Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients
Abstract
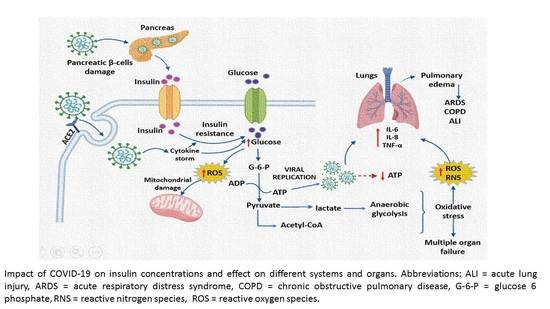
:1. Introduction
2. Materials and Methods
2.1. Study Type
2.2. Collection of Samples to Verify Infection by SARS-CoV-2 upon Admission to the Hospital
2.3. Detection in Plasma of the N and S Protein Antibodies of the SARS-CoV-2 Virus
2.4. Laboratory Tests
2.5. Glucose, Insulin, and HOMA-IR Concentrations
2.6. 8-Isoprostane, Vitamin D, H2S, and 3-Nitrotyrosine Concentrations
2.7. Oxidative Stress Markers
2.7.1. Nitrites (NO2–)
2.7.2. Lipid Peroxidation Levels (LPO)
2.7.3. Evaluation of Total Antioxidant Capacity (TAC)
2.7.4. Thiol Concentrations
2.7.5. Glutathione Levels (GSH)
2.7.6. Selenium
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Detection of Antibodies against the N and S Proteins of the SARS-CoV-2 Virus
3.3. Glucose, Insulin and HOMA-IR Concentrations
3.4. 8-Isoprostane, Vitamin D, H2S, and 3-Nitrotyrosine Concentrations
3.5. Oxidative Stress Markers
3.6. Selenium
4. Discussion
5. Conclusions
5.1. Study Limitations
5.2. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soto, M.E.; Guarner-Lans, V.; Soria-Castro, E.; Manzano-Pech, L.; Pérez-Torres, I. Is antioxidant therapy a useful complementary measure for COVID-19 treatment? An algorithm for its application. Medicina 2020, 56, 386. [Google Scholar] [CrossRef] [PubMed]
- Landstra, C.P.; de Koning, E.J.P. COVID-19 and diabetes: Understanding the interrelationship and risks for a severe course. Front. Endocrinol. 2021, 12, 649525. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Manzano-Pech, L.; Rubio-Ruíz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative stress and its association with cardiometabolic disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S.; Stephan, G. COVID-19 and diabetes: The why, the what and the how. J. Diabetes Complicat. 2020, 34, 107637. [Google Scholar] [CrossRef] [PubMed]
- Soria-Castro, E.; Soto, M.E.; Guarner-Lans, V.; Rojas, G.; Perezpeña-Diazconti, M.; Críales-Vera, S.A.; Manzano-Pech, L.; Pérez-Torres, I. The kidnapping of mitochondrial function associated with the SARS-CoV-2 infection. Histol. Histopathol. 2021, 36, 947–965. [Google Scholar]
- Mahrooz, A.; Muscogiuri, G.; Buzzetti, R.; Maddaloni, E. The complex combination of COVID-19 and diabetes: Pleiotropic changes in glucose metabolism. Endocrine 2021, 72, 317–325. [Google Scholar] [CrossRef]
- Barberis, E.; Timo, S.; Amede, E.; Vanella, V.V.; Puricelli, C.; Cappellano, G.; Raineri, D.; Cittone, M.G.; Rizzi, E.; Pedrinelli, A.R.; et al. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 8623. [Google Scholar] [CrossRef]
- Ceriello, A.; De Nigris, V.; Prattichizzo, F. Why is hyperglycaemia worsening COVID-19 and its prognosis? Diabetes Obes. Metab. 2020, 22, 1951–1952. [Google Scholar] [CrossRef]
- Paul, A.K.; Hossain, M.K.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; de Lourdes, P.M.; et al. Does oxidative stress management help alleviation of COVID-19 symptoms in patients experiencing diabetes? Nutrients 2022, 14, 321. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Mesta, F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Chavarría, A.P.; Vázquez, R.R.V.; Cherit, J.G.D.; Bello, H.H.; Suastegui, H.C.; Moreno-Castañeda, L.; Alanís-Estrada, G.; Hernández, F.; González-Marcos, O.; Saucedo-Orozco, H.; et al. Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Comput. Struct. Biotechnol. J. 2021, 19, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility, and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horovitz, J.H.; Carrico, C.J.; Shires, G.T. Pulmonary response to major injury. Arch. Surg. 1974, 108, 349–355. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Jiménez, I.U.; Díaz-Díaz, E.; Castro, J.S.; Ramos, J.P.; León, M.C.; Alvarado-Ríos, J.A.; Auriostigue, B.J.C.; Correa-Rotter, R.; Aguilar, S.C.A.; Larrea, F. Circulating concentrations of advanced glycation end products, its association with the development of diabetes mellitus. Arch. Med. Res. 2017, 48, 360–369. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt Ueber einige Azoverbindungen. Ber. Deutsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef] [Green Version]
- Manzano-Pech, L.G.; Caballero-Chacón, S.C.; Guarner-Lans, V.; Díaz-Díaz, E.; Moreno, G.A.; Pérez-Torres, I. Effect of oophorosalpingo-hysterectomy on serum antioxidant enzymes in female dogs. Sci. Rep. 2019, 9, 9674. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef]
- Soto, M.E.; Manzano-Pech, L.G.; Guarner-Lans, V.; Díaz-Galindo, J.A.; Vásquez, X.; Castrejón-Tellez, V.; Gamboa, R.; Huesca, C.; Fuentevilla-Alvárez, G.; Pérez-Torres, I. Oxidant/antioxidant profile in the thoracic aneurysm of patients with the Loeys-Dietz syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 5392454. [Google Scholar] [CrossRef] [PubMed]
- González-Fiallo, S.; Mena-Rodríguez, I.; Doeste-Hernández, V.M.; Castro-Batista, P.; Espinosa-Reyes, S. COVID-19 rapid test validation. Isla de la Juventud, Cuba. Vaccimonitor 2021, 30, 105–114. [Google Scholar]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.A.; Groß, R.; Conzelmann, C.; Krüger, J.; Merle, U.; Steinhart, J.; Weil, T.; Koepke, L.; Bozzo, C.P.; Read, C.; et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021, 3, 149–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Xia, H.; Guo, J.; He, K.; Huang, C.; Luo, R.; Chen, Y.; Xu, K.; Gao, H.; et al. Identification of monocytes associated with severe COVID-19 in the PBMCs of severely infected patients through single-cell transcriptome sequencing. Engineering 2021, in press. [Google Scholar] [CrossRef]
- Tang, X.; Uhl, S.; Zhang, T.; Xue, D.; Bo, L.; Vandana, J.J.; Acklin, J.A.; Bonnycastle, L.L.; Narisu, N.; Erdos, M.R.; et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell. Metab. 2021, 33, 1577–1591.e7. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV 2 infects human pancreatic beta cells and elicits beta cell impairment. Cell. Metab. 2021, 33, 1565–1576.e5. [Google Scholar] [CrossRef]
- Montefusco, L.; Ben-Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef]
- Huang, J.F.; Yu, M.L.; Dai, C.Y.; Chuang, W.L. Glucose abnormalities in hepatitis C virus infection. Kaohsiung J. Med. Sci. 2013, 29, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, Y.; Mizuta, T. Interaction between hepatitis C virus and metabolic factors. World. J. Gastroenterol. 2014, 20, 2888–2901. [Google Scholar] [CrossRef]
- Douglas, M.W.; George, J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World. J. Gastroenterol. 2009, 15, 4356–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, R.P. Beta-cell deterioration during diabetes: What’s in the gun? Trends Endocrinol. Metab. 2009, 20, 388–393. [Google Scholar]
