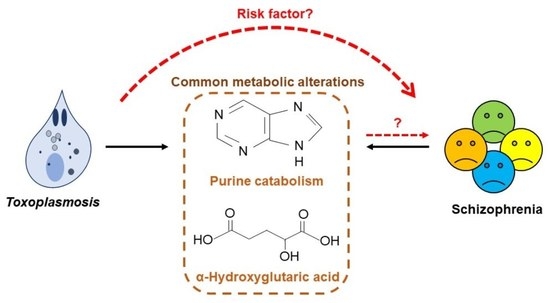
Metabolomic Profiling Reveals Common Metabolic Alterations in Plasma of Patients with Toxoplasma Infection and Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment and Sample Collection
2.2. Serological Assay
2.3. Metabolomic Analysis
3. Results
3.1. Demographic and Clinical Data of the Participants
3.2. Integrity of Metabolomic Data
3.3. Metabolite Changes in Toxoplasma Infection and Schizophrenia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuglewicz, A.J.; Piotrowski, P.; Stodolak, A. Relationship between toxoplasmosis and schizophrenia: A review. Adv. Clin. Exp. Med. 2017, 26, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.B. Congenital toxoplasmosis. J. Pediatr. Infect. Dis. Soc. 2014, 3, S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Nimir, A.R.; Osman, E.; Ibrahim, I.A.A.; Saliem, A.M. Is it toxoplasma encephalitis, HIV encephalopathy or brain tuberculoma? BMJ Case Rep. 2013, 2013, bcr2013008803. [Google Scholar] [CrossRef] [PubMed]
- Torrey, E.F.; Yolken, R.H. Toxoplasma gondii and schizophrenia. Emerg. Infect. Dis. 2003, 9, 1375–1380. [Google Scholar] [CrossRef]
- Torrey, E.F.; Bartko, J.J.; Yolken, R.H. Toxoplasma gondii and other risk factors for schizophrenia: An update. Schizophr. Bull. 2012, 38, 642–647. [Google Scholar] [CrossRef]
- Emelia, O.; Amal, R.N.; Ruzanna, Z.Z.; Shahida, H.; Azzubair, Z.; Tan, K.S.; Noor Aadila, S.; Siti, N.A.M.; Aisah, M.Y. Seroprevalence of anti-Toxoplasma gondii IgG antibody in patients with schizophrenia. Trop. Biomed. 2012, 29, 151–159. [Google Scholar]
- Flegr, J.; Horáček, J. Negative Effects of Latent Toxoplasmosis on Mental Health. Front. Psychiatry 2020, 31, 749–759. [Google Scholar] [CrossRef]
- Omar, A.; Bakar, O.C.; Adam, N.F.; Osman, H.; Osman, A.; Suleiman, A.H.; Manaf, M.R.A.; Selamat, M.I. Seropositivity and serointensity of Toxoplasma gondii antibodies and DNA among patients with schizophrenia. Korean J. Parasitol. 2015, 53, 29–34. [Google Scholar] [CrossRef]
- Yolken, R.H.; Dickerson, F.B.; Fuller Torrey, E. Toxoplasma and schizophrenia. Parasite Immunol. 2009, 31, 706–715. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Büsselberg, D.; Zhu, X.Q. The known and missing links between Toxoplasma gondii and schizophrenia. Metab. Brain Dis. 2016, 31, 749–759. [Google Scholar] [CrossRef]
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-Mcintyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A technology platform for identifying knowns and unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- El Mouhawess, A.; Hammoud, A.; Zoghbi, M.; Hallit, S.; Haddad, C.; El Haddad, K.; El Khoury, S.; Tannous, J.; Obeid, S.; Halabi, M.A.; et al. Relationship between Toxoplasma gondii seropositivity and schizophrenia in the Lebanese population: Potential implication of genetic polymorphism of MMP-9. BMC Psychiatry 2020, 20, 264. [Google Scholar] [CrossRef]
