071398
Hao X, Xie D, Jiang D, Zhu L, Shen L, Gan M, Bai L. Effect of Slightly Acidic Electrolyzed Water on Growth, Diarrhea and Intestinal Bacteria of Newly Weaned Piglets. Genes. 2023; 14(7):1398. https://doi.org/10.3390/genes14071398
Chicago/Turabian StyleHao, Xiaoxia, Dan Xie, Dongmei Jiang, Li Zhu, Linyuan Shen, Mailin Gan, and Lin Bai. 2023. "Effect of Slightly Acidic Electrolyzed Water on Growth, Diarrhea and Intestinal Bacteria of Newly Weaned Piglets" Genes 14, no. 7: 1398. https://doi.org/10.3390/genes14071398