- Clark, A.L.; Mirmira, R.G. SARS-CoV-2 infection of islet β cells: Evidence and implications. Cell. Rep. Med. 2021, 2, 100380. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.G.; Cheng, X.; Qin, J.J.; Zhang, X.J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef]
- Yu, Y.; Maguire, T.G.; Alwine, J.C. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J. Virol. 2011, 85, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanti, J.F.; Jager, J. Cellular mechanisms of insulin resistance: Role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr. Opin. Pharmacol. 2009, 9, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Aytug, S.; Reich, D.; Sapiro, L.E.; Bernstein, D.; Begum, N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: A mechanism for increased prevalence of type 2 diabetes. Hepatology 2003, 38, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Fujie, H.; Miyoshi, H.; Tsutsumi, T.; Tsukamoto, K.; Kimura, S.; Moriya, K.; Koike, K. Hepatitis C virus infection and diabetes: Direct involvement of the virus in the development of insulin resistance. Gastroenterology 2004, 126, 840–848. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Gutiérrez-Alvarez, Y.; Guarner-Lans, V.; Díaz-Díaz, E.; Manzano-Pech, L.; Caballero-Chacón, S.D.C. Intra-abdominal fat adipocyte hypertrophy through a progressive alteration of lipolysis and lipogenesis in metabolic syndrome rats. Nutrients 2019, 11, 1529. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, F.; Khodadadi, I.; Vafaei, S.A.; Abbasi-Oshaghi, E.; Tayebinia, H.; Farahani, F. Importance of hyperglycemia in COVID-19 intensive-care patients: Mechanism and treatment strategy. Prim. Care. Diabetes 2021, 15, 409–416. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res. Clin. Pract. 2020, 167, 108382. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.; Khaliq, O.P.; Moodley, J.; Naicker, T. Insulin resistance in COVID-19 and diabetes. Prim. Care. Diabetes 2021, 15, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 diabetes mellitus on organ metabolism and the immune system. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Magro, D.O.; Evangelista-Poderoso, R.; Saad, M.J.A. Diabetes, obesity, and insulin resistance in COVID-19: Molecular interrelationship and therapeutic implications. Diabetol. Metab. Syndr. 2021, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Cory, T.J.; Emmons, R.S.; Yarbro, J.R.; Davis, K.L.; Pence, B.D. Metformin suppresses monocyte immunometabolic activation by SARS-CoV-2 spike protein subunit 1. Front. Immunol. 2021, 12, 733921. [Google Scholar] [CrossRef]
- Zangiabadian, M.; Nejadghaderi, S.A.; Zahmatkesh, M.M.; Hajikhani, B.; Mirsaeidi, M.; Nasiri, M.J. The Efficacy and potential mechanisms of metformin in the treatment of COVID-19 in the diabetics: A systematic review. Front. Endocrinol. 2021, 12, 645194. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Toyoda, S.; Nishitani, S.; Fukuhara, A.; Kita, S.; Otsuki, M.; Shimomura, I. Possible involvement of adipose tissue in patients with older age, obesity, and diabetes with SARS-CoV-2 infection (COVID-19) via GRP78 (BIP/HSPA5): Significance of hyperinsulinemia management in COVID-19. Diabetes 2021, 70, 2745–2755. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Guarner-Lans, V.; Soria-Castro, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Domínguez-Cherit, J.G.; Herrera-Bello, H.; Castillejos-Suastegui, H.; Moreno-Castañeda, L.; et al. Alteration in the lipid profile and the desaturases activity in patients with severe pneumonia by SARS-CoV-2. Front. Physiol. 2021, 12, 667024. [Google Scholar] [CrossRef]
