Acne Transcriptomics: Fundamentals of Acne Pathogenesis and Isotretinoin Treatment
Abstract
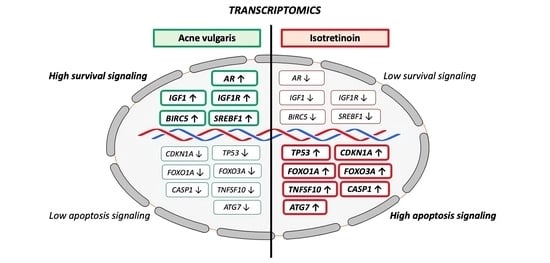
:1. Introduction
2. Growth Factor Signaling in Acne: Activating the Kinase AKT
2.1. Insulin-like Growth Factor 1
2.2. IGF-1-PI3K-AKT-Mediated Downregulation of FoxO1 and FoxO3
2.3. IGF-1-IGF1R-PI3K-AKT-MDM2-Mediated Downregulation of P53
2.4. IGF-1-IGF1R-PI3K-AKT-Mediated Activation of mTORC1
2.5. Insulin-INSR-PI3K-AKT-Mediated Activation of mTORC1
2.6. FGFR2-PI3K-AKT-Mediated Activation of mTORC1
2.7. Androgen Receptor Signaling Converges with the PI3K-Mediated Activation of AKT
3. Hypoxia-Inducible Factor-1α and Leptin
4. Infundibular GATA-Binding Protein 6
5. Transforming Growth Factor β
6. Cutibacterium Acnes
7. Transcriptomic Effects of Isotretinoin Treatment
7.1. Isotretinoin’s In Vitro versus In Vivo Gene-Regulatory Effects
7.2. Isotretinoin’s Potential Impact on Sebocyte Stem and Progenitor Cells
7.3. P53/FoxO1-Mediated Suppression of IGF-1/IGF1R/PI3K/AKT/mTORC1 Signaling
7.4. P53/FoxO1 Upregulation Suppresses AR Signaling
7.5. GATA6 Upregulation Suppresses Comedogenesis
7.6. Perilipin 2-Mediated Suppression of Comedogenesis
7.7. Sebum Suppression, Sebocyte Autophagy and Apoptosis
7.8. Teratogenicity and Neural Crest Cell Apoptosis
7.9. Depression and Impaired Hippocampal Neurogenesis
7.10. Reduced Ovarian Reserve and Granulosa Cell Apoptosis
7.11. Hypertriglyceridemia
7.12. Increased Transepidermal Water Loss and Dry Skin
7.13. Intracranial Hypertension
7.14. Inflammatory Flare upon the Initiation of Isotretinoin Treatment
8. Limitations of Immortalized Human Sebocytes
9. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| AIFM1 | apoptosis-inducing factor, mitochondria associated |
| AKT | Akt kinase (protein kinase B) |
| ALOX5 | arachidonate 5-lipoxigenate |
| APOB | apolipoprotein B |
| APOCIII | apolipoprotein CIII |
| AQP1 | aquaporin 1 |
| AQP3 | aquaporin 3 |
| AR | androgen receptor |
| ARE | androgen response element |
| ATG7 | autophagy-related 7 |
| ATP | adenosine triphosphate |
| ATRA | all-trans retinoic acid |
| BIRC5 | baculoviral IAP repeat-containing protein |
| BLIMP1 | B lymphocyte-induced maturation protein 1 |
| BMP2 | bone morphogenic protein 2 |
| C. acnes | Cutibacterium acnes |
| CASP1 | caspase 1 |
| CDKN1A | cyclin-dependent kinase inhibitor 1A (p21) |
| ChP | choroid plexus |
| CRABP2 | cellular retinoic acid binding protein 2 |
| DEPTOR | DEP-domain containing mTOR-interacting protein |
| EFTUD2 | elongation factor Tu GTP-binding domain-containing 2 |
| FABP5 | fatty acid binding protein 5 |
| FADS2 | fatty acid desaturase 2 |
| FASLG | Fas ligand |
| FGFR2 | fibroblast growth factor receptor 2 |
| FLG | filaggrin |
| FoxO1 | forkhead box O1 |
| FoxO3 | forkhead box O3 |
| FSH | follicle stimulating hormone |
| FSHB | FSH β-polypeptide downregulation |
| FST | follistatin |
| GAP | GTPase-activating protein |
| GATA6 | GATA binding protein 6 |
| GC | granulosa cell |
| GH | growth hormone |
| HIF-1α | hypoxia-inducible factor 1α |
| HIF-2α | hypoxia-inducible factor 2α |
| IGF-1 | insulin-like growth factor 1 |
| IGF1R | insulin-like growth factor receptor |
| IL-1β | interleukin-1β |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IL-17 | interleukin 17 |
| INSR | insulin receptor |
| Isotretinoin | 13-cis retinoic acid |
| LD | lipid droplet |
| LEP | leptin |
| LH | luteinizing hormone |
| LHB | β-subunit of LH downregulation |
| MDM2 | MDM2 protooncogene |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| mTORC2 | mechanistic target of rapamycin complex 2 |
| MTTP | microsomal triglyceride transfer protein |
| NC | neural crest |
| NCC | neural crest cell |
| OVOL1 | ovo-like 1 |
| PGN | peptidoglycan |
| PI3K | phosphoinositide 3-kinase |
| PLIN2 | perilipin 2 |
| POMC | proopiomelanocortin |
| PRDM1 | PR domain-containing protein 1 (BLIMP1) |
| PRKAB1 | protein kinase, AMP-activated, noncatalytic, β-1 |
| PPARβ/δ | peroxisome proliferator-activated receptor β/δ |
| PPARγ | peroxisome proliferator-activated receptor γ |
| PTEN | phosphatase and tensin homolog |
| RAI1 | retinoic acid-inducible gene 1 |
| RAPTOR | regulatory associated protein of mTOR |
| RAR | retinoic acid receptor |
| RARE | retinoic acid response element |
| RICTOR | rapamycin-insensitive companion of mTOR |
| RHEB | Ras protein homolog enriched in brain |
| RORγt | retinoic acid receptor-related orphan receptor-γt |
| SG | sebaceous gland |
| SMAD2 | SMAD family member 2 |
| SREBF1 | sterol regulatory element-binding transcription factor 1 |
| STAT3 | signal transducer and activator of transcription 3 |
| SV | simian virus |
| TCOF1 | treacle ribosome biogenesis factor 1 |
| TGFβ | transforming growth factor beta |
| TGFB2 | transforming growth factor beta 2 |
| TLR2 | toll-like receptor 2 |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor-α |
| TNFSF10 | tumor necrosis factor ligand superfamily, member 10 |
| TP53 | tumor protein |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand (TNFSF10) |
| TSC1 | TSC complex subunit 1 (hamartin) |
| TSC2 | TSC complex subunit 2 (tuberin) |
| TSH | thyroid stimulating hormone |
| VLDL | very low-density lipoprotein |
References
- Cong, T.X.; Hao, D.; Wen, X.; Li, X.H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef]
- Plewig, G.; Melnik, B.; Chen, W. (Eds.) Acne Pathogenesis. In Plewig and Kligman’s Acne and Rosacea; Springer: Cham, Switzerland, 2019; pp. 45–61. [Google Scholar] [CrossRef]
- Benito, M.; Valverde, A.M.; Lorenzo, M. IGF-I: A mitogen also involved in differentiation processes in mammalian cells. Int. J. Biochem. Cell Biol. 1996, 28, 499–510. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.I. IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef]
- Christoforidis, A.; Maniadaki, I.; Stanhope, R. Growth hormone/insulin-like growth factor-1 axis during puberty. Pediatr. Endocrinol. Rev. 2005, 3, 5–10. [Google Scholar]
- Upners, E.N.; Busch, A.S.; Almstrup, K.; Petersen, J.H.; Assens, M.; Main, K.M.; Jensen, R.B.; Juul, A. Does height and IGF-I determine pubertal timing in girls? Pediatr. Res. 2021, 90, 176–183. [Google Scholar] [CrossRef]
- Racine, H.L.; Serrat, M.A. The actions of IGF-1 in the growth plate and its role in postnatal bone elongation. Curr. Osteoporos. Rep. 2020, 18, 210–227. [Google Scholar] [CrossRef]
- Fellowes, H.M.; Billewicz, W.Z.; Thomson, A.M. Is acne a sign of normal puberty? A longitudinal study. J. Biosoc. Sci. 1981, 13, 401–407. [Google Scholar] [CrossRef]
- Deplewski, D.; Rosenfield, R.L. Role of hormones in pilosebaceous unit development. Endocr. Rev. 2000, 21, 363–392. [Google Scholar] [CrossRef]
- Cappel, M.; Mauger, D.; Thiboutot, D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch. Dermatol. 2005, 143, 333–338. [Google Scholar] [CrossRef]
- Vora, S.; Ovhal, A.; Jerajani, H.; Nair, N.; Chakrabortty, A. Correlation of facial sebum to serum insulin-like growth factor-1 in patients with acne. Br. J. Dermatol. 2008, 159, 990–991. [Google Scholar] [CrossRef]
- Seleit, I.; Bakry, O.A.; Abdou, A.G.; Hashim, A. Body mass index, selected dietary factors, and acne severity: Are they related to in situ expression of insulin-like growth factor-1? Anal. Quant. Cytopathol. Histpathol. 2014, 36, 267–278. [Google Scholar]
- Burris, J.; Shikany, J.M.; Rietkerk, W.; Woolf, K. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: A short-duration, 2-week randomized controlled trial. J. Acad. Nutr. Diet. 2018, 118, 1874–1885. [Google Scholar] [CrossRef]
- Qin, L.Q.; He, K.; Xu, J.Y. Milk consumption and circulating insulin-like growth factor-I level: A systematic literature review. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S7), 330–340. [Google Scholar] [CrossRef]
- Watling, C.Z.; Kelly, R.K.; Tong, T.Y.N.; Piernas, C.; Watts, E.L.; Tin Tin, S.; Knuppel, A.; Schmidt, J.A.; Travis, R.C.; Key, T.J.; et al. Associations of circulating insulin-like growth factor-I with intake of dietary proteins and other macronutrients. Clin. Nutr. 2021, 40, 4685–4693. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp. Dermatol. 2009, 18, 833–841. [Google Scholar] [CrossRef]
- Meixiong, J.; Ricco, C.; Vasavda, C.; Ho, B.K. Diet and acne: A systematic review. JAAD Int. 2022, 7, 95–112. [Google Scholar] [CrossRef]
- Ben-Amitai, D.; Laron, Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 950–954. [Google Scholar] [CrossRef]
- Klinger, B.; Anin, S.; Silbergeld, A.; Eshet, R.; Laron, Z. Development of hyperandrogenism during treatment with insulin-like growth factor-I (IGF-I) in female patients with Laron syndrome. Clin. Endocrinol. 1998, 48, 81–87. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Melnik, B.C. FoxO1—The key for the pathogenesis and therapy of acne? J. Dtsch. Dermatol. Ges. 2010, 8, 105–114. [Google Scholar] [CrossRef]
- Mirdamadi, Y.; Thielitz, A.; Wiede, A.; Goihl, A.; Papakonstantinou, E.; Hartig, R.; Zouboulis, C.C.; Reinhold, D.; Simeoni, L.; Bommhardt, U.; et al. Insulin and insulin-like growth factor-1 can modulate the phosphoinositide-3-kinase/Akt/FoxO1 pathway in SZ95 sebocytes in vitro. Mol. Cell Endocrinol. 2015, 415, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yanase, T.; Morinaga, H.; Okabe, T.; Nomura, M.; Daitoku, H.; Fukamizu, A.; Kato, S.; Takayanagi, R.; Nawata, H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J. Biol. Chem. 2007, 282, 7329–7338. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, W.; O-Sullivan, I.; Williams, J.B.; Dong, Q.; Park, E.A.; Raghow, R.; Unterman, T.G.; Elam, M.B. FoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1c. J. Biol. Chem. 2012, 287, 20132–20143. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Imamura, T.; Sonoda, N.; Sears, D.D.; Patsouris, D.; Kim, J.J.; Olefsky, J.M. FOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J. Biol. Chem. 2009, 284, 12188–12197. [Google Scholar] [CrossRef]
- Yang, G.; Lim, C.Y.; Li, C.; Xiao, X.; Radda, G.K.; Li, C.; Cao, X.; Han, W. FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1. J. Biol. Chem. 2009, 284, 3719–3727. [Google Scholar] [CrossRef]
- Törőcsik, D.; Kovács, D.; Camera, E.; Lovászi, M.; Cseri, K.; Nagy, G.G.; Molinaro, R.; Rühl, R.; Tax, G.; Szabó, K.; et al. Leptin promotes a proinflammatory lipid profile and induces inflammatory pathways in human SZ95 sebocytes. Br. J. Dermatol. 2014, 171, 1326–1335. [Google Scholar] [CrossRef]
- Chen, W.C.; Zouboulis, C.C. Hormones and the pilosebaceous unit. Dermatoendocrinology 2009, 1, 81–86. [Google Scholar] [CrossRef]
- Smith, T.M.; Gilliland, K.; Clawson, G.A.; Thiboutot, D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J. Investig. Dermatol. 2008, 128, 1286–1293. [Google Scholar] [CrossRef]
