Basal Ganglia Compensatory White Matter Changes on DTI in Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects/Participants
2.2. MRI Data Acquisition
- (1)
- T1-weighted 3D MPRAGE had the following parameters: voxel size of 0.85 × 0.85 × 0.85 mm3, 192 sagittal slices, TE of 4.73 ms, TR of 2000 ms, flip angle of 10°, FOV of 326 mm, and TA:10:42 min.
- (2)
- 3D T2-weighted FLAIR had the following parameters: voxel size of 1 × 1 × 1 mm3, 176 sagittal slices, TE of 422 ms, TR of 6000 ms, FOV of 256 mm, and TA: 6:38.
- (3)
- Diffusion-weighted images using SE EPI sequence had the parameters: voxel size of 2 × 2 × 2 mm3, TR of 6000 ms, TE of 93 ms, 44 axial slices, three averages, FOV of 256 mm, number of diffusion directions 20, and two b values: 0, 1000 s/mm2, TA: 6:38 min.
2.3. DTI Analysis
2.4. Anatomical Considerations
2.5. DTI Data Reconstruction
2.6. Tractography
2.7. Measured Parameters
2.8. FreeSurfer Volume Analysis
2.9. Statistical Analysis
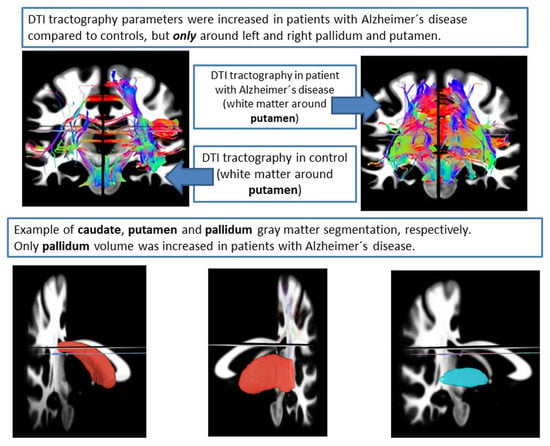
3. Results
3.1. DTI Analysis
3.2. Number of Tracts (NT)
3.3. Normalized Quantitative Anisotropy (nQA)
3.4. FreeSurfer Volume Analysis
3.5. Pearson Correlation Coefficients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cho, H.; Kim, J.H.; Kim, C.; Ye, B.S.; Kim, H.J.; Yoon, C.W.; Noh, Y.; Kim, G.H.; Kim, Y.J.; Kim, C.H.; et al. Shape changes of the basal ganglia and thalamus in Alzheimer’s disease: A three-year longitudinal study. J. Alzheimer’s Dis. 2014, 40, 285–925. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Ling, H.W.; Zhang, Y.; Huang, J.; Zhang, H.; Wang, T.; Yan, F.H. Pattern of cerebral hyperperfusion in Alzheimer’s disease and amnestic mild cognitive impairment using voxel-based analysis of 3D arterial spin-labeling imaging: Initial experience. Clin. Interv. Aging 2014, 9, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Kochunov, P.; Chiappelli, J.; McMahon, R.; Muellerklein, F.; Wijtenburg, S.A.; White, M.G.; Rowland, L.M.; Hong, L.E. Accelerated white matter aging in schizophrenia: Role of white matter blood perfusion. Neurobiol. Aging 2014, 35, 2411–2418. [Google Scholar] [CrossRef]
- Wen, W.; Sachdev, P.; Shnier, R.; Brodaty, H. Effect of white matter hyperintensities on cortical cerebral blood volume using perfusion MRI. Neuroimage 2004, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, M.J.; Rhee, H.Y.; Ryu, C.W.; Kim, E.J.; Petersen, E.T.; Jahng, G.H. Regional cerebral perfusion in patients with Alzheimer’s disease and mild cognitive impairment: Effect of APOE epsilon4 allele. Neuroradiology 2013, 55, 25–34. [Google Scholar] [CrossRef]
- Andica, C.; Kamagata, K.; Hatano, T.; Saito, Y.; Ogaki, K.; Hattori, N.; Aoki, S. MR biomarkers of degenerative brain disorders derived from diffusion imaging. J. Magn. Reson. Imaging 2020, 52, 1620–1636. [Google Scholar] [CrossRef]
- Kuchtova, B.; Wurst, Z.; Mrzilkova, J.; Ibrahim, I.; Tintera, J.; Bartos, A.; Musil, V.; Kieslich, K.; Zach, P. Compensatory Shift of Subcallosal Area and Paraterminal Gyrus White Matter Parameters on DTI in Patients with Alzheimer Disease. Curr. Alzheimer Res. 2018, 15, 590–599. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Grothe, M.J.; Barthel, H.; Sepulcre, J.; Dyrba, M.; Sabri, O.; Teipel, S.J. In vivo staging of regional amyloid deposition. Neurology 2017, 89, 2031–2038. [Google Scholar] [CrossRef]
- Bartos, A.; Raisova, M. The Mini-Mental State Examination: Czech Norms and Cutoffs for Mild Dementia and Mild Cognitive Impairment due to Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2016, 42, 50–57. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.C.; Tseng, W.Y. NTU-90: A high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 2011, 58, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [PubMed]
- RC Team. A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 6 March 2023).