- Montuschi, P.; Corradi, M.; Ciabattoni, G.; Nightingale, J.; Kharitonov, S.A.; Barnes, P.J. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am. J. Respir. Crit. Care. Med. 1999, 160, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Praticò, D.; Basili, S.; Vieri, M.; Cordova, C.; Violi, F.; Fitzgerald, G.A. Chronic obstructive pulmonary disease is associated with an increase in urinary levels of isoprostane F2α-III, an index of oxidant stress. Am. J. Respir. Crit. Care. Med. 1998, 158, 1709–1714. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.C.; Chen, Y.Y.; Hsiao, T.C.; Chou, H.C.; Kuo, H.P.; Feng, P.H.; Ho, S.C.; Chen, J.K.; Chuang, K.J.; Lee, K.Y. Alteration in angiotensin-converting enzyme 2 by PM1 during the development of emphysema in rats. ERJ. Open. Res. 2020, 6, 00174–02020. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alblihed, M.; Guerreiro, S.G.; Cruz-Martins, N.; Batiha, G.E. COVID-19 in relation to hyperglycemia and diabetes mellitus. Front. Cardiovasc. Med. 2021, 8, 644095. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, N.; Youssefi, M.; Azad, F.J.; Farzad, F.; Yavari, Z.; Avval, F.Z. Total antioxidant capacity as a marker of severity of COVID-19 infection: Possible prognostic and therapeutic clinical application. J. Med.Virol. 2022, 94, 1558–1565. [Google Scholar] [CrossRef]
- Kouhpayeh, S.; Shariati, L.; Boshtam, M.; Rahimmanesh, I.; Mirian, M.; Esmaeili, Y.; Najaflu, M.; Khanahmad, N.; Zeinalian, M.; Trovato, M.; et al. The Molecular basis of COVID-19 pathogenesis, conventional and nanomedicine therapy. Int. J. Mol. Sci. 2021, 22, 5438. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Kaur, S.; Kaur, C.; Prabha, P.K.; Bhatacharya, A.; Sarma, P.; Medhi, B. Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: A systematic review. Indian. J. Pharmacol. 2021, 53, 236–243. [Google Scholar] [PubMed]
- Wang, J.; Mei, F.; Bai, L.; Zhou, S.; Liu, D.; Yao, L.; Ahluwalia, A.; Ghiladi, R.A.; Su, L.; Shu, T.; et al. Serum nitrite and nitrate: A potential biomarker for post-COVID-19 complications? Free. Radic. Biol. Med. 2021, 175, 216–225. [Google Scholar] [CrossRef]
- Kalem, A.K.; Kayaaslan, B.; Neselioglu, S.; Eser, F.; Hasanoglu, İ.; Aypak, A.; Akinci, E.; Akca, H.N.; Erel, O.; Guner, R.A. Useful and sensitive marker in the prediction of COVID-19 and disease severity: Thiol. Free. Radic. Biol. Med. 2021, 166, 11–17. [Google Scholar] [CrossRef]
- Ibrahim, H.; Perl, A.; Smith, D.; Lewis, T.; Kon, Z.; Goldenberg, R.; Yarta, K.; Staniloae, C.; Williams, M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin. Immunol. 2020, 219, 108544. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Offringa, A.K.; van Eijk, L.E.; Abdulle, A.E.; Hillebrands, J.L.; van der Voort, P.H.J.; van Goor, H.; van Hezik, E.J. N-Acetylcysteine and hydrogen sulfide in coronavirus disease 2019. Antioxid. Redox. Signal. 2021, 35, 1207–1225. [Google Scholar] [CrossRef]
- Parimala, N.; Pandey, V.; Phanithi, P.B. Role of nitric oxide and hydrogen sulfide in ischemic stroke and the emergent epigenetic underpinnings. Mol. Neurobiol. 2019, 56, 1749–1769. [Google Scholar]
- Ming, Y.; Lai, C.L. SARS-CoV-2 infection: Can ferroptosis be a potential treatment target for multiple organ involvement? Cell Death Discov. 2020, 6, 130. [Google Scholar]