- Mastrofrancesco, A.; Ottaviani, M.; Cardinali, G.; Flori, E.; Briganti, S.; Ludovici, M.; Zouboulis, C.C.; Lora, V.; Camera, E.; Picardo, M. Pharmacological PPARγ modulation regulates sebogenesis and inflammation in SZ95 human sebocytes. Biochem. Pharmacol. 2017, 138, 96–106. [Google Scholar] [CrossRef]
- Kwon, H.H.; Yoon, J.Y.; Hong, J.S.; Jung, J.Y.; Park, M.S.; Suh, D.H. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: A randomized, controlled trial. Acta Derm. Venereol. 2012, 92, 241–246. [Google Scholar] [CrossRef]
- Mayo, L.D.; Donner, D.B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 11598–11603. [Google Scholar] [CrossRef] [PubMed]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, Y.; Kishishita, S.; Obata, T.; Isazawa, Y.; Suzuki, T.; Tanaka, K.; Masuyama, N.; Gotoh, Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J. Biol. Chem. 2002, 277, 21843–21850. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. p53: Key conductor of all anti-acne therapies. J. Transl. Med. 2017, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Dibble, C.C.; Manning, B.D. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol. 2013, 15, 555–564. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Manning, B.D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Huang, J.; Manning, B.D. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef]
- Melnik, B. Dietary intervention in acne: Attenuation of increased mTORC1 signaling promoted by Western diet. Dermatoendocrinology 2012, 4, 20–32. [Google Scholar] [CrossRef]
- Melnik, B.C.; Zouboulis, C.C. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp. Dermatol. 2013, 22, 311–315. [Google Scholar] [CrossRef]
- Monfrecola, G.; Lembo, S.; Caiazzo, G.; De Vita, V.; Di Caprio, R.; Balato, A.; Fabbrocini, G. Mechanistic target of rapamycin (mTOR) expression is increased in acne patients’ skin. Exp. Dermatol. 2016, 25, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Agamia, N.F.; Abdallah, D.M.; Sorour, O.; Mourad, B.; Younan, D.N. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br. J. Dermatol. 2016, 174, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Lembo, S.; Di Caprio, R.; Balato, A.; Caiazzo, G.; Fabbrocini, G.; Skroza, N.; Tolino, E.; Potenza, C. The increase of mTOR expression is consistent with FoxO1 decrease at gene level in acne but not in psoriasis. Arch. Dermatol. Res. 2020, 312, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: Lessons learnt from Laron syndrome. Nutr. Metab. 2011, 8, 41. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk—A nutrient system of mammalian evolution promoting mTORC1-dependent translation. Int. J. Mol. Sci. 2015, 16, 17048–17087. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 2013, 126, 1713–1719. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Anno, T.; Manning, B.D.; Luo, Z.; Kandror, K.V. The mammalian target of rapamycin complex 1 regulates leptin biosynthesis in adipocytes at the level of translation: The role of the 5′-untranslated region in the expression of leptin messenger ribonucleic acid. Mol. Endocrinol. 2008, 22, 2260–2267. [Google Scholar] [CrossRef]
- Chen, W.; Obermayer-Pietsch, B.; Hong, J.B.; Melnik, B.C.; Yamasaki, O.; Dessinioti, C.; Ju, Q.; Liakou, A.I.; Al-Khuzaei, S.; Katsambas, A.; et al. Acne-associated syndromes: Models for better understanding of acne pathogenesis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 637–646. [Google Scholar] [CrossRef]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef]
- Cai, W.; Sakaguchi, M.; Kleinridders, A.; Gonzalez-Del Pino, G.; Dreyfuss, J.M.; O’Neill, B.T.; Ramirez, A.K.; Pan, H.; Winnay, J.N.; Boucher, J.; et al. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nat. Commun. 2017, 8, 14892. [Google Scholar] [CrossRef]
- Hoyt, G.; Hickey, M.S.; Cordain, L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br. J. Nutr. 2005, 93, 175–177. [Google Scholar] [CrossRef]
- Hoppe, C.; Mølgaard, C.; Vaag, A.; Barkholt, V.; Michaelsen, K.F. High intakes of milk, but not meat, increase s-insulin and insulin resistance in 8-year-old boys. Eur. J. Clin. Nutr. 2005, 59, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Macut, D.; Bjekić-Macut, J.; Rahelić, D.; Doknić, M. Insulin and the polycystic ovary syndrome. Diabetes Res. Clin. Pract. 2017, 130, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Theda, C.; Maestri, N.E.; Meyers, G.A.; Fryburg, J.S.; Dufresne, C.; Cohen, M.M., Jr.; Jabs, E.W. Analysis of phenotypic features and FGFR2 mutations in Apert syndrome. Am. J. Hum. Genet. 1995, 57, 321–328. [Google Scholar] [PubMed]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Solomon, L.M.; Fretzin, D.; Pruzansky, S. Pilosebaceous abnormalities in Apert’s syndrome. Arch. Dermatol. 1970, 102, 381–385. [Google Scholar] [CrossRef]
- Krafchik, B. Acne in Apert syndrome. Clin. Plast. Surg. 1991, 18, 407. [Google Scholar] [CrossRef]
- Munro, C.S.; Wilkie, A.O. Epidermal mosaicism producing localised acne: Somatic mutation in FGFR2. Lancet 1998, 352, 704–705. [Google Scholar] [CrossRef]
- Melnik, B.C.; Vakilzadeh, F.; Aslanidis, C.; Schmitz, G. Unilateral segmental acneiform naevus: A model disorder towards understanding fibroblast growth factor receptor 2 function in acne? Br. J. Dermatol. 2008, 158, 1397–1399. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, B.; Wu, Z. Segmental hypopigmented acneiform nevus with FGFR2 gene mutation. An. Bras. Dermatol. 2023, 98, 710–712. [Google Scholar] [CrossRef]
- Melnik, B.; Schmitz, G. FGFR2 signaling and the pathogenesis of acne. J. Dtsch. Dermatol. Ges. 2008, 6, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Role of FGFR2-signaling in the pathogenesis of acne. Dermatoendocrinology 2009, 1, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Schmitz, G.; Zouboulis, C.C. Anti-acne agents attenuate FGFR2 signal transduction in acne. J. Investig. Dermatol. 2009, 129, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wei, Z.; Ju, Q.; Chen, W. Sex hormones and acne: State of the art. J. Dtsch. Dermatol. Ges. 2021, 19, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Belgorosky, A.; Baquedano, M.S.; Guercio, G.; Rivarola, M.A. Expression of the IGF and the aromatase/estrogen receptor systems in human adrenal tissues from early infancy to late puberty: Implications for the development of adrenarche. Rev. Endocr. Metab. Disord. 2009, 10, 51–61. [Google Scholar] [CrossRef]
- De Mellow, J.S.; Handelsman, D.J.; Baxter, R.C. Short-term exposure to insulin-like growth factors stimulates testosterone production by testicular interstitial cells. Acta Endocrinol. 1987, 115, 483–489. [Google Scholar] [CrossRef]
- Lin, T.; Haskell, J.; Vinson, N.; Terracio, L. Direct stimulatory effects of insulin-like growth factor-I on Leydig cell steroidogenesis in primary culture. Biochem. Biophys. Res. Commun. 1986, 137, 950–956. [Google Scholar] [CrossRef]
- Kasson, B.G.; Hsueh, A.J. Insulin-like growth factor-I augments gonadotropin-stimulated androgen biosynthesis by cultured rat testicular cells. Mol. Cell Endocrinol. 1987, 52, 27–34. [Google Scholar] [CrossRef]
- Cara, J.F.; Rosenfield, R.L. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology 1988, 123, 733–739. [Google Scholar] [CrossRef]
- Cara, J.F. Insulin-like growth factors, insulin-like growth factor binding proteins and ovarian androgen production. Horm. Res. 1994, 42, 49–54. [Google Scholar] [CrossRef]
- Horton, R.; Pasupuletti, V.; Antonipillai, I. Androgen induction of steroid 5 alpha-reductase may be mediated via insulin-like growth factor-I. Endocrinology 1993, 133, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Grino, P.B.; Griffin, J.E.; Wilson, J.D. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology 1990, 126, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen receptor structure, function and biology: From bench to bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar] [PubMed]
- Barrault, C.; Garnier, J.; Pedretti, N.; Cordier-Dirikoc, S.; Ratineau, E.; Deguercy, A.; Bernard, F.X. Androgens induce sebaceous differentiation in sebocyte cells expressing a stable functional androgen receptor. J. Steroid. Biochem. Mol. Biol. 2015, 152, 34–44. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Zhao, J.; Pan, J.; Wu, Q.; Zhang, Y.; Bauman, W.A.; Cardozo, C.P. Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology 2007, 148, 2984–2993. [Google Scholar] [CrossRef]
- Pandini, G.; Mineo, R.; Frasca, F.; Roberts, C.T., Jr.; Marcelli, M.; Vigneri, R.; Belfiore, A. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res. 2005, 65, 1849–1857. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, T.; Dizeyi, N.; Chen, S.; Wang, H.; Swanson, K.D.; Cai, C.; Balk, S.P.; Yuan, X. Androgen receptor enhances p27 degradation in prostate cancer cells through rapid and selective TORC2 activation. J. Biol. Chem. 2012, 287, 2090–2098. [Google Scholar] [CrossRef]
- Koryakina, Y.; Ta, H.Q.; Gioeli, D. Androgen receptor phosphorylation: Biological context and functional consequences. Endocr. Relat. Cancer 2014, 21, T131–T145. [Google Scholar] [CrossRef]
- Alimirah, F.; Panchanathan, R.; Chen, J.; Zhang, X.; Ho, S.M.; Choubey, D. Expression of androgen receptor is negatively regulated by p53. Neoplasia 2007, 9, 1152–1159. [Google Scholar] [CrossRef]
- Imperato-McGinley, J.; Gautier, T.; Cai, L.Q.; Yee, B.; Epstein, J.; Pochi, P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J. Clin. Endocrinol. Metab. 1993, 76, 524–528. [Google Scholar] [CrossRef]
- Danby, F.W. Ductal hypoxia in acne: Is it the missing link between comedogenesis and inflammation? J. Am. Acad. Dermatol. 2014, 70, 948–949. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Is sebocyte-derived leptin the missing link between hyperseborrhea, ductal hypoxia, inflammation and comedogenesis in acne vulgaris? Exp. Dermatol. 2016, 25, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.E.; O’Neill, L.A. HIF1α and metabolic reprogramming in inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Grosfeld, A.; Andre, J.; Hauguel-De Mouzon, S.; Berra, E.; Pouyssegur, J.; Guerre-Millo, M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J. Biol. Chem. 2002, 277, 42953–42957. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Stallmeyer, B.; Kämpfer, H.; Kolb, N.; Pfeilschifter, J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J. Clin. Investig. 2000, 106, 501–509. [Google Scholar] [CrossRef]
- Wong, W.J.; Richardson, T.; Seykora, J.T.; Cotsarelis, G.; Simon, M.C. Hypoxia-inducible factors regulate filaggrin expression and epidermal barrier function. J. Investig. Dermatol. 2015, 135, 454–461. [Google Scholar] [CrossRef]