- Fisher, R.A. Frequency Distribution of the Values of the Correlation Coefficient in Samples from an Indefinitely Large Population. Biometrika 1915, 10, 507–521. [Google Scholar] [CrossRef]
- Jones, D.K.; Cercignani, M. Twenty-five Pitfalls in the Analysis of Diffusion MRI Data. NMR Biomed. 2010, 23, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, K.; Klodowski, K.; Figiel, H.; Krzyzak, A.T. A theoretical validation of the B-matrix spatial distribution approach to diffusion tensor imaging. Magn. Reson. Imaging 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Borkowski, K.; Krzyżak, A.T. Analysis and correction of errors in DTI-based tractography due to diffusion gradient inhomogeneity. J. Magn. Reson. 2018, 296, 5–11. [Google Scholar] [CrossRef]
- Yeh, F.C.; Verstynen, T.D.; Wang, Y.; Fernandez-Miranda, J.C.; Tseng, W.Y. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lu, T.; Qiu, B.; Tang, Y.; Ou, S.; Tie, X.; Sun, C.; Xu, K. Differences between generalized q-sampling imaging and diffusion tensor imaging in the preoperative visualization of the nerve fiber tracts within peritumoral edema in brain. Neurosurgery 2013, 73, 1044–1053. [Google Scholar] [CrossRef]
- Tucholka, A.; Grau-Rivera, O.; Falcon, C.; Rami, L.; Sanchez-Valle, R.; Llado, A.; Gispert, J.D.; Molinuevo, J.L. Structural Connectivity Alterations Along the Alzheimer’s Disease Continuum: Reproducibility Across Two Independent Samples and Correlation with Cerebrospinal Fluid Amyloid-beta and Tau. J. Alzheimer’s Dis. 2018, 61, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.R.; Wichmann, T. Circuits and circuit disorders of the basal ganglia. Arch. Neurol. 2007, 64, 20–24. [Google Scholar] [CrossRef]
- Grillner, S.; Robertson, B. The Basal Ganglia Over 500 Million Years. Curr. Biol. 2016, 26, R1088–R1100. [Google Scholar] [CrossRef]
- Pasquini, J.; Durcan, R.; Wiblin, L.; Gersel Stokholm, M.; Rochester, L.; Brooks, D.J.; Burn, D.; Pavese, N. Clinical implications of early caudate dysfunction in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1098–1104. [Google Scholar] [CrossRef]
- Sanjari Moghaddam, H.; Dolatshahi, M.; Mohebi, F.; Aarabi, M.H. Structural white matter alterations as compensatory mechanisms in Parkinson’s disease: A systematic review of diffusion tensor imaging studies. J. Neurosci. Res. 2020, 98, 1398–1416. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.R.; Parkinson, A.; Jung, J.; Ryan, S.E.; Morgan, P.S.; Hollis, C.; Jackson, G.M. Compensatory neural reorganization in Tourette syndrome. Curr. Biol. 2011, 21, 580–585. [Google Scholar] [CrossRef]
- Ji, E.; Guevara, P.; Guevara, M.; Grigis, A.; Labra, N.; Sarrazin, S.; Hamdani, N.; Bellivier, F.; Delavest, M.; Leboyer, M.; et al. Increased and Decreased Superficial White Matter Structural Connectivity in Schizophrenia and Bipolar Disorder. Schizophr. Bull. 2019, 45, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Xekardaki, A.; Giannakopoulos, P.; Haller, S. White Matter Changes in Bipolar Disorder, Alzheimer Disease, and Mild Cognitive Impairment: New Insights from DTI. J. Aging Res. 2011, 2011, 286564. [Google Scholar] [CrossRef]
- Wright, N.; Alhindi, A.; Millikin, C.; Modirrousta, M.; Udow, S.; Borys, A.; Anang, J.; Hobson, D.E.; Ko, J.H. Elevated caudate connectivity in cognitively normal Parkinson’s disease patients. Sci. Rep. 2020, 10, 17978. [Google Scholar] [CrossRef]