- Yao, J.K.; Dougherty, G.G.; Reddy, R.D.; Keshavan, M.S.; Montrose, D.M.; Matson, W.R.; McEvoy, J.; Kaddurah-Daouk, R. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naïve patients with schizophrenia. PLoS ONE 2010, 5, e9508. [Google Scholar] [CrossRef]
- Ghérardi, A.; Sarciron, M.E. Molecules targeting the purine salvage pathway in Apicomplexan parasites. Trends Parasitol. 2007, 23, 384–389. [Google Scholar] [CrossRef]
- De-Koning, H.P.; Al-Salabi, M.I.; Cohen, A.M.; Coombs, G.H.; Wastling, J.M. Identification and characterisation of high affinity nucleoside and nucleobase transporters in Toxoplasma gondii. Int. J. Parasitol. 2003, 33, 821–831. [Google Scholar] [CrossRef]
- Chaudhary, K.; Darling, J.A.; Fohl, L.M.; Sullivan, W.J.; Donald, R.G.K.; Pfefferkorn, E.R.; Ullman, B.; Roost, D.S. Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 2004, 279, 31224–31227. [Google Scholar] [CrossRef]
- Chaudhary, K.; Li, M.T.; Kim, K.; Roos, D.S. Toxoplasma gondii purine nucleoside phosphorylase biochemical characterization, inhibitor profiles, and comparison with the Plasmodium falciparum ortholog. J. Biol. Chem. 2006, 281, 25652–25658. [Google Scholar] [CrossRef]
- Krug, E.C.; Marr, J.J.; Berens, R.L. Purine metabolism in Toxoplasma gondii. J. Biol. Chem. 1989, 264, 10601–10607. [Google Scholar] [CrossRef]
- Steen, N.E.; Dieset, I.; Hope, S.; Vedal, T.S.J.; Smeland, O.B.; Matson, W.; Kaddurah-Daouk, R.; Agartz, I.; Melle, I.; Djurovic, S.; et al. Metabolic dysfunctions in the kynurenine pathway, noradrenergic and purine metabolism in schizophrenia and bipolar disorders. Psychol. Med. 2020, 50, 595–606. [Google Scholar] [CrossRef]
- Napoli, E.; Tassone, F.; Wong, S.; Angkustsiri, K.; Simon, T.J.; Song, G.; Giulivi, C. Mitochondrial citrate transporter-dependent metabolic signature in the 22q11.2 deletion syndrome. J. Biol. Chem. 2015, 290, 23240–23253. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, N.; Futamura, T.; Kakumoto, K.; Salehi, A.M.; Sellgren, C.M.; Holmén-Larsson, J.; Jakobsson, J.; Pålsson, E.; Landén, M.; Hashimoto, K. Blood metabolomics analysis identifies abnormalities in the citric acid cycle, urea cycle, and amino acid metabolism in bipolar disorder. BBA Clin. 2016, 5, 151–158. [Google Scholar] [CrossRef]



| TP+Sz | TN+Sz | TP+NSz | TN+NSz | |
|---|---|---|---|---|
| Number (N) | 15 | 15 | 15 | 15 |
| General | ||||
| Age in years, mean ± SD | 44.1 ± 11.5 | 34 ± 7.8 a | 36.3 ± 12.5 | 32.4 ± 8.6 a |
| Max age, years | 60 | 45 | 65 | 50 |
| Min age, years | 19 | 23 | 19 | 23 |
| Gender, N | ||||
| Male | 8 | 8 | 7 | 6 |
| Female | 7 | 7 | 8 | 9 |
| Clinical features (N of participants) | ||||
| Age of onset Teenagers (13–17 years old) Young adults (18–35 years old) | 0 15 | 3 12 | NA | NA |
| Disease onset Recent onset psychosis (≤24 months) Established (>24 months) DIP | 1 14 0 | 0 13 2 | NA | NA |