- Kurokawa, I.; Mayer-da-Silva, A.; Gollnick, H.; Orfanos, C.E. Monoclonal antibody labeling for cytokeratins and filaggrin in the human pilosebaceous unit of normal, seborrhoeic and acne skin. J. Investig. Dermatol. 1988, 91, 566–571. [Google Scholar] [CrossRef]
- Lee, M.; Lee, E.; Jin, S.H.; Ahn, S.; Kim, S.O.; Kim, J.; Choi, D.; Lim, K.M.; Lee, S.T.; Noh, M. Leptin regulates the pro-inflammatory response in human epidermal keratinocytes. Arch. Dermatol. Res. 2018, 310, 351–362. [Google Scholar] [CrossRef]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef]
- Dang, E.V.; Barbi, J.; Yang, H.Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.R.; et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Khin, P.P.; Lim, O.K.; Jun, H.S. LPA/LPAR1 signaling induces PGAM1 expression via AKT/mTOR/HIF-1α pathway and increases aerobic glycolysis, contributing to keratinocyte proliferation. Life Sci. 2022, 311, 121201. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Zhu, R.; Tang, Z.; Man, X.Y. A prediction model identifying glycolysis signature as therapeutic target for psoriasis. Front. Immunol. 2023, 14, 1188745. [Google Scholar] [CrossRef]
- Downie, M.M.; Kealey, T. Human sebaceous glands engage in aerobic glycolysis and glutaminolysis. Br. J. Dermatol. 2004, 151, 320–327. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Kelhälä, H.L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef]
- Sitohang, I.B.S.; Soebaryo, R.W.; Kanoko, M. Acne lesion extraction versus oral doxycycline for moderate acne vulgaris: A randomized clinical trial. J. Clin. Aesthet. Dermatol. 2021, 14, E61–E65. [Google Scholar]
- Choi, K.; Jin, M.; Zouboulis, C.C.; Lee, Y. Increased lipid accumulation under hypoxia in SZ95 human sebocytes. Dermatology 2021, 237, 131–141. [Google Scholar] [CrossRef]
- Li, C.H.; Liao, P.L.; Yang, Y.T.; Huang, S.H.; Lin, C.H.; Cheng, Y.W.; Kang, J.J. Minocycline accelerates hypoxia-inducible factor-1 alpha degradation and inhibits hypoxia-induced neovasculogenesis through prolyl hydroxylase, von Hippel-Lindau-dependent pathway. Arch. Toxicol. 2014, 88, 659–671. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Hekmatjah, J.; Kircik, L.H. Oral tetracyclines and acne: A systematic review for dermatologists. J. Drugs Dermatol. 2020, 19, s6–s13. [Google Scholar]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of acne vulgaris: A review. JAMA 2021, 326, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro-Porro, M. Azelaic acid. J. Am. Acad. Dermatol. 1987, 17, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Obacz, J.; Pastorekova, S.; Vojtesek, B.; Hrstka, R. Cross-talk between HIF and p53 as mediators of molecular responses to physiological and genotoxic stresses. Mol. Cancer 2013, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Kaluzová, M.; Kaluz, S.; Lerman, M.I.; Stanbridge, E.J. DNA damage is a prerequisite for p53-mediated proteasomal degradation of HIF-1alpha in hypoxic cells and downregulation of the hypoxia marker carbonic anhydrase IX. Mol. Cell Biol. 2004, 24, 5757–5766. [Google Scholar] [CrossRef]
- Choy, M.K.; Movassagh, M.; Bennett, M.R.; Foo, R.S. PKB/Akt activation inhibits p53-mediated HIF1A degradation that is independent of MDM2. J. Cell Physiol. 2010, 222, 635–639. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, A.R.; Durden, D.L. MDM2 regulates hypoxic hypoxia-inducible factor 1α stability in an E3 ligase, proteasome, and PTEN-phosphatidylinositol 3-kinase-AKT-dependent manner. J. Biol. Chem. 2014, 289, 22785–22797. [Google Scholar] [CrossRef]
- Agamia, N.F.; Sorror, O.A.; Sayed, N.M.; Ghazala, R.A.; Echy, S.M.; Moussa, D.H.; Melnik, B.C. Overexpression of hypoxia-inducible factor-1α in hidradenitis suppurativa: The link between deviated immunity and metabolism. Arch. Dermatol. Res. 2023, 315, 2107–2118. [Google Scholar] [CrossRef]
- Donati, G.; Rognoni, E.; Hiratsuka, T.; Liakath-Ali, K.; Hoste, E.; Kar, G.; Kayikci, M.; Russell, R.; Kretzschmar, K.; Mulder, K.W.; et al. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat. Cell Biol. 2017, 19, 603–613. [Google Scholar] [CrossRef]
- Swanson, J.B.; Vagnozzi, A.N.; Veniaminova, N.A.; Wong, S.Y. Loss of Gata6 causes dilation of the hair follicle canal and sebaceous duct. Exp. Dermatol. 2019, 28, 345–349. [Google Scholar] [CrossRef]
- Oulès, B.; Philippeos, C.; Segal, J.; Tihy, M.; Vietri Rudan, M.; Cujba, A.M.; Grange, P.A.; Quist, S.; Natsuga, K.; Deschamps, L.; et al. Contribution of GATA6 to homeostasis of the human upper pilosebaceous unit and acne pathogenesis. Nat. Commun. 2020, 11, 5067. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Medzhitov, R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 2014, 157, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Collins, S.L.; Sun, I.H.; Tam, A.J.; Patel, C.H.; Arwood, M.L.; Chan-Li, Y.; Powell, J.D.; Horton, M.R. mTORC2 signaling selectively regulates the generation and function of tissue-resident peritoneal macrophages. Cell Rep. 2017, 20, 2439–2454. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Merenick, B.L.; Ding, M.; Fetalvero, K.M.; Wagner, R.J.; Mai, A.; Gleim, S.; Tucker, D.F.; Birnbaum, M.J.; et al. Phosphorylation of GATA-6 is required for vascular smooth muscle cell differentiation after mTORC1 inhibition. Sci. Signal. 2015, 8, ra44. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.M.; Gilliland, K.L.; Cong, Z.; Thiboutot, D.M. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J. Investig. Dermatol. 2006, 126, 2178–2189. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Perlman, H.; Suzuki, E.; Simonson, M.; Smith, R.C.; Walsh, K. GATA-6 induces p21(Cip1) expression and G1 cell cycle arrest. J. Biol. Chem. 1998, 273, 13713–13718. [Google Scholar] [CrossRef]
- Xu, X.; Ge, S.; Jia, R.; Zhou, Y.; Song, X.; Zhang, H.; Fan, X. Hypoxia-induced miR-181b enhances angiogenesis of retinoblastoma cells by targeting PDCD10 and GATA6. Oncol. Rep. 2015, 33, 2789–2796. [Google Scholar] [CrossRef]
- Navarini, A.A.; Simpson, M.A.; Weale, M.; Knight, J.; Carlavan, I.; Reiniche, P.; Burden, D.A.; Layton, A.; Bataille, V.; Allen, M.; et al. Genome-wide association study identifies three novel susceptibility loci for severe acne vulgaris. Nat. Commun. 2014, 5, 4020. [Google Scholar] [CrossRef]
- Mitchell, B.L.; Saklatvala, J.R.; Dand, N.; Hagenbeek, F.A.; Li, X.; Min, J.L.; Thomas, L.; Bartels, M.; Jan Hottenga, J.; Lupton, M.K.; et al. Genome-wide association meta-analysis identifies 29 new acne susceptibility loci. Nat. Commun. 2022, 13, 702. [Google Scholar] [CrossRef]
- McNairn, A.J.; Doucet, Y.; Demaude, J.; Brusadelli, M.; Gordon, C.B.; Uribe-Rivera, A.; Lambert, P.F.; Bouez, C.; Breton, L.; Guasch, G. TGFβ signaling regulates lipogenesis in human sebaceous glands cells. BMC Dermatol. 2013, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, J.; Ying, J.; Xiao, D.; O’Keefe, R.J. FoxO1 is a crucial mediator of TGF-β/TAK1 signaling and protects against osteoarthritis by maintaining articular cartilage homeostasis. Proc. Natl. Acad. Sci. USA 2020, 117, 30488–30497. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhuo, F.; Han, B.; Li, W.; Jiang, B.; Zhang, K.; Jian, X.; Chen, Z.; Li, H.; Huang, H.; et al. The updates and implications of cutaneous microbiota in acne. Cell Biosci. 2023, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Kistowska, M.; Meier, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Coenye, T.; He, L.; Kabashima, K.; Kobayashi, T.; Niemann, C.; Nomura, T.; Oláh, A.; Picardo, M.; Quist, S.R.; et al. Sebaceous immunobiology—Skin homeostasis, pathophysiology, coordination of innate immunity and inflammatory response and disease associations. Front. Immunol. 2022, 13, 1029818. [Google Scholar] [CrossRef]
- Isard, O.; Knol, A.C.; Ariès, M.F.; Nguyen, J.M.; Khammari, A.; Castex-Rizzi, N.; Dréno, B. Propionibacterium acnes activates the IGF-1/IGF-1R system in the epidermis and induces keratinocyte proliferation. J. Investig. Dermatol. 2011, 131, 59–66. [Google Scholar] [CrossRef]
- Jugeau, S.; Tenaud, I.; Knol, A.C.; Jarrousse, V.; Quereux, G.; Khammari, A.; Dreno, B. Induction of toll-like receptors by Propionibacterium acnes. Br. J. Dermatol. 2005, 153, 1105–1113. [Google Scholar] [CrossRef]
- Marks, K.E.; Flaherty, S.; Patterson, K.M.; Stratton, M.; Martinez, G.J.; Reynolds, J.M. Toll-like receptor 2 induces pathogenicity in Th17 cells and reveals a role for IPCEF in regulating Th17 cell migration. Cell Rep. 2021, 35, 109303. [Google Scholar] [CrossRef]
- Jahns, A.C.; Eilers, H.; Alexeyev, O.A. Transcriptomic analysis of Propionibacterium acnes biofilms in vitro. Anaerobe 2016, 42, 111–118. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Spittaels, K.J.; Achermann, Y. The role of biofilm formation in the pathogenesis and antimicrobial susceptibility of Cutibacterium acnes. Biofilm 2021, 4, 100063. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, C.H.; Li, T.T.; Zouboulis, C.C.; Hsu, H.C. Cell-free extracts of Propionibacterium acnes stimulate cytokine production through activation of p38 MAPK and Toll-like receptor in SZ95 sebocytes. Life Sci. 2015, 139, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.R.; Zhang, J.A.; Zhang, Q.; Permatasari, F.; Xu, Y.; Wu, D.; Yin, Z.Q.; Luo, D. Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1β, and tumor necrosis factor-α via a NF-κB-dependent mechanism in HaCaT keratinocytes. Mediat. Inflamm. 2013, 2013, 530429. [Google Scholar] [CrossRef] [PubMed]
- Fogh, K.; Voorhees, J.J.; Aström, A. Expression, purification, and binding properties of human cellular retinoic acid-binding protein type I and type II. Arch. Biochem. Biophys. 1993, 300, 751–755. [Google Scholar] [CrossRef]
- Allenby, G.; Bocquel, M.T.; Saunders, M.; Kazmer, S.; Speck, J.; Rosenberger, M.; Lovey, A.; Kastner, P.; Grippo, J.F.; Chambon, P.; et al. Retinoic acid receptors and retinoid X receptors: Interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 1993, 90, 30–34. [Google Scholar] [CrossRef]
- Levin, A.A.; Bosakowski, T.; Kazmer, S.; Grippo, J.F. 13-cis retinoic acid does not bind to retinoic acid receptors alpha, beta and gamma. Toxicologist 1992, 12, 181. [Google Scholar]
- Ott, F.; Bollag, W.; Geiger, J.M. Oral 9-cis-retinoic acid versus 13-cis-retinoic acid in acne therapy. Dermatology 1996, 193, 124–126. [Google Scholar] [CrossRef]
- McCormick, A.M.; Kroll, K.D.; Napoli, J.L. 13-cis-retinoic acid metabolism in vivo. The major tissue metabolites in the rat have the all-trans configuration. Biochemistry 1983, 22, 3933–3940. [Google Scholar] [CrossRef]