- Deeb, W.; Salvato, B.; Almeida, L.; Foote, K.D.; Amaral, R.; Germann, J.; Rosenberg, P.B.; Tang-Wai, D.F.; Wolk, D.A.; Burke, A.D.; et al. Fornix-Region Deep Brain Stimulation-Induced Memory Flashbacks in Alzheimer’s Disease. N. Engl. J. Med. 2019, 381, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Kilimann, I.; Grothe, M.; Heinsen, H.; Alho, E.J.; Grinberg, L.; Amaro, E., Jr.; Dos Santos, G.A.; da Silva, R.E.; Mitchell, A.J.; Frisoni, G.B.; et al. Subregional basal forebrain atrophy in Alzheimer’s disease: A multicenter study. J. Alzheimer’s Dis. 2014, 40, 687–700. [Google Scholar] [CrossRef] [PubMed]


| AD Group | Control Group | p Values | |
|---|---|---|---|
| Numbers of participants | 10 | 10 | |
| Age at scan (years) | 70.1 ± 6.5 | 67.6 ± 4.2 | n.s |
| Education (years) | 13 ± 1 | 14 ± 6 | n.s |
| Male/female sex | 6/10 | 5/10 | n.s |
| MMSE score (0–30 pts.) | 21 ± 3 | 29 ± 8 | p < 0.001 |
| NT (Unit) | nQA (Unit) | TV (mm3) | GFA (Unit) | QA (Unit) | TL (mm) | |
|---|---|---|---|---|---|---|
| right caudate ctrl | 10,945 ± 2816 * | 0.13 ± 0.05 | 46,594 ± 16,341 | 0.1 ± 0.004 | 0.6 ± 0.13 | 71 ± 11.1 |
| right caudate AD | 7667 ± 3557 * | 0.17 ± 0.05 | 39,382 ± 18,303 | 0.09 ± 0.006 | 0.62 ± 0.13 | 68.6 ± 15 |
| left caudate ctrl | 13,873 ± 3813 | 0.13 ± 0.05 | 55,388 ± 16,471 | 0.1 ± 0.005 | 0.61 ± 0.13 | 77.6 ± 8.1 |
| left caudate AD | 10,527 ± 5558 | 0.18 ± 0.06 | 46,135 ± 21,453 | 0.1 ± 0.01 | 0.66 ± 0.15 | 74.6 ± 16.2 |
| right pallidum ctrl | 18,202 ± 3649 ** | 0.15 ± 0.06 * | 87,140 ± 30,651 | 0.11 ± 0.003 | 0.68 ± 0.1 | 110 ± 16.8 |
| right pallidum AD | 24,882 ± 5633 ** | 0.21 ± 0.05 * | 107,239 ± 23,390 | 0.11 ± 0.003 | 0.74 ± 0.14 | 115.8 ± 16 |
| left pallidum ctrl | 20,728 ± 4002 | 0.15 ± 0.06 * | 92,296 ± 23,687 | 0.11 ± 0.005 | 0.7 ± 0.09 | 116.3 ± 13.4 |
| left pallidum AD | 24,105 ± 6108 | 0.21 ± 0.05 * | 102,759 ± 23,521 | 0.11 ± 0.005 | 0.74 ± 0.15 | 117.8 ± 19.3 |
| right putamen ctrl | 27,172 ± 5618 ** | 0.13 ± 0.05 * | 102,896 ± 35,726 | 0.1 ± 0.004 | 0.62 ± 0.12 | 98.2 ± 15.9 |
| right putamen AD | 38,715 ± 9724 ** | 0.19 ± 0.05 * | 127,691 ± 30,643 | 0.1 ± 0.005 | 0.7 ± 0.12 | 104 ± 9.7 |
| left putamen ctrl | 35,368 ± 4250 * | 0.15 ± 0.06 * | 115,491 ± 23,729 | 0.1 ± 0.004 | 0.68 ± 0.11 | 104.6 ± 11.3 |
| left putamen AD | 42,603 ± 9387 * | 0.2 ± 0.05 * | 128,184 ± 29,395 | 0.1 ± 0.007 | 0.74 ± 0.16 | 107.8 ± 15 |
| Structure | AD Patients | Controls | p-Value |
|---|---|---|---|
| left caudate | 3016.7 | 3304.8 | n.s. |
| left putamen | 3656.5 | 4302.2 | p = 0.01 |
| left pallidum | 1843.1 | 1747.9 | n.s. |
| right caudate | 3043.4 | 3321.4 | n.s. |
| right putamen | 3487.3 | 4283.9 | p = 0.01 |
| right pallidum | 1897.5 | 1813.8 | n.s. |
| brain segmentation volume | 10.1 × 105 | 10.37 × 105 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurst, Z.; Birčák Kuchtová, B.; Křemen, J.; Lahutsina, A.; Ibrahim, I.; Tintěra, J.; Bartoš, A.; Brabec, M.; Rai, T.; Zach, P.; et al. Basal Ganglia Compensatory White Matter Changes on DTI in Alzheimer’s Disease. Cells 2023, 12, 1220. https://doi.org/10.3390/cells12091220
Wurst Z, Birčák Kuchtová B, Křemen J, Lahutsina A, Ibrahim I, Tintěra J, Bartoš A, Brabec M, Rai T, Zach P, et al. Basal Ganglia Compensatory White Matter Changes on DTI in Alzheimer’s Disease. Cells. 2023; 12(9):1220. https://doi.org/10.3390/cells12091220
Chicago/Turabian StyleWurst, Zdeněk, Barbora Birčák Kuchtová, Jan Křemen, Anastasiya Lahutsina, Ibrahim Ibrahim, Jaroslav Tintěra, Aleš Bartoš, Marek Brabec, Tanya Rai, Petr Zach, and et al. 2023. "Basal Ganglia Compensatory White Matter Changes on DTI in Alzheimer’s Disease" Cells 12, no. 9: 1220. https://doi.org/10.3390/cells12091220