| No. of hospitalizations (severity) 0–6 times ≥7 times | 12 3 | 12 3 | NA | NA |
| Treatment-resistant schizophrenia Yes No | 1 14 | 3 12 | NA | NA |
| Family history of psychiatric illness, N Yes No | 8 7 | 9 6 | 0 15 | 1 14 |
| Duration of illness 1–8 years 9–16 years ≥17 years | 3 6 6 | 6 4 5 | NA | NA |
| On medication for schizophrenia Yes No | 15 0 | 15 0 | NA | NA |
| Drugs with anti-toxoplasmic activity Yes No | 6 9 | 13 2 | NA | NA |
| Illicit drugs Yes No | 2 13 | 4 11 | 0 15 | 0 15 |
| Lifestyle (N of participants) | ||||
| Having cat(s) as pet Yes No | 7 8 | 8 7 | 8 7 | 10 5 |
| Smoking Yes No | 5 10 | 6 9 | 1 14 | 0 15 |
| Alcohol intake Yes No | 4 11 | 2 13 | 0 15 | 1 14 |
| Numbers | TP+NSz vs. TN+NSz | TP+Sz vs. TN+Sz | TN+Sz vs. TN+NSz | TP+Sz vs. TP+NSz |
|---|---|---|---|---|
| Total MFs | ||||
| Positive ion mode | 1031 | 1031 | 1031 | 1031 |
| Negative ion mode | 611 | 611 | 611 | 611 |
| DeMFs | ||||
| Positive ion mode | 36 | 0 | 46 | 4 |
| Negative ion mode | 21 | 0 | 30 | 1 |
| Total identified DeMFs * | 6 | 0 | 7 | 1 |
| Metabolites | Molecular Weight | Retention Time (min) | Database ID | Fold Change * (FDR) | |||
|---|---|---|---|---|---|---|---|
| TP+NSz/ TN+NSz | TP+Sz/ TN+Sz | TN+Sz/ TN+NSz | TP+Sz/ TP+NSz | ||||
| 3,3′-Thiopropionic acid | 178.02893 | 1.77 | mzc3298 | −6.9 (7.8 × 10−5) | - | −5.2 (2.1 × 10−4) | - |
| α-Hydroxyglutaric acid | 148.03596 | 0.72 | mzc372 | +2.1 (0.016) | - | +2.8 (1.8 × 10−7) | - |
| Adenosine monophosphate | 347.06249 | 0.72 | mzc252 | −2.2 (0.017) | - | −2.8 (3.6 × 10−4) | - |
| Caprolactam | 113.08415 | 2.86 | mzc2867 | - | - | +1.9 (5.7 × 10−8) | +2.0 (2.4 × 10−8) |
| Hypoxanthine | 136.03832 | 0.82 | mzc441 | −4.2 (0.017) | - | −12.3 (3.4 × 10−8) | - |
| Inosine | 268.08026 | 1.00 | mzc1234 | −5.1 (0.013) | - | −148.8 (4.7 × 10−10) | - |
| Triisopropanolamine | 191.15198 | 0.95 | mzc2688 | −2.6 (0.002) | - | - | - |
| Xanthine | 152.03229 | 0.87 | mzc781 | - | - | −1.7 (0.049) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, E.; Mohammad Zahariluddin, A.S.; Sharip, S.; Md Idris, Z.; Tan, J.K. Metabolomic Profiling Reveals Common Metabolic Alterations in Plasma of Patients with Toxoplasma Infection and Schizophrenia. Genes 2022, 13, 1482. https://doi.org/10.3390/genes13081482
Osman E, Mohammad Zahariluddin AS, Sharip S, Md Idris Z, Tan JK. Metabolomic Profiling Reveals Common Metabolic Alterations in Plasma of Patients with Toxoplasma Infection and Schizophrenia. Genes. 2022; 13(8):1482. https://doi.org/10.3390/genes13081482
Chicago/Turabian StyleOsman, Emelia, Anis Safirah Mohammad Zahariluddin, Shalisah Sharip, Zulkarnain Md Idris, and Jen Kit Tan. 2022. "Metabolomic Profiling Reveals Common Metabolic Alterations in Plasma of Patients with Toxoplasma Infection and Schizophrenia" Genes 13, no. 8: 1482. https://doi.org/10.3390/genes13081482