- Creech Kraft, J.; Eckhoff, C.; Kochhar, D.M.; Bochert, G.; Chahoud, I.; Nau, H. Isotretinoin (13-cis-retinoic acid) metabolism, cis-trans isomerization, glucuronidation, and transfer to the mouse embryo: Consequences for teratogenicity. Teratog. Carcinog. Mutagen. 1991, 11, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Tzimas, G.; Bürgin, H.; Collins, M.D.; Hummler, H.; Nau, H. The high sensitivity of the rabbit to the teratogenic effects of 13-cis-retinoic acid (isotretinoin) is a consequence of prolonged exposure of the embryo to 13-cis-retinoic acid and 13-cis-4-oxo-retinoic acid, and not of isomerization to all-trans-retinoic acid. Arch. Toxicol. 1994, 68, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S. Cellular metabolism and actions of 13-cis-retinoic acid. J. Am. Acad. Dermatol. 2001, 45, S129–S135. [Google Scholar] [CrossRef] [PubMed]
- Nau, H. Teratogenicity of isotretinoin revisited: Species variation and the role of all-trans-retinoic acid. J. Am. Acad. Dermatol. 2001, 45, S183–S187. [Google Scholar] [CrossRef] [PubMed]
- Layton, A. The use of isotretinoin in acne. Dermatoendocrinology 2009, 1, 162–169. [Google Scholar] [CrossRef]
- Tsukada, M.; Schröder, M.; Roos, T.C.; Chandraratna, R.A.; Reichert, U.; Merk, H.F.; Orfanos, C.E.; Zouboulis, C.C. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J. Investig. Dermatol. 2000, 115, 321–327. [Google Scholar] [CrossRef]
- Nelson, A.M.; Zhao, W.; Gilliland, K.L.; Zaenglein, A.L.; Liu, W.; Thiboutot, D.M. Isotretinoin temporally regulates distinct sets of genes in patient skin. J. Investig. Dermatol. 2009, 129, 1038–1042. [Google Scholar] [CrossRef]
- Kovács, D.; Hegyi, K.; Szegedi, A.; Deák, D.; Póliska, S.; Rühl, R.; Zouboulis, C.C.; Törőcsik, D. Isotretinoin is indirectly effective in sebocytes. Br. J. Dermatol. 2020, 182, 1052–1054. [Google Scholar] [CrossRef]
- Sitzmann, J.H.; Bauer, F.W.; Cunliffe, W.J.; Holland, B.; Lemotte, P.K. In situ hybridization analysis of CRABP II expression in sebaceous follicles from 13-cis retinoic acid-treated acne patients. Br. J. Dermatol. 1995, 133, 241–248. [Google Scholar] [CrossRef]
- Durand, B.; Saunders, M.; Leroy, P.; Leid, M.; Chambon, P. All-trans and 9-cis retinoic acid induction of CRABPII transcription is mediated by RAR-RXR heterodimers bound to DR1 and DR2 repeated motifs. Cell 1992, 71, 73–85. [Google Scholar] [CrossRef]
- Aström, A.; Pettersson, U.; Chambon, P.; Voorhees, J.J. Retinoic acid induction of human cellular retinoic acid-binding protein-II gene transcription is mediated by retinoic acid receptor-retinoid X receptor heterodimers bound to one far upstream retinoic acid-responsive element with 5-base pair spacing. J. Biol. Chem. 1994, 269, 22334–22339. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.T.; Kaplan, A.; Cromie, M.A.; Kang, S.; Voorhees, J.J. Retinoid induction of CRABP II mRNA in human dermal fibroblasts: Use as a retinoid bioassay. J. Investig. Dermatol. 1996, 106, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 2007, 129, 723–733. [Google Scholar] [CrossRef]
- Nelson, A.M.; Zhao, W.; Gilliland, K.L.; Zaenglein, A.L.; Liu, W.; Thiboutot, D.M. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J. Clin. Investig. 2008, 118, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J.; Wagner, J.A. Retinoids regulate stem cell differentiation. J. Cell Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef]
- Geueke, A.; Niemann, C. Stem and progenitor cells in sebaceous gland development, homeostasis and pathologies. Exp. Dermatol. 2021, 30, 588–597. [Google Scholar] [CrossRef]
- Hou, X.; Wei, Z.; Zouboulis, C.C.; Ju, Q. Aging in the sebaceous gland. Front. Cell Dev. Biol. 2022, 10, 909694. [Google Scholar] [CrossRef]
- Veniaminova, N.A.; Jia, Y.Y.; Hartigan, A.M.; Huyge, T.J.; Tsai, S.Y.; Grachtchouk, M.; Nakagawa, S.; Dlugosz, A.A.; Atwood, S.X.; Wong, S.Y. Distinct mechanisms for sebaceous gland self-renewal and regeneration provide durability in response to injury. Cell Rep. 2023, 42, 113121. [Google Scholar] [CrossRef]
- Gudas, L.J. Retinoids induce stem cell differentiation via epigenetic changes. Semin. Cell Dev. Biol. 2013, 24, 701–705. [Google Scholar] [CrossRef]
- Gudas, L.J. Retinoid metabolism: New insights. J. Mol. Endocrinol. 2022, 69, T37–T49. [Google Scholar] [CrossRef]
- Horsley, V.; O’Carroll, D.; Tooze, R.; Ohinata, Y.; Saitou, M.; Obukhanych, T.; Nussenzweig, M.; Tarakhovsky, A.; Fuchs, E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 2006, 126, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.; Mukha, D.; Maor, I.I.; Sedov, E.; Koren, E.; Yosefzon, Y.; Shlomi, T.; Fuchs, Y. Blimp1+ cells generate functional mouse sebaceous gland organoids in vitro. Nat. Commun. 2019, 10, 2348. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nakasaki, M.; Todorova, D.; Lake, B.; Yuan, C.Y.; Jamora, C.; Xu, Y. p53 Induces skin aging by depleting Blimp1+ sebaceous gland cells. Cell Death Dis. 2014, 5, e1141. [Google Scholar] [CrossRef] [PubMed]
- Cottle, D.L.; Kretzschmar, K.; Schweiger, P.J.; Quist, S.R.; Gollnick, H.P.; Natsuga, K.; Aoyagi, S.; Watt, F.M. c-MYC-induced sebaceous gland differentiation is controlled by an androgen receptor/p53 axis. Cell Rep. 2013, 3, 427–441. [Google Scholar] [CrossRef]
- Lo Celso, C.; Berta, M.A.; Braun, K.M.; Frye, M.; Lyle, S.; Zouboulis, C.C.; Watt, F.M. Characterization of bipotential epidermal progenitors derived from human sebaceous gland: Contrasting roles of c-Myc and beta-catenin. Stem Cells 2008, 26, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Jiang, J.; Lim, C.A.; Wu, Q.; Ng, H.H.; Chin, K.C. BLIMP1 regulates cell growth through repression of p53 transcription. Proc. Natl. Acad. Sci. USA 2007, 104, 1841–1846. [Google Scholar] [CrossRef]
- Wang, S.R.; Hu, R.D.; Ma, M.; You, X.; Cui, H.; He, Y.; Xu, D.; Zhao, Z.B.; Selmi, C.; Gershwin, M.E.; et al. FoxO1 suppresses IL-10 producing B cell differentiation via negatively regulating Blimp-1 expression and contributes to allergic asthma progression. Mucosal Immunol. 2022, 15, 459–470. [Google Scholar] [CrossRef]
- Vallerand, I.A.; Lewinson, R.T.; Farris, M.S.; Sibley, C.D.; Ramien, M.L.; Bulloch, A.G.M.; Patten, S.B. Efficacy and adverse events of oral isotretinoin for acne: A systematic review. Br. J. Dermatol. 2018, 178, 76–85. [Google Scholar] [CrossRef]
- Bagatin, E.; Costa, C.S. The use of isotretinoin for acne—An update on optimal dosing, surveillance, and adverse effects. Expert Rev. Clin. Pharmacol. 2020, 13, 885–897. [Google Scholar] [CrossRef]
- Melnik, B.C. Isotretinoin and FoxO1: A scientific hypothesis. Dermatoendocrinology 2011, 3, 141–165. [Google Scholar] [CrossRef]
- Melnik, B.C. Is nuclear deficiency of FoxO1 due to increased growth factor/PI3K/Akt-signalling in acne vulgaris reversed by isotretinoin treatment? Br. J. Dermatol. 2010, 162, 1398–1400. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. The role of transcription factor FoxO1 in the pathogenesis of acne vulgaris and the mode of isotretinoin action. G. Ital. Dermatol. Venereol. 2010, 145, 559–571. [Google Scholar] [PubMed]
- Shi, G.; Liao, P.Y.; Cai, X.L.; Pi, X.X.; Zhang, M.F.; Li, S.J.; Quan, J.H.; Fan, Y.M. FoxO1 enhances differentiation and apoptosis in human primary keratinocytes. Exp. Dermatol. 2018, 27, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Jung, J.Y.; Park, K.; Hossini, A.M.; Zouboulis, C.C.; Lee, S.E. Autophagy regulates lipid production and contributes to the sebosuppressive effect of retinoic acid in human SZ95 sebocytes. J. Dermatol. Sci. 2020, 98, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Agamia, N.F.; Hussein, O.M.; Abdelmaksoud, R.E.; Abdalla, D.M.; Talaat, I.M.; Zaki, E.I.; El Tawdy, A.; Melnik, B.C. Effect of oral isotretinoin on the nucleo-cytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp. Dermatol. 2018, 27, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Pappas, K.; Xu, J.; Zairis, S.; Resnick-Silverman, L.; Abate, F.; Steinbach, N.; Ozturk, S.; Saal, L.H.; Su, T.; Cheung, P.; et al. p53 maintains baseline expression of multiple tumor suppressor genes. Mol. Cancer Res. 2017, 15, 1051–1062. [Google Scholar] [CrossRef]
- Renault, V.M.; Thekkat, P.U.; Hoang, K.L.; White, J.L.; Brady, C.A.; Kenzelmann Broz, D.; Venturelli, O.S.; Johnson, T.M.; Oskoui, P.R.; Xuan, Z.; et al. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene 2011, 30, 3207–3221. [Google Scholar] [CrossRef]
- Agamia, N.F.; El Mulla, K.F.; Alsayed, N.M.; Ghazala, R.M.; El Maksoud, R.E.A.; Abdelmeniem, I.M.; Talaat, I.M.; Zaki, I.I.; Sabah, R.M.; Melnik, B.C. Isotretinoin treatment upregulates the expression of p53 in the skin and sebaceous glands of patients with acne vulgaris. Arch. Dermatol. Res. 2023, 315, 1355–1365. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- He, W.; Zhang, A.; Qi, L.; Na, C.; Jiang, R.; Fan, Z.; Chen, J. FOXO1, a potential therapeutic target, regulates autophagic flux, oxidative stress, mitochondrial dysfunction, and apoptosis in human cholangiocarcinoma QBC939 cells. Cell Physiol. Biochem. 2018, 45, 1506–1514. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, W.; Zhou, F.; Gong, P.; Xiong, Y. The role of FOXO1-mediated autophagy in the regulation of bone formation. Cell Cycle 2023, 22, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Galluzzi, L.; Morselli, E.; Kepp, O.; Malik, S.A.; Kroemer, G. Autophagy regulation by p53. Curr. Opin. Cell Biol. 2010, 22, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Green, D.R. Mechanisms of p53-dependent apoptosis. Biochem. Soc. Trans. 2001, 29, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Di, J.; Cao, H.; Bai, J.; Zheng, J. p53-mediated autophagic regulation: A prospective strategy for cancer therapy. Cancer Lett. 2015, 363, 101–107. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Duan, L.; Maki, C.G. The IGF-1R/AKT pathway determines cell fate in response to p53. Transl. Cancer Res. 2016, 5, 664–675. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, H.; Levine, A.J.; Jin, S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. USA 2005, 102, 8204–8209. [Google Scholar] [CrossRef]
- Feng, Z.; Levine, A.J. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010, 20, 427–434. [Google Scholar] [CrossRef]
- Feng, Z. p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb. Perspect. Biol. 2010, 2, a001057. [Google Scholar] [CrossRef]
- Goetz, E.M.; Shankar, B.; Zou, Y.; Morales, J.C.; Luo, X.; Araki, S.; Bachoo, R.; Mayo, L.D.; Boothman, D.A. ATM-dependent IGF-1 induction regulates secretory clusterin expression after DNA damage and in genetic instability. Oncogene 2011, 30, 3745–3754. [Google Scholar] [CrossRef]