- Grishin, A.M.; Dolgova, N.V.; Landreth, S.; Fisette, O.; Pickering, I.J.; George, G.N.; Falzarano, D.; Cygler, M. Disulfide bonds play a critical role in the structure and function of the receptor-binding domain of the SARS-CoV-2 Spike antigen. J. Mol. Biol. 2022, 434, 167357. [Google Scholar] [CrossRef] [PubMed]
- Erel, O.; Neşelioğlu, S.; Tunçay, M.E.; Oğuz, E.F.; Eren, F.; Akkuş, M.S.; Güner, H.R.; Ateş, İ. A sensitive indicator for the severity of COVID-19: Thiol. Turk. J. Med. Sci. 2021, 51, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Mösbauer, K.; Fritsch, V.N.; Adrian, L.; Bernhardt, J.; Gruhlke, M.C.H.; Slusarenko, A.J.; Niemeyer, D.; Antelmann, H. The effect of allicin on the proteome of SARS-CoV-2 infected Calu-3 Cells. Front. Microbiol. 2021, 12, 746795. [Google Scholar] [CrossRef] [PubMed]
- Yang, G. H2S as a potential defense against COVID-19? Am. J. Physiol. Cell. Physiol. 2020, 319, C244–C249. [Google Scholar] [CrossRef] [PubMed]
- Renieris, G.; Katrini, K.; Damoulari, C.; Akinosoglou, K.; Psarrakis, C.; Kyriakopoulou, M.; Dimopoulos, G.; Lada, M.; Koufargyris, P.; Giamarellos-Bourboulis, E.J. Serum hydrogen sulfide and outcome association in pneumonia by the SARS-CoV-2 coronavirus. Shock 2020, 54, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Ghelani, D.; Alesi, S.; Mousa, A. Vitamin D and COVID-19: An overview of recent evidence. Int. J. Mol. Sci. 2021, 22, 10559. [Google Scholar] [CrossRef]
- Cooper, I.D.; Crofts, C.A.P.; DiNicolantonio, J.J.; Malhotra, A.; Elliott, B.; Kyriakidou, Y.; Brookler, K.H. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: Rationale for clinical management. Open Heart 2020, 7, e001356. [Google Scholar] [CrossRef]
- Song, Y.; Qayyum, S.; Greer, R.A.; Slominski, R.M.; Raman, C.; Slominski, A.T.; Song, Y. Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: A computational study. J. Biomol. Struct. Dyn. 2021, 1–17. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al-Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kotur, N.; Skakic, A.; Klaassen, K.; Gasic, V.; Zukic, B.; Skodric-Trifunovic, V.; Stjepanovic, M.; Zivkovic, Z.; Ostojic, O.; Stevanovic, G.; et al. Association of vitamin D, zinc and selenium related genetic variants with COVID-19 disease severity. Front. Nutr. 2021, 8, 689419. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratta-Pasini, A.M.; Stranieri, C.; Girelli, D.; Busti, F.; Cominacini, L. Is Ferroptosis a key component of the process leading to multiorgan damage in COVID-19? Antioxidants 2021, 10, 1677. [Google Scholar] [CrossRef]
- Herzenberg, L.A.; De Rosa, S.C.; Dubs, J.G.; Roederer, M.; Anderson, M.T.; Ela, S.W.; Deresinski, S.C.; Herzenberg, L.A. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA 1997, 94, 1967–1972. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Warnock, M.; Harbaugh, A.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Knight, J.S.; Kanthi, Y.; Lawrence, D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021, 11, 1580. [Google Scholar] [CrossRef]






| Total n = 61 (100) Number and Percentage | Moderate n = 34 (56) Number and Percentage | Severe n = 27 (44) Number and Percentage | p | |
|---|---|---|---|---|
| Women | 17 (28) | 11 (32) | 6 (22) | NS |
| Men | 44 (72) | 23 (68) | 21 (78) | NS |
| Age | 56 ± 13 | 54 ± 12 | 59 ± 14 | NS |
| BMI (kg/m2) | 29 ± 4 | 29 ± 4 | 29 ± 4 | NS |
| Temperature (°C) | 36.6 ± 0.46 | 36.5 ± 0.43 | 36.7 ± 0.49 | NS |