- Karadag, A.S.; Ertugrul, D.T.; Tutal, E.; Akin, K.O. Short-term isotretinoin treatment decreases insulin-like growth factor-1 and insulin-like growth factor binding protein-3 levels: Does isotretinoin affect growth hormone physiology? Br. J. Dermatol. 2010, 162, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Karnieli, E.; Rauscher, F.J.; LeRoith, D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc. Natl. Acad. Sci. USA 1996, 93, 8318–8323. [Google Scholar] [CrossRef] [PubMed]
- Sarfstein, R.; Werner, H. Tumor suppressor p53 regulates insulin receptor (INSR) gene expression via direct binding to the INSR promoter. Oncotarget 2020, 11, 2424–2437. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hu, W.; de Stanchina, E.; Teresky, A.K.; Jin, S.; Lowe, S.; Levine, A.J. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: Stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007, 67, 3043–3053. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Qu, R.; Liu, D.; Xiong, X.; Liang, T.; Zhao, Y. The cross talk between p53 and mTOR pathways in response to physiological and genotoxic stresses. Front. Cell Dev. Biol. 2021, 9, 775507. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Dai, X.; Gong, L.; Chen, X.; Wang, L.; Xiong, X.; Zhao, Y. DEPTOR is a direct p53 target that suppresses cell growth and chemosensitivity. Cell Death Dis. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Guseva, N.V.; Rokhlin, O.W.; Glover, R.A.; Cohen, M.B. P53 and the proteasome regulate androgen receptor activity. Cancer Biol Ther. 2012, 13, 553–558. [Google Scholar] [CrossRef]
- Boudou, P.; Soliman, H.; Chivot, M.; Villette, J.M.; Vexiau, P.; Belanger, A.; Fiet, J. Effect of oral isotretinoin treatment on skin androgen receptor levels in male acneic patients. J. Clin. Endocrinol. Metab. 1995, 80, 1158–1161. [Google Scholar] [CrossRef]
- Melnik, B.C. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin. Dermatol. 2018, 36, 29–40. [Google Scholar] [CrossRef]
- Huang, P.; Chandra, V.; Rastinejad, F. Retinoic acid actions through mammalian nuclear receptors. Chem. Rev. 2014, 114, 233–254. [Google Scholar] [CrossRef]
- Najt, C.P.; Devarajan, M.; Mashek, D.G. Perilipins at a glance. J. Cell Sci. 2022, 135, jcs259501. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, M.; Fröhlich, T.; Arnold, G.J.; Müller, U.; Leonhardt, H.; Zouboulis, C.C.; Schneider, M.R. Characterization of the sebocyte lipid droplet proteome reveals novel potential regulators of sebaceous lipogenesis. Exp. Cell Res. 2015, 332, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R. Lipid droplets and associated proteins in sebocytes. Exp. Cell Res. 2016, 340, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, M.; Camera, E.; Picardo, M.; Zouboulis, C.C.; Chan, L.; Chang, B.H.; Schneider, M.R. PLIN2, the major perilipin regulated during sebocyte differentiation, controls sebaceous lipid accumulation in vitro and sebaceous gland size in vivo. Biochim. Biophys. Acta 2013, 1830, 4642–4649. [Google Scholar] [CrossRef]
- Bell, M.; Wang, H.; Chen, H.; McLenithan, J.C.; Gong, D.W.; Yang, R.Z.; Yu, D.; Fried, S.K.; Quon, M.J.; Londos, C.; et al. Consequences of lipid droplet coat protein downregulation in liver cells: Abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes 2008, 57, 2037–2045. [Google Scholar] [CrossRef]
- Brasaemle, D.L. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007, 48, 2547–2559. [Google Scholar] [CrossRef]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Sorg, O.; Nocera, T.; Fontao, F.; Castex-Rizzi, N.; Garidou, L.; Lauze, C.; Le Digabel, J.; Josse, G.; Saurat, J.H. Lipid droplet proteins in acne skin: A sound target for the maintenance of low comedogenic sebum and acne-prone skin health. JID Innov. 2021, 1, 100057. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Roy, A.M.; Baliga, M.S. Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol. Cancer Ther. 2005, 4, 207–216. [Google Scholar] [CrossRef]
- Fan, S.; Qi, M.; Yu, Y.; Li, L.; Yao, G.; Tashiro, S.; Onodera, S.; Ikejima, T. P53 activation plays a crucial role in silibinin induced ROS generation via PUMA and JNK. Free Radic. Res. 2012, 46, 310–319. [Google Scholar] [CrossRef]
- Fan, S.; Yu, Y.; Qi, M.; Sun, Z.; Li, L.; Yao, G.; Tashiro, S.; Onodera, S.; Ikejima, T. P53-mediated GSH depletion enhanced the cytotoxicity of NO in silibinin-treated human cervical carcinoma HeLa cells. Free Radic. Res. 2012, 46, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Vasuri, F.; Trisolino, G.; Bellavista, E.; Santoro, A.; Degiovanni, A.; Martucci, E.; D’Errico-Grigioni, A.; Caporossi, D.; Capri, M.; et al. Increased Plin2 expression in human skeletal muscle is associated with sarcopenia and muscle weakness. PLoS ONE 2013, 8, e73709. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Vasuri, F.; Bertaggia, E.; Armani, A.; Santoro, A.; Bellavista, E.; Degiovanni, A.; D’Errico-Grigioni, A.; Trisolino, G.; Capri, M.; et al. Differential expression of perilipin 2 and 5 in human skeletal muscle during aging and their association with atrophy-related genes. Biogerontology 2015, 16, 329–340, Erratum in Biogerontology 2015, 16, 341. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Apoptosis may explain the pharmacological mode of action and adverse effects of isotretinoin, including teratogenicity. Acta Derm. Venereol. 2017, 97, 173–181. [Google Scholar] [CrossRef]
- Nelson, A.M.; Cong, Z.; Gilliland, K.L.; Thiboutot, D.M. TRAIL contributes to the apoptotic effect of 13-cis retinoic acid in human sebaceous gland cells. Br. J. Dermatol. 2011, 165, 526–533. [Google Scholar] [CrossRef]
- Kuribayashi, K.; Krigsfeld, G.; Wang, W.; Xu, J.; Mayes, P.A.; Dicker, D.T.; Wu, G.S.; El-Deiry, W.S. TNFSF10 (TRAIL), a p53 target gene that mediates p53-dependent cell death. Cancer Biol. Ther. 2008, 7, 2034–2038. [Google Scholar] [CrossRef]
- Assaf, H.A.; Abdel-Maged, W.M.; Elsadek, B.E.; Hassan, M.H.; Adly, M.A.; Ali, S.A. Survivin as a novel biomarker in the pathogenesis of acne vulgaris and its correlation to insulin-like growth factor-I. Dis. Markers 2016, 2016, 7040312. [Google Scholar] [CrossRef]
- Aksoy Saraç, G.; Kader, S.; Akdağ, T. Elevated survivin levels in patients with acne vulgaris. J. Cosmet. Dermatol. 2022, 21, 1744–1748. [Google Scholar] [CrossRef]
- Mirza, A.; McGuirk, M.; Hockenberry, T.N.; Wu, Q.; Ashar, H.; Black, S.; Wen, S.F.; Wang, L.; Kirschmeier, P.; Bishop, W.R.; et al. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene 2002, 21, 2613–2622. [Google Scholar] [CrossRef]
- Melnik, B.C. Mechanism of action of isotretinoin. In Retinoids in Dermatology, 1st ed.; Karadag, A.S., Aksoy, B., Parish, L.C., Eds.; CRC Press: Boca Raton, FL, USA, 2020; Chapter 4. [Google Scholar]
- Ronca, F.; Yee, K.S.; Yu, V.C. Retinoic acid confers resistance to p53-dependent apoptosis in SH-SY5Y neuroblastoma cells by modulating nuclear import of p53. J. Biol. Chem. 1999, 274, 18128–18134. [Google Scholar] [CrossRef]
- Zheng, A.; Mäntymaa, P.; Säily, M.; Savolainen, E.; Vähäkangas, K.; Koistinen, P. p53 pathway in apoptosis induced by all-trans-retinoic acid in acute myeloblastic leukaemia cells. Acta Haematol. 2000, 103, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.C.; Dragnev, K.H.; Sekula, D.; Christie, A.J.; Dmitrovsky, E.; Spinella, M.J. Retinoic acid activates p53 in human embryonal carcinoma through retinoid receptor-dependent stimulation of p53 transactivation function. Oncogene 2001, 20, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Mrass, P.; Rendl, M.; Mildner, M.; Gruber, F.; Lengauer, B.; Ballaun, C.; Eckhart, L.; Tschachler, E. Retinoic acid increases the expression of p53 and proapoptotic caspases and sensitizes keratinocytes to apoptosis: A possible explanation for tumor preventive action of retinoids. Cancer Res. 2004, 64, 6542–6548. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, F.; Yuan, Y.; Ding, C.; Zhang, L.; Li, Q. All-trans retinoic acid upregulates the expression of p53 via Axin and inhibits the proliferation of glioma cells. Oncol. Rep. 2013, 29, 2269–2274. [Google Scholar] [CrossRef]
- Blanco-Luquin, I.; Lázcoz, P.; Celay, J.; Castresana, J.S.; Encío, I.J. In vitro assessment of the role of p53 on chemotherapy treatments in neuroblastoma cell lines. Pharmaceuticals 2021, 14, 1184. [Google Scholar] [CrossRef]
- Guruvayoorappan, C.; Pradeep, C.R.; Kuttan, G. 13-cis-retinoic acid induces apoptosis by modulating caspase-3, bcl-2, and p53 gene expression and regulates the activation of transcription factors in B16F-10 melanoma cells. J. Environ. Pathol. Toxicol. Oncol. 2008, 27, 197–207. [Google Scholar] [CrossRef]
- Burney, W.; Bosanac, S.S.; Nguyen, C.; Isseroff, R.R.; Sivamani, R.K. Short-term exposure of human sebocytes to 13-cis-retinoic acid induces acnegenic changes. Br. J. Dermatol. 2018, 179, 1201–1202. [Google Scholar] [CrossRef]
- Lee, I.H.; Kawai, Y.; Fergusson, M.M.; Rovira, I.I.; Bishop, A.J.; Motoyama, N.; Cao, L.; Finkel, T. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 2012, 336, 225–228, Erratum in Science 2012, 337, 911. Erratum in Science 2013, 341, 457. [Google Scholar] [CrossRef]
- Kenzelmann Broz, D.; Spano Mello, S.; Bieging, K.T.; Jiang, D.; Dusek, R.L.; Brady, C.A.; Sidow, A.; Attardi, L.D. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 2013, 27, 1016–1031. [Google Scholar] [CrossRef]
- White, E. Autophagy and p53. Cold Spring Harb. Perspect. Med. 2016, 6, a026120. [Google Scholar] [CrossRef]
- McCormick, F.; Clark, R.; Harlow, E.; Tjian, R. SV40 T antigen binds specifically to a cellular 53 K protein in vitro. Nature 1981, 292, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Dobbelstein, M.; Roth, J. The large T antigen of simian virus 40 binds and inactivates p53 but not p73. J. Gen. Virol. 1998, 79, 3079–3083. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Comite, H.; Mescon, H.; Pochi, P.E. Isotretinoin in the treatment of acne: Histologic changes, sebum production, and clinical observations. Arch. Dermatol. 1982, 118, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Lammer, E.J.; Armstrong, D.L. Malformations in hindbrain structures among humans exposed to isotretinoin (13-cis-retinoic acid) during early embryogenesis. In Retinoids in Normal Development and Teratogenesis; Morriss-Kay, G., Ed.; Oxford University Press: New York, NY, USA, 1991; pp. 281–295. [Google Scholar]
- Draghici, C.C.; Miulescu, R.G.; Petca, R.C.; Petca, A.; Dumitrașcu, M.C.; Șandru, F. Teratogenic effect of isotretinoin in both fertile females and males (Review). Exp. Ther. Med. 2021, 21, 534. [Google Scholar] [CrossRef]
- Akhtar, R.S.; Geng, Y.; Klocke, B.J.; Roth, K.A. Neural precursor cells possess multiple p53-dependent apoptotic pathways. Cell Death Differ. 2006, 13, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Overexpression of p53 explains isotretinoin’s teratogenicity. Exp. Dermatol. 2018, 27, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Rinon, A.; Molchadsky, A.; Nathan, E.; Yovel, G.; Rotter, V.; Sarig, R.; Tzahor, E. p53 coordinates cranial neural crest cell growth and epithelial-mesenchymal transition/delamination processes. Development 2011, 138, 1827–1838. [Google Scholar] [CrossRef]
- Jones, N.C.; Lynn, M.L.; Gaudenz, K.; Sakai, D.; Aoto, K.; Rey, J.P.; Glynn, E.F.; Ellington, L.; Du, C.; Dixon, J.; et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat. Med. 2008, 14, 125–133. [Google Scholar] [CrossRef]
- Berenguer, M.; Darnaudery, M.; Claverol, S.; Bonneu, M.; Lacombe, D.; Rooryck, C. Prenatal retinoic acid exposure reveals candidate genes for craniofacial disorders. Sci. Rep. 2018, 8, 17492. [Google Scholar] [CrossRef]
- Beauchamp, M.C.; Djedid, A.; Bareke, E.; Merkuri, F.; Aber, R.; Tam, A.S.; Lines, M.A.; Boycott, K.M.; Stirling, P.C.; Fish, J.L.; et al. Mutation in Eftud2 causes craniofacial defects in mice via mis-splicing of Mdm2 and increased p53. Hum. Mol. Genet. 2021, 30, 739–757. [Google Scholar] [CrossRef]
- Roy, S.; Ng, T. Blimp-1 specifies neural crest and sensory neuron progenitors in the zebrafish embryo. Curr. Biol. 2004, 14, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Ludot, M.; Mouchabac, S.; Ferreri, F. Inter-relationships between isotretinoin treatment and psychiatric disorders: Depression, bipolar disorder, anxiety, psychosis and suicide risks. World J. Psychiatry 2015, 5, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Sobreira, G.; Velosa, J.; Telles Correia, D.; Filipe, P. Association of isotretinoin with depression and suicide: A review of current literature. J. Cutan. Med. Surg. 2018, 22, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Metekoglu, S.; Oral, E.; Ucar, C.; Akalin, M. Does isotretinoin cause depression and anxiety in acne patients? Dermatol. Ther. 2019, 32, e12795. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Wang, W.; Ai, M.; Zhang, Q.; Kuang, L. Use of isotretinoin and risk of depression in patients with acne: A systematic review and meta-analysis. BMJ Open 2019, 9, e021549, Erratum in BMJ Open 2019, 9, e021549corr1. [Google Scholar] [CrossRef]
- Bremner, J.D. Isotretinoin and neuropsychiatric side effects: Continued vigilance is needed. J. Affect. Disord. Rep. 2021, 6, 100230. [Google Scholar] [CrossRef]
- Kridin, K.; Ludwig, R.J. Isotretinoin and the risk of psychiatric disturbances: A global study shedding new light on a debatable story. J. Am. Acad. Dermatol. 2023, 88, 388–394. [Google Scholar] [CrossRef]
- Duman, R.S. Depression: A case of neuronal life and death? Biol. Psychiatry 2004, 56, 140–145. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Depression, antidepressants, and the shrinking hippocampus. Proc. Natl. Acad. Sci. USA 2001, 98, 12320–12322. [Google Scholar] [CrossRef]
- Vaidya, V.A.; Fernandes, K.; Jha, S. Regulation of adult hippocampal neurogenesis: Relevance to depression. Expert Rev. Neurother. 2007, 7, 853–864. [Google Scholar] [CrossRef]
- Apple, D.M.; Fonseca, R.S.; Kokovay, E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017, 1655, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Leal, W. Adult hippocampal neurogenesis and affective disorders: New neurons for psychic well-being. Front. Neurosci. 2021, 15, 594448. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Rajkowska, G.; Du, F.; Seraji-Bozorgzad, N.; Manji, H.K. Enhancement of hippocampal neurogenesis by lithium. J. Neurochem. 2000, 75, 1729–1734. [Google Scholar] [CrossRef]
- Kim, J.S.; Chang, M.Y.; Yu, I.T.; Kim, J.H.; Lee, S.H.; Lee, Y.S.; Son, H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J. Neurochem. 2004, 89, 324–336. [Google Scholar] [CrossRef]
- Palmos, A.B.; Duarte, R.R.R.; Smeeth, D.M.; Hedges, C.; Nixon, D.F.; Thuret, S.; Powell, T.R. Lithium treatment and human hippocampal neurogenesis. Transl. Psychiatry 2021, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Song, L.; Jope, R.S. Lithium attenuates p53 levels in human neuroblastoma SH-SY5Y cells. Neuroreport 1999, 10, 1123–1125. [Google Scholar] [CrossRef]
- Chen, R.W.; Chuang, D.M. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J. Biol. Chem. 1999, 274, 6039–6042. [Google Scholar] [CrossRef]
- Mao, Z.; Liu, L.; Zhang, R.; Li, X. Lithium reduces FoxO3a transcriptional activity by decreasing its intracellular content. Biol. Psychiatry 2007, 62, 1423–1430. [Google Scholar] [CrossRef]
- Okrasinski, H. Lithium acne. Dermatologica 1977, 154, 251–253. [Google Scholar] [CrossRef]
- Oztas, P.; Aksakal, A.B.; Oztas, M.O.; Onder, M. Severe acne with lithium. Ann. Pharmacother. 2001, 35, 961–962. [Google Scholar] [CrossRef]
- Scarfi, F.; Arunachalam, M. Lithium acne. CMAJ 2013, 85, 1525. [Google Scholar] [CrossRef] [PubMed]
- Crandall, J.; Sakai, Y.; Zhang, J.; Koul, O.; Mineur, Y.; Crusio, W.E.; McCaffery, P. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.N.; Pinali, D.; Olds, K.; Lu, N.; Appleby, L.; Doan, L.; Lane, M.A. 13-Cis-retinoic acid decreases hypothalamic cell number in vitro. Neurosci. Res. 2010, 68, 185–190. [Google Scholar] [CrossRef]
- Hu, P.; Wang, Y.; Liu, J.; Meng, F.T.; Qi, X.R.; Chen, L.; van Dam, A.M.; Joëls, M.; Lucassen, P.J.; Zhou, J.N. Chronic retinoic acid treatment suppresses adult hippocampal neurogenesis, in close correlation with depressive-like behavior. Hippocampus 2016, 26, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Haybaeck, J.; Postruznik, M.; Miller, C.L.; Dulay, J.R.; Llenos, I.C.; Weis, S. Increased expression of retinoic acid-induced gene 1 in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and major depression. Neuropsychiatr. Dis. Treat. 2015, 11, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Chowdhari, S.; Saini, N. Gene expression profiling reveals the role of RIG1 like receptor signaling in p53 dependent apoptosis induced by PUVA in keratinocytes. Cell Signal. 2016, 28, 25–33. [Google Scholar] [CrossRef]
- Xiang, H.; Hochman, D.W.; Saya, H.; Fujiwara, T.; Schwartzkroin, P.A.; Morrison, R.S. Evidence for p53-mediated modulation of neuronal viability. J. Neurosci. 1996, 16, 6753–6765. [Google Scholar] [CrossRef]
- Morrison, R.S.; Kinoshita, Y. The role of p53 in neuronal cell death. Cell Death Differ. 2000, 7, 868–879. [Google Scholar] [CrossRef]
- Morrison, R.S.; Kinoshita, Y.; Johnson, M.D.; Guo, W.; Garden, G.A. p53-dependent cell death signaling in neurons. Neurochem. Res. 2003, 28, 15–27. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Z.; Xia, J.; Cui, Z.; Li, L.; Qi, Z.; Liu, W. Gene expression profile associated with Asmt knockout-induced depression-like behaviors and exercise effects in mouse hypothalamus. Biosci. Rep. 2022, 42, BSR20220800. [Google Scholar] [CrossRef]
- Xiang, H.; Kinoshita, Y.; Knudson, C.M.; Korsmeyer, S.J.; Schwartzkroin, P.A.; Morrison, R.S. Bax involvement in p53-mediated neuronal cell death. J. Neurosci. 1998, 18, 1363–1373. [Google Scholar] [CrossRef]
- Wang, H.; Quirion, R.; Little, P.J.; Cheng, Y.; Feng, Z.P.; Sun, H.S.; Xu, J.; Zheng, W. Forkhead box O transcription factors as possible mediators in the development of major depression. Neuropharmacology 2015, 99, 527–537. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Mehta, V.; Singh, S.; Sharma, N.; Bungau, S. Elucidating the possible role of FoxO in depression. Neurochem. Res. 2021, 46, 2761–2775. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.H.; Ding, Z.; Narurkar, R.; Ramkissoon, S.; Muller, F.; Kamoun, W.S.; Chae, S.S.; Zheng, H.; Ying, H.; Mahoney, J.; et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 2009, 5, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Ishigami, T.; Kawano, T. Transcriptional regulation of neuronal genes and its effect on neural functions: Expression and function of forkhead transcription factors in neurons. J. Pharmacol. Sci. 2005, 98, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Park, J.; Tran, H.; Hu, L.S.; Hemmings, B.A.; Greenberg, M.E. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell Biol. 2001, 21, 952–965. [Google Scholar] [CrossRef]
- Essaghir, A.; Dif, N.; Marbehant, C.Y.; Coffer, P.J.; Demoulin, J.B. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J. Biol. Chem. 2009, 284, 10334–10342. [Google Scholar] [CrossRef]
- Zheng, W.H.; Kar, S.; Quirion, R. Insulin-like growth factor-1-induced phosphorylation of the forkhead family transcription factor FKHRL1 is mediated by Akt kinase in PC12 cells. J. Biol. Chem. 2000, 275, 39152–39158. [Google Scholar] [CrossRef]
- Polter, A.; Yang, S.; Zmijewska, A.A.; van Groen, T.; Paik, J.H.; Depinho, R.A.; Peng, S.L.; Jope, R.S.; Li, X. Forkhead box, class O transcription factors in brain: Regulation and behavioral manifestation. Biol. Psychiatry 2009, 65, 150–159. [Google Scholar] [CrossRef]
- Zheng, W.H.; Kar, S.; Quirion, R. Insulin-like growth factor-1-induced phosphorylation of transcription factor FKHRL1 is mediated by phosphatidylinositol 3-kinase/Akt kinase and role of this pathway in insulin-like growth factor-1-induced survival of cultured hippocampal neurons. Mol. Pharmacol. 2002, 62, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Hwang, I.; Muller, F.L.; Paik, J.H. Functional regulation of FoxO1 in neural stem cell differentiation. Cell Death Differ. 2015, 22, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.S.; Ertugrul, D.T.; Tutal, E.; Akin, K.O. Isotretinoin influences pituitary hormone levels in acne patients. Acta Derm. Venereol. 2011, 91, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.S.; Takci, Z.; Ertugrul, D.T.; Bilgili, S.G.; Balahoroglu, R.; Takir, M. The effect of different doses of isotretinoin on pituitary hormones. Dermatology 2015, 230, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Sharma, S.C.; Falender, A.E.; Lo, Y.H. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: Evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol. Endocrinol. 2002, 16, 580–599. [Google Scholar] [CrossRef] [PubMed]
- Skarra, D.V.; Arriola, D.J.; Benson, C.A.; Thackray, V.G. Forkhead box O1 is a repressor of basal and GnRH-induced Fshb transcription in gonadotropes. Mol. Endocrinol. 2013, 27, 1825–1839. [Google Scholar] [CrossRef]
- Arriola, D.J.; Mayo, S.L.; Skarra, D.V.; Benson, C.A.; Thackray, V.G. FOXO1 transcription factor inhibits luteinizing hormone β gene expression in pituitary gonadotrope cells. J. Biol. Chem. 2012, 287, 33424–33435. [Google Scholar] [CrossRef]
- Thackray, V.G. Fox tales: Regulation of gonadotropin gene expression by forkhead transcription factors. Mol. Cell Endocrinol. 2014, 385, 62–70. [Google Scholar] [CrossRef]