| Laboratory at the admission Median (Min–Max) range | ||||
| PAO2 (58.5–67.1 mmHg) | 66.9 (34–223) | 67 (34–223) | 66.4 (62–93) | NS |
| PCO2 (30.4–40 mmHg) | 31.7 (12.2–81.2) | 32 (12.2–81.2) | 31.6 (22–71) | NS |
| PAO2/FIO2 (>164) | 128 (26.8–299) | 145 (26.8–281) | 113 (30–299) | NS |
| SpO2/FIO2 (>300) | 138 (50–280) | 157 (88–240) | 128 (50–280) | 0.007 |
| Urea (<40 mg/dL) | 16 (5.6–106.7) | 29.2 (16–60) | 30 (13–224) | NS |
| Creatinine (mg/dL) | 0.90 (0.5–5.3) | 1 (0.5–2.5) | 0.8 (0.5–5.3) | NS |
| Ureic Nitrogen (7–25 mg/L) | 16 (5.6–196-7) | 15.7 (7.5–36) | 17 (5.6–106.7) | NS |
| TC (<200 mg/dL) | 136 (69–217) | 140 (69–217) | 132 (86–190) | NS |
| HDL (mg/dL) | 31 (14–60) | 32 (14–60) | 31 (14–45) | NS |
| LDL (mg/dL) | 70 (28–140) | 64 (35–135) | 79 (28–140) | NS |
| DHL (mg/dL) | 253 (124–515) | 233 (128–412) | 255 (124–515) | NS |
| TB (mg/dL) | 0.60 (0.12–4.10) | 0.50 (0.12–1.3) | 0.70 (0.33–4.10) | 0.02 |
| DB (mg/dL) | 0.20 (0.10–1.20) | 0.20 (0.10–1.2) | 0.20 (0.10–0.8) | NS |
| TG (<150 mg/dL) | 133 (62–726) | 137 (62–726) | 133 (77–328) | NS |
| Leukocytes (3.5–10.3 × 103/µL) | 8.8 (2–25) | 8.2 (2–14) | 11.5 (3–25) | 0.003 |
| Lymphocytes (0.99–3.2 × 103/µL) | 0.8 (0.14–9.6) | 0.92 (0.42–9.6) | 0.69 (0.14–8.2) | 0.01 |
| Platelets (150,000–500,000 × 103/µL) | 244 (16–576) | 226 (16–576) | 254 (122–412) | 0.057 |
| Ferritin (11–307 ng/mL) | 541 (147–2592) | 513 (147–2100) | 592 (175–2592) | NS |
| IL-6 (pg/mL9) | 67 (7.8–638.5) | 30.2 (7.8–304) | 94 (7.8–639) | 0.003 |
| Index N/L | 11.5 (1–89) | 10 (3–89) | 13 (1–83) | NS |
| D-Dimer (0–24 µg/mL) | 700 (136–16440) | 615 (136–5130) | 810 (210–16,640) | 0.08 |
| CRP (1–3 mg/L) | 146 (20–2450) | 280 (20–1380) | 146 (32–2450) | NS |
| Comorbidities (%) | ||||
| DM | 6 (10) | 4 (7) | 2 (3) | NS |
| SAH | 5 (8) | 3 (5) | 2 (3) | NS |
| Dyslipidemia | 11 (18) | 8 (13) | 3 (5) | NS |
| DM + Dyslipidemia | 5 (8) | 2 (3) | 3 (5) | NS |
| DM + SAH | 5 (8) | 2 (3) | 3 (5) | NS |
| SAH + Dyslipidemia | 3 (5) | 0 | 2 (5) | NS |
| SM | 9 (15) | 6 (10) | 3 (5) | NS |
| Healthy Subjects without comorbidities | 17 (28) | 8 (13) | 9 (15) | NS |
| Normal weight | 13 (21) | 6 (18) | 7 (26) | NS |
| Overweight | 24 (39) | 17 (50) | 7 (26) | 0.06 |
| Obesity | 24 (39) | 11 (33) | 13 (48) | NS |
| COPD | 1 (1.6) | 0 | 1 (4) | NS |
| ECKD | 2 (3.3) | 0 | 2 (8) | NS |
| Norepinephrine | 18 (29) | 1 (3) | 17 (63) | 0.0001 |
| Enteral nutrition | 26 (43) | 22 (65) | 4 (15) | 0.001 |
| Deaths | 1 (1.6) | 0 | 1 (4) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, M.E.; Guarner-Lans, V.; Díaz-Díaz, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Aisa-Álvarez, A.; Saucedo-Orozco, H.; Pérez-Torres, I. Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients. Cells 2022, 11, 932. https://doi.org/10.3390/cells11060932
Soto ME, Guarner-Lans V, Díaz-Díaz E, Manzano-Pech L, Palacios-Chavarría A, Valdez-Vázquez RR, Aisa-Álvarez A, Saucedo-Orozco H, Pérez-Torres I. Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients. Cells. 2022; 11(6):932. https://doi.org/10.3390/cells11060932
Chicago/Turabian StyleSoto, María Elena, Verónica Guarner-Lans, Eulises Díaz-Díaz, Linaloe Manzano-Pech, Adrían Palacios-Chavarría, Rafael Ricardo Valdez-Vázquez, Alfredo Aisa-Álvarez, Huitzilihuitl Saucedo-Orozco, and Israel Pérez-Torres. 2022. "Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients" Cells 11, no. 6: 932. https://doi.org/10.3390/cells11060932