- Abali, R.; Yuksel, M.A.; Aktas, C.; Celik, C.; Guzel, S.; Erfan, G.; Sahin, O. Decreased ovarian reserve in female Sprague-Dawley rats induced by isotretinoin (retinoic acid) exposure. Reprod. Biomed. Online 2013, 27, 184–191. [Google Scholar] [CrossRef]
- Aksoy, H.; Cinar, L.; Acmaz, G.; Aksoy, U.; Aydin, T.; Vurdem, U.E.; Oz, L.; Idem Karadag, O.; Kartal, D. The effect of isotretinoin on ovarian reserve based on hormonal parameters, ovarian volume, and antral follicle count in women with acne. Gynecol. Obstet. Investig. 2015, 79, 78–82. [Google Scholar] [CrossRef]
- Liu, Z.; Castrillon, D.H.; Zhou, W.; Richards, J.S. FOXO1/3 depletion in granulosa cells alters follicle growth, death and regulation of pituitary FSH. Mol. Endocrinol. 2013, 27, 238–252. [Google Scholar] [CrossRef]
- Shen, M.; Liu, Z.; Li, B.; Teng, Y.; Zhang, J.; Tang, Y.; Sun, S.C.; Liu, H. Involvement of FoxO1 in the effects of follicle-stimulating hormone on inhibition of apoptosis in mouse granulosa cells. Cell Death Dis. 2014, 5, e1475. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Gao, B.W.; Wang, J.; Ren, Q.L.; Chen, J.F.; Ma, Q.; Zhang, Z.J.; Xing, B.S. Critical role of FoxO1 in granulosa cell apoptosis caused by oxidative stress and protective effects of grape seed procyanidin B2. Oxid. Med. Cell Longev. 2016, 2016, 6147345. [Google Scholar] [CrossRef]
- Cui, C.; Han, S.; Yin, H.; Luo, B.; Shen, X.; Yang, F.; Liu, Z.; Zhu, Q.; Li, D.; Wang, Y. FOXO3 is expressed in ovarian tissues and acts as an apoptosis initiator in granulosa cells of chickens. Biomed. Res. Int. 2019, 2019, 6902906. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Rudd, M.D.; Hernandez-Gonzalez, I.; Gonzalez-Robayna, I.; Fan, H.Y.; Zeleznik, A.J.; Richards, J.S. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol. Endocrinol. 2009, 23, 649–661, Erratum in Mol. Endocrinol. 2009, 23, 1522. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Benco, A.; Tandlmajerova, A.; Vasícek, D.; Kotwica, J.; Darlak, K.; Valenzuela, F. Transcription factor p53 can regulate proliferation, apoptosis and secretory activity of luteinizing porcine ovarian granulosa cell cultured with and without ghrelin and FSH. Reproduction 2008, 136, 611–618. [Google Scholar] [CrossRef]
- Abdelhamed, A.; Ezz El-Dawla, R.; Karadag, A.S.; Agamia, N.F.; Melnik, B.C. The impact of isotretinoin on the pituitary-ovarian axis: An interpretative review of the literature. Reprod. Toxicol. 2021, 104, 85–95. [Google Scholar] [CrossRef]
- Marsden, J. Hyperlipidaemia due to isotretinoin and etretinate: Possible mechanisms and consequences. Br. J. Dermatol. 1986, 114, 401–407. [Google Scholar] [CrossRef]
- Melnik, B.; Bros, U.; Plewig, G. Characterization of apoprotein metabolism and atherogenic lipoproteins during oral isotretinoin treatment. Dermatologica 1987, 175 (Suppl. 1), 158–168. [Google Scholar] [CrossRef]
- Gustafson, S.; Vahlquist, C.; Sjöblom, L.; Eklund, A.; Vahlquist, A. Metabolism of very low density lipoproteins in rats with isotretinoin (13-cis retinoic acid)-induced hyperlipidemia. J. Lipid Res. 1990, 31, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Newberry, E.P.; Norris, J.Y.; Xie, Y.; Luo, J.; Kennedy, S.M.; Davidson, N.O. ApoB100 is required for increased VLDL-triglyceride secretion by microsomal triglyceride transfer protein in ob/ob mice. J. Lipid Res. 2008, 49, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, S.O.; Borén, J. Apolipoprotein B secretory regulation by degradation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1334–1338. [Google Scholar] [CrossRef]
- Ashur-Fabian, O.; Har-Zahav, A.; Shaish, A.; Wiener Amram, H.; Margalit, O.; Weizer-Stern, O.; Dominissini, D.; Harats, D.; Amariglio, N.; Rechavi, G. apoB and apobec1, two genes key to lipid metabolism, are transcriptionally regulated by p53. Cell Cycle 2010, 9, 3761–3770. [Google Scholar] [CrossRef] [PubMed]
- Kamagate, A.; Dong, H.H. FoxO1 integrates insulin signaling to VLDL production. Cell Cycle 2008, 7, 3162–3170. [Google Scholar] [CrossRef]
- Altomonte, J.; Cong, L.; Harbaran, S.; Richter, A.; Xu, J.; Meseck, M.; Dong, H.H. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J. Clin. Investig. 2004, 114, 1493–1503. [Google Scholar] [CrossRef]
- Peng, S.; Li, W.; Hou, N.; Huang, N. A Review of FoxO1-regulated metabolic diseases and related drug discoveries. Cells 2020, 9, 184. [Google Scholar] [CrossRef]
- Vu-Dac, N.; Gervois, P.; Torra, I.P.; Fruchart, J.C.; Kosykh, V.; Kooistra, T.; Princen, H.M.; Dallongeville, J.; Staels, B. Retinoids increase human apo C-III expression at the transcriptional level via the retinoid X receptor. Contribution to the hypertriglyceridemic action of retinoids. J. Clin. Investig. 1998, 102, 625–632. [Google Scholar] [CrossRef]
- Mendivil, C.O.; Zheng, C.; Furtado, J.; Lel, J.; Sacks, F.M. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 239–245, Erratum in Arterioscler. Thromb. Vasc. Biol. 2010, 30, e171. [Google Scholar] [CrossRef]
- Larsson, M.; Vorrsjö, E.; Talmud, P.; Lookene, A.; Olivecrona, G. Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J. Biol. Chem. 2013, 288, 33997–34008. [Google Scholar] [CrossRef]
- Larsson, M.; Allan, C.M.; Jung, R.S.; Heizer, P.J.; Beigneux, A.P.; Young, S.G.; Fong, L.G. Apolipoprotein C-III inhibits triglyceride hydrolysis by GPIHBP1-bound LPL. J. Lipid Res. 2017, 58, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Çölgeçen, E.; Özyurt, K.; Ferahbaş Kesikoğlu, A. The effect of systemic isotretinoin treatment on skin biophysical parameters among patients with acne vulgaris. Turk. J. Med. Sci. 2016, 46, 1641–1644. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Liao, W.; Jiang, P.; Xu, W.; Jin, X. Effect of retinoic acid on aquaporin 3 expression in keratinocytes. Genet. Mol. Res. 2016, 15, 15016951. [Google Scholar] [CrossRef]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the epidermis: More than skin deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef]
- Choudhary, V.; Olala, L.O.; Kagha, K.; Pan, Z.Q.; Chen, X.; Yang, R.; Cline, A.; Helwa, I.; Marshall, L.; Kaddour-Djebbar, I.; et al. Regulation of the glycerol transporter, aquaporin-3, by histone deacetylase-3 and p53 in keratinocytes. J. Investig. Dermatol. 2017, 137, 1935–1944. [Google Scholar] [CrossRef]
- Fraunfelder, F.W.; Fraunfelder, T.; Corbett, J.J. Isotretinoin-associated intracranial hypertension. Ophthalmology 2004, 111, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Reifenrath, J.; Rupprecht, C.; Gmeiner, V.; Haslinger, B. Intracranial hypertension after rosacea treatment with isotretinoin. Neurol. Sci. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Uldall, M.; Bhatt, D.K.; Kruuse, C.; Juhler, M.; Jansen-Olesen, I.; Jensen, R.H. Choroid plexus aquaporin 1 and intracranial pressure are increased in obese rats: Towards an idiopathic intracranial hypertension model? Int. J. Obes. 2017, 41, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Bushue, N.; Wan, Y.J. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298. [Google Scholar] [CrossRef]
- González-Marrero, I.; Hernández-Abad, G.; González-Gómez, M.; Soto-Viera, M.; Carmona-Calero, E.M.; Castañeyra-Ruiz, L.; Castañeyra-Perdomo, A. Altered expression of AQP1 and AQP4 in brain barriers and cerebrospinal fluid may affect cerebral water balance during chronic hypertension. Int. J. Mol. Sci. 2022, 23, 12277. [Google Scholar] [CrossRef]
- Stiebel-Kalish, H.; Eyal, S.; Steiner, I. The role of aquaporin-1 in idiopathic and drug-induced intracranial hypertension. Med. Hypotheses 2013, 81, 1059–1062. [Google Scholar] [CrossRef]
- Lv, W.; Xue, L.; Liang, L.; Liu, D.; Li, C.; Liao, J.; Jin, Y. Endotoxin induced acute kidney injury modulates expression of AQP1, P53 and P21 in rat kidney, heart, lung and small intestine. PLoS ONE 2023, 18, e0288507. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Rodríguez, I.; Sánchez Silva, R.; Martins, A.P.; Soveral, G.; Toledo-Aral, J.J.; López-Barneo, J.; Echevarría, M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1α. PLoS ONE 2011, 6, e28385. [Google Scholar] [CrossRef] [PubMed]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef]
- ElAttar, Y.; Mourad, B.; Alngomy, H.A.; Shams El Deen, A.; Ismail, M. Study of interleukin-1 beta expression in acne vulgaris and acne scars. J. Cosmet. Dermatol. 2022, 21, 4864–4870. [Google Scholar] [CrossRef]
- Thanh, L.T.V.; Anh, L.V.; Giang, T.H.; Hung, T.Q.; Trung, V.T.; Vuong, N.L. Immunohistochemical expression of interleukin 1 beta in papule biopsies from patients with acne vulgaris. Dermatol. Reports 2022, 14, 9444. [Google Scholar] [CrossRef]
- Denes, A.; Lopez-Castejon, G.; Brough, D. Caspase-1: Is IL-1 just the tip of the ICEberg? Cell Death Dis. 2012, 3, e338. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Ogura, Y.; Szczepanik, M.; Lara-Tejero, M.; Lichtenberger, G.S.; Grant, E.P.; Bertin, J.; Coyle, A.J.; Galán, J.E.; Askenase, P.W.; et al. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006, 24, 317–327. [Google Scholar] [CrossRef]
- Gupta, S.; Radha, V.; Furukawa, Y.; Swarup, G. Direct transcriptional activation of human caspase-1 by tumor suppressor p53. J. Biol. Chem. 2001, 276, 10585–10588. [Google Scholar] [CrossRef]
- Borghi, A.; Mantovani, L.; Minghetti, S.; Virgili, A.; Bettoli, V. Acute acne flare following isotretinoin administration: Potential protective role of low starting dose. Dermatology 2009, 218, 178–180. [Google Scholar] [CrossRef]
- Fakih, A.; Goens, J.; Grozdev, I.; Dangoisse, C.; Richert, B. Acne fulminans induced by a low dose isotretinoin: Case report and review of the literature. Dermatol. Online J. 2020, 26, 13030/qt14h2419w. [Google Scholar] [CrossRef]
- Yorulmaz, A.; Hayran, Y. Isotretinoin-induced acne fulminans. J. Cutan. Med. Surg. 2022, 26, 435. [Google Scholar] [CrossRef]
- Lee, K.H.; Kang, T.B. The molecular links between cell death and inflammasome. Cells 2019, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.P.; Crawford, L.V. T antigen is bound to a host protein in SV40-transformed cells. Nature 1979, 278, 261–263. [Google Scholar] [CrossRef]
- Linzer, D.I.; Levine, A.J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 1979, 17, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kress, M.; May, E.; Cassingena, R.; May, P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J. Virol. 1979, 31, 472–483. [Google Scholar] [CrossRef]
- DeLeo, A.B.; Jay, G.; Appella, E.; Dubois, G.C.; Law, L.W.; Old, L.J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc. Natl. Acad. Sci. USA 1979, 76, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Seltmann, H.; Neitzel, H.; Orfanos, C.E. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95). J. Investig. Dermatol. 1999, 113, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.; Jabara, S.; McAllister, J.M.; Sivarajah, A.; Gilliland, K.; Cong, Z.; Clawson, G. Human skin is a steroidogenic tissue: Steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J. Investig. Dermatol. 2003, 120, 905–914. [Google Scholar] [CrossRef]
- Bargonetti, J.; Reynisdóttir, I.; Friedman, P.N.; Prives, C. Site-specific binding of wild-type p53 to cellular DNA is inhibited by SV40 T antigen and mutant p53. Genes Dev. 1992, 6, 1886–1898. [Google Scholar] [CrossRef]
- Lane, D.P. Cancer. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef]
- Vousden, K.H.; Ryan, K.M. p53 and metabolism. Nat. Rev. Cancer 2009, 9, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Ezra, O.; Rivlin, N.; Molchadsky, A.; Madar, S.; Goldfinger, N.; Rotter, V. p53, a novel regulator of lipid metabolism pathways. J. Hepatol. 2012, 56, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Linares, L.K.; Rueda-Rincon, N.; Bloch, K.; Di Michele, M.; De Blasio, C.; Fau, C.; Gayte, L.; Blanchet, E.; Mairal, A.; et al. The multifunctional protein E4F1 links P53 to lipid metabolism in adipocytes. Nat. Commun. 2021, 12, 7037. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Hu, W.; Feng, Z. Tumor suppressor p53 and metabolism. J. Mol. Cell Biol. 2019, 11, 284–292. [Google Scholar] [CrossRef]
- Lacroix, M.; Riscal, R.; Arena, G.; Linares, L.K.; Le Cam, L. Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer. Mol. Metab. 2020, 33, 2–22. [Google Scholar] [CrossRef]
- Yahagi, N.; Shimano, H.; Matsuzaka, T.; Najima, Y.; Sekiya, M.; Nakagawa, Y.; Ide, T.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. p53 Activation in adipocytes of obese mice. J. Biol. Chem. 2003, 278, 25395–25400. [Google Scholar] [CrossRef]
- Oren, M. Decision making by p53: Life, death and cancer. Cell Death Differ. 2003, 10, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef]
- Shen, Y.; White, E. p53-dependent apoptosis pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar] [CrossRef]
- Zamzami, N.; Kroemer, G. p53 in apoptosis control: An introduction. Biochem. Biophys. Res. Commun. 2005, 331, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Meulmeester, E.; Jochemsen, A.G. p53: A guide to apoptosis. Curr. Cancer Drug Targets 2008, 8, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Seltmann, H.; Fimmel, S.; Müller-Decker, K.; Tsukada, M.; Bogdanoff, B.; Mandt, N.; Blume-Peytavi, U.; Orfanos, C.E.; Zouboulis, C.C. Differentiation and apoptosis in human immortalized sebocytes. J. Investig. Dermatol. 2003, 120, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Eliasz, S.; De Marco, M.A.; Rudzinski, J.; Zhang, L.; Carbone, M. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 2008, 68, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Agamia, N.F.; Chen, W.; De Vita, V.; Karadag, A.S.; Plewig, G.; Schmitz, G. Isotretinoin’s paradoxical effects in immortalized sebocytes. Br. J. Dermatol. 2019, 180, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Coronel, L.; Somalanka, B.; Raju, A.; Aning, O.A.; An, O.; Ho, Y.S.; Chen, S.; Mak, S.Y.; Hor, P.Y.; et al. Mitochondrial uncoupling reveals a novel therapeutic opportunity for p53-defective cancers. Nat. Commun. 2018, 9, 3931. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Benoit, A.M.; Downing, D.T.; Strauss, J.S. Suppression of sebum secretion with 13-cis-retinoic acid: Effect on individual skin surface lipids and implications for their anatomic origin. J. Investig. Dermatol. 1984, 82, 74–78. [Google Scholar] [CrossRef]
- Landthaler, M.; Kummermehr, J.; Wagner, A.; Plewig, G. Inhibitory effects of 13-cis-retinoic acid on human sebaceous glands. Arch. Dermatol. Res. 1980, 269, 297–309. [Google Scholar] [CrossRef]
- Hoover, E.; Aslam, S.; Krishnamurthy, K. Physiology, sebaceous glands. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fischer, H.; Fumicz, J.; Rossiter, H.; Napirei, M.; Buchberger, M.; Tschachler, E.; Eckhart, L. Holocrine secretion of sebum is a unique DNase2-dependent mode of programmed cell death. J. Investig. Dermatol. 2017, 137, 587–594. [Google Scholar] [CrossRef]
- Tarayrah-Ibraheim, L.; Maurice, E.C.; Hadary, G.; Ben-Hur, S.; Kolpakova, A.; Braun, T.; Peleg, Y.; Yacobi-Sharon, K.; Arama, E. DNase II mediates a parthanatos-like developmental cell death pathway in Drosophila primordial germ cells. Nat. Commun. 2021, 12, 2285. [Google Scholar] [CrossRef]
- Xiong, C.; Ling, H.; Hao, Q.; Zhou, X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023, 30, 876–884. [Google Scholar] [CrossRef]
- Stambolsky, P.; Weisz, L.; Shats, I.; Klein, Y.; Goldfinger, N.; Oren, M.; Rotter, V. Regulation of AIF expression by p53. Cell Death Differ. 2006, 13, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. The TRAIL to acne pathogenesis: Let’s focus on death pathways. Exp. Dermatol. 2017, 26, 270–272. [Google Scholar] [CrossRef]
- Cordain, L.; Lindeberg, S.; Hurtado, M.; Hill, K.; Eaton, S.B.; Brand-Miller, J. Acne vulgaris: A disease of Western civilization. Arch. Dermatol. 2002, 138, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172 (Suppl. 1), 3–12. [Google Scholar] [CrossRef] [PubMed]
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers 2015, 1, 15029. [Google Scholar] [CrossRef]
- Dréno, B.; Bettoli, V.; Araviiskaia, E.; Sanchez Viera, M.; Bouloc, A. The influence of exposome on acne. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 812–819. [Google Scholar] [CrossRef]
- Melnik, B.C. Diet in acne: Further evidence for the role of nutrient signalling in acne pathogenesis. Acta Derm. Venereol. 2012, 92, 228–231. [Google Scholar] [CrossRef]
- Melnik, B.C. Linking diet to acne metabolomics, inflammation, and comedogenesis: An update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 371–388. [Google Scholar] [CrossRef]
- Peck, G.L.; Olsen, T.G.; Yoder, F.W.; Strauss, J.S.; Downing, D.T.; Pandya, M.; Butkus, D.; Arnaud-Battandier, J. Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N. Engl. J. Med. 1979, 300, 329–333. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Ma, Y.; Wright, G.M.; Huang, M.; Irby, R.; Briggs, J.; Karras, J.; Cress, W.D.; Pardoll, D.; et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell Biol. 2005, 25, 7432–7440. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Weiskirchen, R.; Schmitz, G. The endocrine and epigenetic impact of persistent cow milk consumption on prostate carcinogenesis. J. Transl. Genet. Genom. 2022, 6, 1–45. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Carrera-Bastos, P.; Cordain, L.; Leitzmann, C.; Weiskirchen, R.; Schmitz, G. The role of cow’s milk consumption in breast cancer initiation and progression. Curr. Nutr. Rep. 2023, 12, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Lifetime impact of cow’s milk on overactivation of mTORC1: From fetal to childhood overgrowth, acne, diabetes, cancers, and neurodegeneration. Biomolecules 2021, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, S.; Giovannucci, E.; Isaacs, W.B.; Willett, W.C.; Platz, E.A. Acne and risk of prostate cancer. Int. J. Cancer 2007, 121, 2688–2692. [Google Scholar] [CrossRef]
- Ugge, H.; Udumyan, R.; Carlsson, J.; Andrén, O.; Montgomery, S.; Davidsson, S.; Fall, K. Acne in late adolescence and risk of prostate cancer. Int. J. Cancer 2018, 142, 1580–1585. [Google Scholar] [CrossRef]
- Murphy, J.D.; Sandler, D.; White, A.J.; O’Brien, K.M. Severe acne and risk of breast cancer. Breast Cancer Res. Treat. 2019, 177, 487–495. [Google Scholar] [CrossRef]
- Peck, G.L.; Gross, E.G.; Butkus, D.; DiGiovanna, J.J. Chemoprevention of basal cell carcinoma with isotretinoin. J. Am. Acad. Dermatol. 1982, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.; Holdener, E.E. Retinoids in cancer prevention and therapy. Ann. Oncol. 1992, 3, 513–526. [Google Scholar] [CrossRef]
- Bettoli, V.; Zauli, S.; Virgili, A. Retinoids in the chemoprevention of non-melanoma skin cancers: Why, when and how. J. Dermatolog. Treat. 2013, 24, 235–237. [Google Scholar] [CrossRef]
- Veal, G.J.; Tweddle, D.A.; Visser, J.; Errington, J.; Buck, H.; Marange, J.; Moss, J.; Joseph, S.; Mulla, H. Pharmacokinetics and safety of a novel oral liquid formulation of 13-cis retinoic acid in children with neuroblastoma: A randomized crossover clinical trial. Cancers 2021, 13, 1868. [Google Scholar] [CrossRef] [PubMed]
- Pile, H.D.; Sadiq, N.M. Isotretinoin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]



| Gene | Gene Name | References |
|---|---|---|
| AR | Androgen receptor ↓ | [80] |
| IGF1 | Insulin-like growth factor 1 ↓ | [191] |
| IGF1R | Insulin-like growth factor I receptor ↓ | [193] |
| BIRC5 | Baculoviral IAP repeat-containing protein 5 ↓ | [220] |
| SREBF1 | Sterol regulatory element-binding transcription factor 1 ↓ | [350] |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21) ↑ | [117] |
| FOXO1A | Forkhead box O1A ↑ | [177] |
| FOXO3A | Forkhead box O3A ↑ | [178] |
| PTEN | Phosphatase and tensin homog ↑ | [195] |
| PRKAB1 | Protein kinase, AMP-activated, noncatalytic, β-1 ↑ | [195] |
| TSC2 | TSC complex subunit 2 ↑ | [195] |
| DEPDC6 | DEP domain-containg protein 6 (DEPTOR) ↑ | [197] |
| TNFSF10 | Tumor necrosis factor ligand superfamily, member 10 ↑ | [217] |
| ATG7 | Autophagy-related 7 ↑ | [231] |
| APOB | Apolipoprotein B ↑ | [307] |
| AQP3 | Aquaporin 3 ↑ | [318] |
| AQP1 | Aquaporin 1 ↑ | [326] |
| CASP1 | Caspase 1 ↑ | [332] |
| ALOX5 | Arachidonate 5-lipoxigenate ↑ | [359] |
| AIFM1 | Apoptosis-inducing factor, mitochondria associated ↑ | [366] |
| Gene Symbol | Gene Name | References |
|---|---|---|
| AR | Androgen receptor ↓ | [23] |
| SREBF1 | Sterol regulatory element-binding transcription factor 1 ↓ | [24] |
| PPARG | Peroxisome proliferator-activated receptor gamma γ ↓ | [25] |
| STAT3 | Signal transducer and activator of transcription ↓ | [26] |
| PRDM1 | PR domain-containing protein 1 (BLIMP1) ↓ | [168] |
| FSHB | FSH β-polypeptide downregulation ↓ | [289] |
| LHB | β-subunit of LH downregulation ↓ | [290] |
| GATA6 | GATA-binding protein 6 ↑ | [112,114] |
| FASLG | Fas ligand ↑ | [278] |
| MTTP | Microsomal triglyceride transfer protein ↑ | [308] |
| APOC3 | Apolipoprotein C-III ↑ | [309,310] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnik, B.C. Acne Transcriptomics: Fundamentals of Acne Pathogenesis and Isotretinoin Treatment. Cells 2023, 12, 2600. https://doi.org/10.3390/cells12222600
Melnik BC. Acne Transcriptomics: Fundamentals of Acne Pathogenesis and Isotretinoin Treatment. Cells. 2023; 12(22):2600. https://doi.org/10.3390/cells12222600
Chicago/Turabian StyleMelnik, Bodo C. 2023. "Acne Transcriptomics: Fundamentals of Acne Pathogenesis and Isotretinoin Treatment" Cells 12, no. 22: 2600. https://doi.org/10.3390/cells12222600